Effects of narrow plant spacing on root distribution and physiological nitrogen use efficiency in summer maize
Wenshun Jiang,Kongjun Wang,Qiuping Wu,Shuting Dong,Peng Liu,Jiwang Zhang*
State Key Laboratory of Crop Biology/Agronomy College,Shandong Agricultural University,Tai'an,Shandong 271018,China
1.Introduction
Population structure is of great importance for maximizing yield in crops.Plant density acts as a key factor in regulating plant competition within the population and optimal plant densities are very important for efficient agronomic practice.Plant spacing varies with the growth of plants and the growing environments[1].To date,diverse planting patterns,such as narrow spacing[2,3],wide–narrow rows [4–6],and multiple-plant hill plots [7],have been developed in maize (Zea mays L.) in pursuit of high grain yields under different growing conditions.Studies addressing the effects of plant spacing on yield have largely focused on improvement of above-ground canopy structure,resulting in photosynthetic rate increases via effective interception of solar radiation[3,6]or better photosynthetic performance of ear leaves [7].These strategies often result in reduction in plant competition for light resources at high planting densities.However,individual plants always compete for nutrition,water and root space [8],and few reports are available regarding root nutrient absorption under different plant spacings.
The fibrous root system of maize radiates outward and more than 90%of the dry root weight in soil is distributed in the top 20 cm,and 60%in the soil region within 10 cm from each plant[9].Mineral nutrient absorption by roots results in the formation of a nutritional gradient zone around each individual.When the nutritional gradient zones of neighboring plants overlap,nutrient concentration in the overlapped area remarkably decreases because of interactions between adjacent roots,resulting in reduced root absorption efficiency[10].It has been demonstrated that root nutrient absorption in the overlapped area varies under different plant spacing strategies.
Competing neighboring roots can deplete soil nutrient resources and thus inhibit root growth.With other things being equal,plants grow roots preferentially in areas free of other roots[11].Plant roots do not interact solely through the depletion of soil resources but may also interact,causing profound consequences for plant growth and competition[12].Schenk provided an excellent summary of direct interactions between roots,and distinguished between two classes of interaction [13].First,roots may exude toxic substances that cause non-specific inhibitory effects on root development of neighboring plants.Second,genetically identical plants may use non-toxic chemical signals that specifically affect the roots of neighbors.Increasing numbers of studies have shown that plants produce more root mass when sharing rooting space with a genetically similar neighbor compared with plants growing alone [11,14].This phenomenon has been described as a“tragedy of commons”[15].However,Hess and Kroon hypothesized that root overproduction in the presence of other plants is consistent with the effects of available larger soil volumes on plants with competition than on those growing alone [12].Earlier,McConnaughay and Loh showed that root mass is a function of the available rooting volume,independent of the available nutrients [16,17].Furthermore,some of the observed root overproduction could not be immediately explained solely based on soil volume and nutrient availability[12].The results observed with competing plants may be an overall effect of the existence of interplant root interactions within a larger space.
Therefore,a thorough understanding of the effects of overlapping roots on maize root growth and nitrogen absorption and utilization will help to explore the effects of plant spacing on maize yields.In recent years,it was proposed that increasing plant populations is a key factor for improvement of maize yields in China [7,18],but few reports are available on competition between above-ground and below-ground factors while increasing plant populations.In this study,the differences between root distribution,nutrient absorption and nitrogen utilization under different conditions of plant spacing and nitrogen availability were investigated to provide guidelines for optimizing plant densities in high yield maize production.
2.Materials and methods
2.1.Plant materials and experimental designs
The field experiment was carried out at the Experimental Farm of Shandong Agricultural University,Tai'an,China (36°18′ N,117°13′E)in 2007 and 2008.Only one maize hybrid,Denghai 661,was used because previous experiments confirmed increased grain yield of this cultivar at high plant densities[18].
A box-type soil column cultivation method was adopted.The soil column measuring 54 cm × 27 cm × 100 cm was made of a PVC plate with bottom sealing,and one side of the planter could be dismantled to facilitate removal of the soil and roots without damage to the study materials.The soil column was placed in an 80 cm deep square pit filled with soil both inside and outside the column and made soil compact by watering.The distance between soil columns was 11 cm,that is,the row width was 65 cm,and surrounded by the board rows(Fig.1).
Two plants were grown in each soil column.Plants in one column were planted under normal spacing(NS,27 cm),and the other under narrow spacing (CS,6 cm).The columns were treated at two nitrogen levels,N0(no N)and N1(7.5 g N plant-1),and for the N1 treatment,nitrogen fertilizer was applied by 20%,50%and 30%at the seedling,male-tetrad and flowering stages,respectively.The experimental design included four treatments(N0 × NS,N0 × CS,N1 × NS and N1 × CS) and 30 separate soil columns were planted in each treatment.
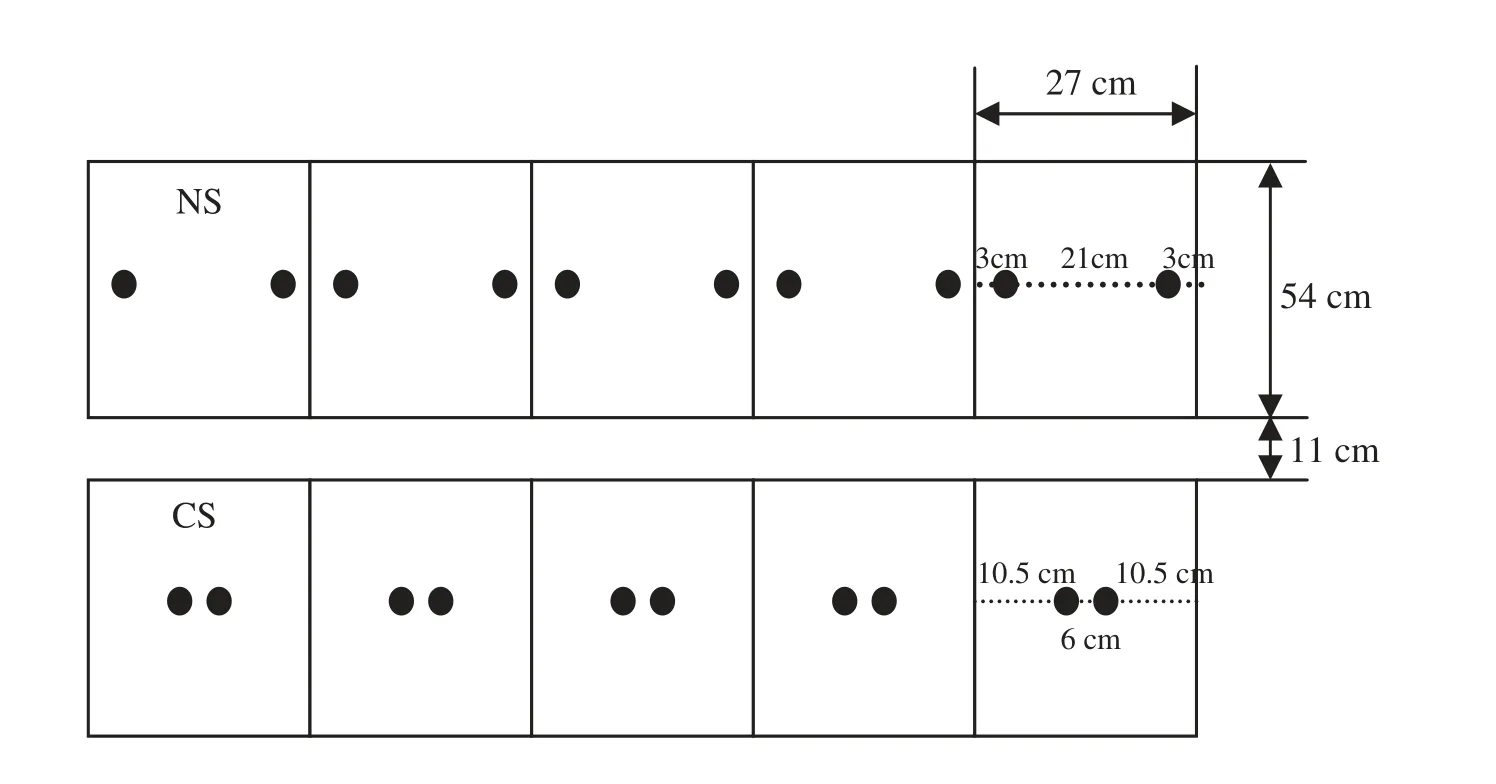
Fig.1-Field arrangement of different experiments.
Samples of the soil columns (top 40 cm) were mixed and screened with 20 mesh sieving.Then they were mixed with clean river sand in a ratio of 3:1 by volume of topsoil to sand.The mixed soil nutrient contents were as follows: organic matter 7.1 g kg-1,total nitrogen (N) 0.62 g kg-1,mean available mineral phosphorous (P) 46 mg kg-1,and exchangeable potassium(K) 59 mg kg-1.
All treatments were fertilized with P and K according to nutrient demand,and each unit of experimental treatment was fertilized with 2.5 g of phosphate (P2O5) and 6.25 g of potash(K2O),with both applied at the seedling stage.Required irrigation was also applied from the outlet of a pump by using plastic pipes.
2.2.Measurements and calculation
At the onset of pollination,three replicates of each treatment were sampled on the same day fortnightly.The above-ground plant parts were divided into leaves,grains and stems(remaining parts except for leaves and grains).Roots were separated from various layers of the soil profile,viz.0–20,20–40,40–70 and >70 cm,and washed to remove all soil residues.Root layers were mixed well after removing impurities,and fine roots were selected and temporarily stored at 0 °C.2,3,5-triphenyl tetrazolium chloride(TTC)reduction was applied to determine root reductive activity [19]; fresh root samples (0.5 g) were exposed to 0.4% TTC and 0.2 mol L-1tricine-HCl buffer(pH 8.4),then placed in a darkroom at 37 °C for 6 h to induce reduction of TTC to triphenyl formazan(TTF),following the method described by Duncan and Widholm [19].To quantify the amount of TTC reduced,we extracted the tissues with 95% ethanol at room temperature for 48 h,and then performed spectrophotometric analysis at 485 nm.The results were expressed as μg TTF g-1root mass h-1.At maturity,the remaining aboveground parts were completely harvested to calculate average dry matter weight per plant and weight distribution.
The remaining roots and above-ground samples were fixed at 105 °C for 30 min.Samples were subsequently baked at 75 °C until a constant weight was reached and recorded.The dried samples were smashed and steamed with H2SO4–H2O2.Nitrogen content was measured using semi-micro-kjeldahl determination[20].
Nitrogen parameters were calculated following the method of Moll et al.[21]:
Nitrogen accumulation (g plant-1) = plant nitrogen content(%) × biomass(g plant-1)
N use efficiency in grain (NUEg) = grain yield / total plant nitrogen accumulation
软件开发过程实现开放化管理,也就是实现对资源的共享,通过公开软件源代码,使得软件产品的标准化工作逐步被推动起来,软件的兼容性问题能够进一步提高,进而能够达到对资源共享的目的。同时,软件开发设计人员在未来可以实现彼此之间的相互交流,实现共同进步,也可以彼此之间通力合作,实现计算机软件行业的不断可持续发展。
N use efficiency per plant (NUEbiomass) = biomass / total plant nitrogen accumulation
N harvest index(NHI) = grain nitrogen content / total plant nitrogen accumulation
N partial factor productivity in grain(PFPN) = grain yield /total plant nitrogen applied
Transfer volume = plant vegetative nitrogen content at flowering–plant vegetative nitrogen content at maturity
Transfer rate = transfer volume/plant vegetative nitrogen content at flowering.
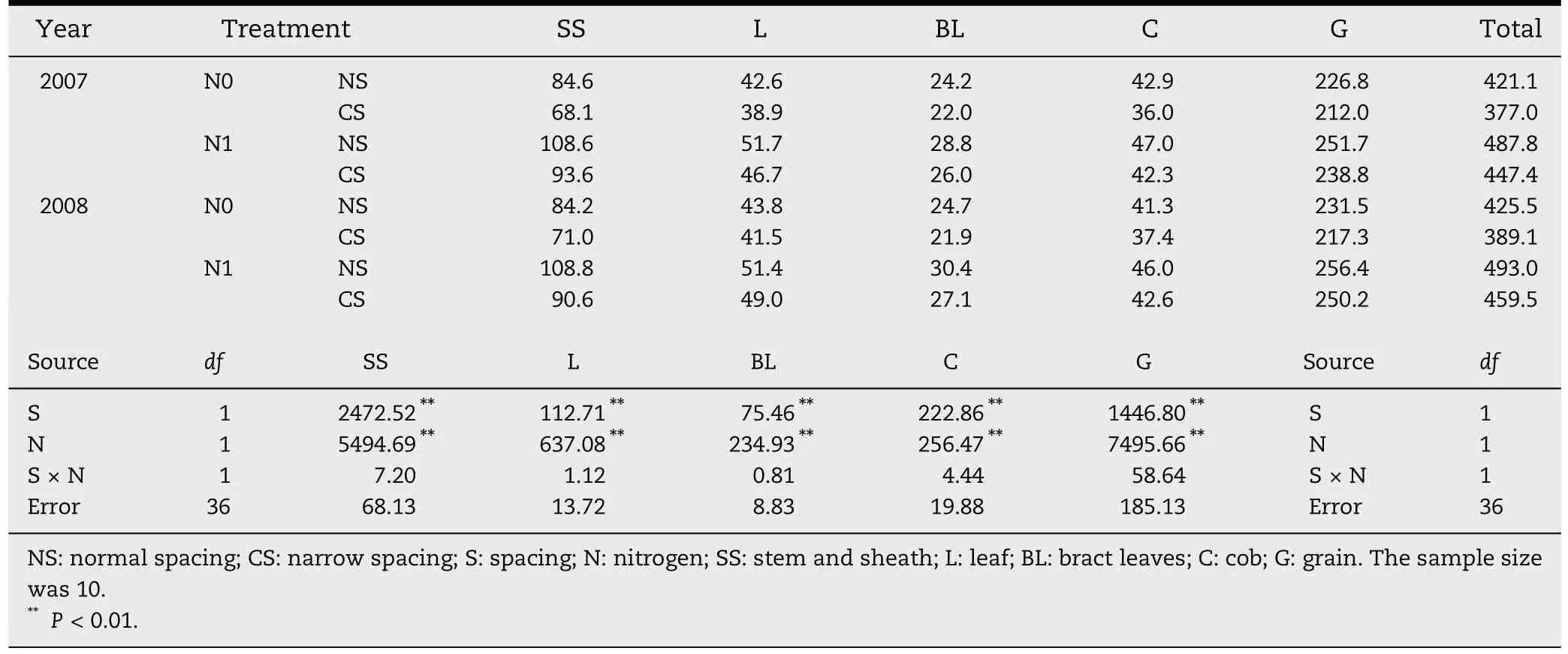
Table 1-Effects of plant spacing and nitrogen treatments on dry matter accumulation in above ground organs(g plant-1).
2.3.Statistical analysis
Data were statistically evaluated by one-way analysis of variance(ANOVA) with the program Data Processing System [22].Duncan's multiple range test was carried out to determine if significant(P <0.05)differences occurred between treatments.
3.Results
3.1.Dry matter accumulation
Significant effects of plant spacing and nitrogen on dry matter accumulation (P <0.01) were detected in each plant part (stem and sheath-SS,leaves-L,bract leaves-BL,cob-C and grain-G).However,no significant interaction was found between plant spacing and nitrogen.Compared with NS,under CS dry matter accumulation of SS,L,BL,C and G decreased respectively by 16.3%,7.1%,10.2%,10.7% and 5.0%,and the average decrease in aboveground dry weight was 8.4%.Further multiple comparisons among all treatments showed that with and without N application,CS did not significantly reduce grain yield,but reduced biomass by 7.5%for N0 and 9.5%for N1(Table 1).
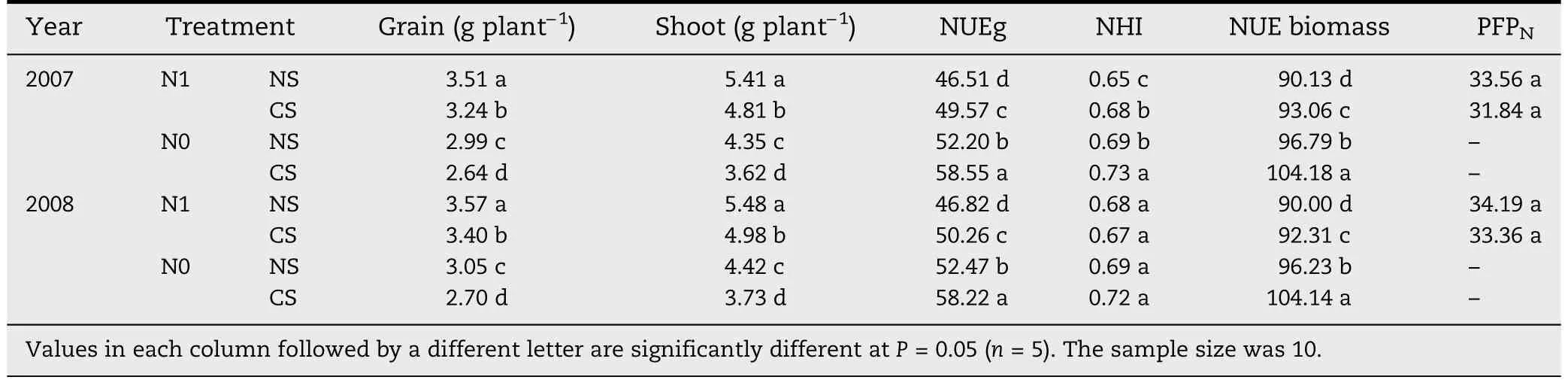
Table 2-Nitrogen accumulation and utilization under different plant spacing treatments.
3.2.Nitrogen accumulation and utilization
3.3.Nitrogen absorption and translocation
Compared with NS,SS nitrogen accumulation at silking and maturity were significantly lower under CS(P <0.05).R nitrogen accumulation was significantly lower for N1 at the maturity stage (P <0.05),and leaf nitrogen accumulation in N0 significantly decreased(P <0.05)under CS.Compared to NS,the total nitrogen accumulation of R,L and SS in CS treatment were significantly lower(P <0.05),with 12.8%and 20.9%decreases at the silking and maturity stages,respectively.However,the nitrogen translocation rates of R,L and SS in CS increased by 23.9%(Table 3).
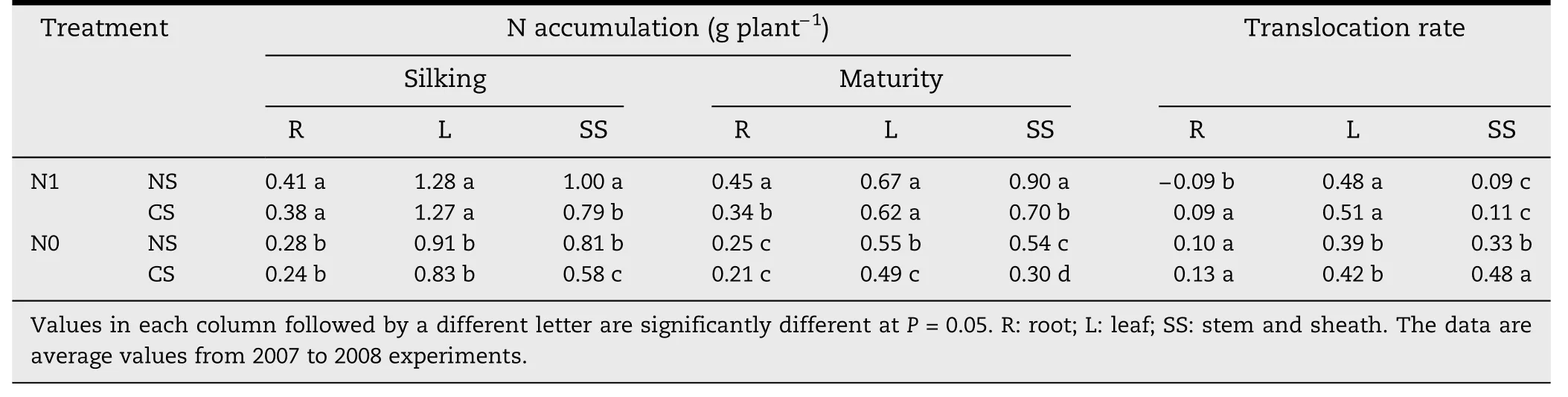
Table 3-Nitrogen accumulation and translocation in roots,leaves,stems and sheaths under different plant spacing treatments.
3.4.Temporal and spatial distribution of dry root weight
Compared with NS,dry root weight of CS was lower in the 0–20 cm root layer at both nitrogen levels,and dry root weights in the 20–40 cm and 40–70 cm layers were also slightly reduced at late grain filling.However,dry root weight at 70–100 cm for the closely spaced plants remained fairly constant during the entire period.Closely spaced plants showed a significant decrease in dry root matter in the 0–20 cm layer; and the ratio of dry root weight/biomass and total dry root weight also showed obvious declines(Fig.2).
N application resulted in a change in dry root weight from each soil layer throughout plant development (to maturity)and exhibited a single-peak curve at specific days post pollination (Fig.2).Dry root weight in the 0–20 cm soil layer peaked at 14 d after pollination,and at 28 d for soils 20–40 cm and below.In the N0 treatment,dry root weight in the 0–20 cm layer peaked 14 d after pollination,but below 20 cm the dry root weight was reduced.Compared with N1,the N0 treatment showed a significant(P <0.05)decrease in dry root weight at 0–20 cm soil depth,but there was a significant(P <0.05)increase in the 70–100 cm layer.Changes in dry root weight in the 20–40 cm and 40–70 cm soil layers were not significantly different;however,the deep root ratio of N0 was significantly higher than that of N1.
3.5.Spatial and temporal distribution of root reductive activity
Root reductive activity is a comprehensive index that reflects root absorption function[13].After pollination,root reductive activity in each soil layer changed as the plants matured(Fig.3),exhibiting single-peak increases before decreasing.Under N1,root reductive activities underwent significant increases in the 0–20 cm and 20–40 cm soil layers,with peaks exhibiting prolonged durations.Root reductive activity in the 70–100 cm layer under N0 showed a steady decrease compared with N1.Under both nitrogen levels,root reductive activity decreased in each layer of closely spaced plants,and the greatest difference between treatments was observed during the grain-filling stage.At late grain filling,differences were not as evident.
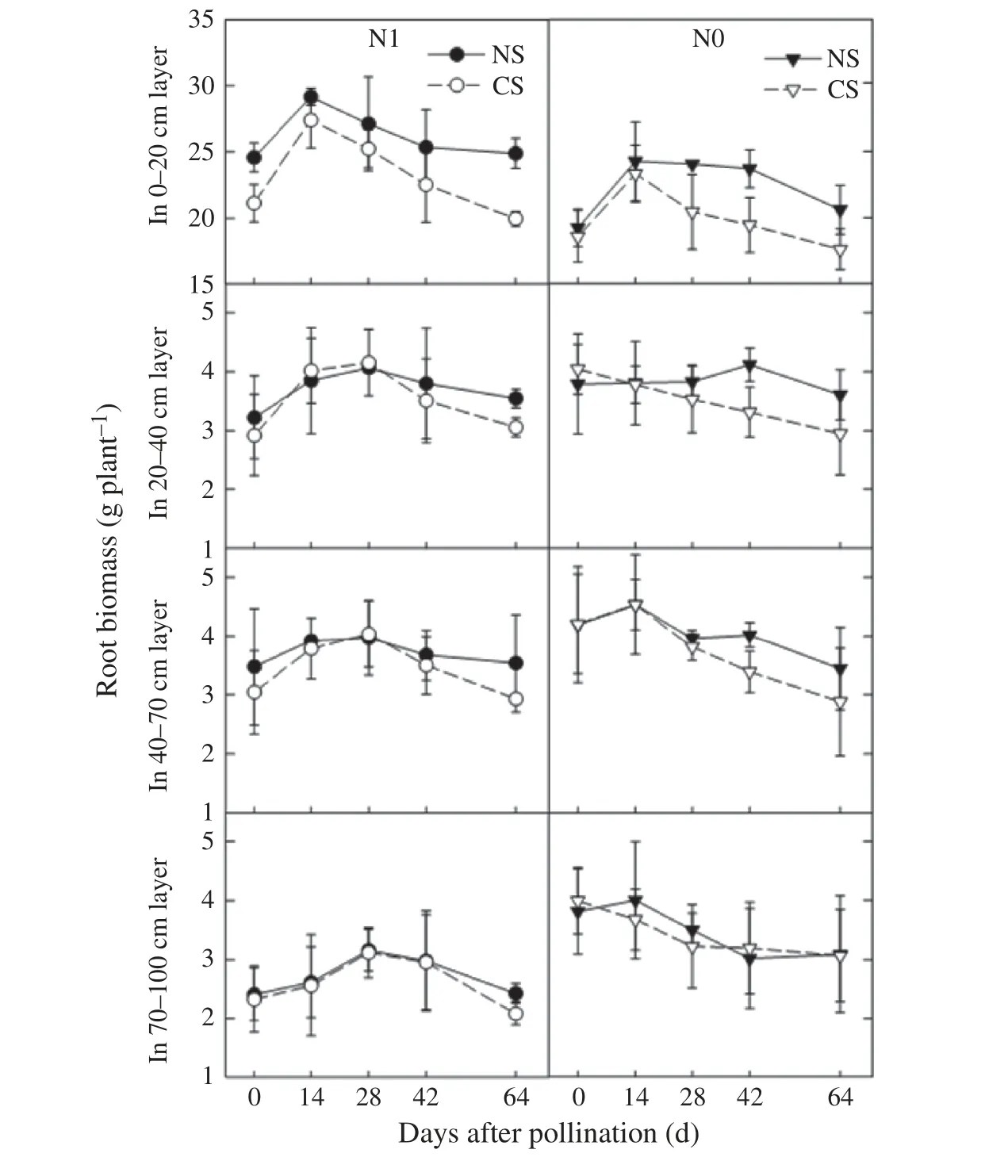
Fig.2-Dynamics of dry root weight in each soil layer with different plant spacing treatments.
4.Discussion
The effects of different plant spacing treatments on maize grain yield are influenced by interactions between aboveground and belowground resource competitions.Compared with competition for light aboveground,nutrient competition in roots includes more than 20 nutrient elements,which have substantial differences in molecular weight,soil oxidation state and mobility,and there are more significant effects of nutrient competition in roots on the growth of plant[8].
Narrow spacing is chosen most often to increase photosynthetic capacity by increasing the interception of available solar radiation,resulting in improved maize yield[6].However,some studies have demonstrated that an increase in solar radiation does not increase but decrease maize production[23,24].In this study,excluding interference due to aboveground competition for light,narrow spaced plants significantly decreased aboveground dry matter accumulation and grain yield by 8.4%and 5.0%,respectively.
Aboveground dry weight and grain production are closely related to nitrogen accumulation,translocation and utilization.Above-ground nitrogen accumulation in the narrow plant spacing treatment was decreased by an average 12.8%.However,compared with the normal spacing treatment,nitrogen translocation in roots,leaves and stem-sheaths significantly increased under narrow spacing following pollination,with increases in nitrogen harvest index and utilization being also observed.In other studies lower nitrogen accumulation treatment exhibited higher translocation rates and nitrogen utilization [25,26],and partially alleviated nitrogen shortage in yield.
Nitrogen uptake relies mainly on root biomass,root spatial distribution and per unit root nitrogen uptake rate [27].In addition,nitrogen uptake by neighboring plants can limit nitrogen accumulation [8].Narrow spacing significantly increased nutrient absorption in areas of adjacent overlapping plants,especially when neighboring plants exhibited similar root architecture.However nutrient concentration in the overlapped areas markedly declined,decreasing nutrient uptake.Sharratt et al.and Barbieri et al.both suggested that uniform plant distributions are conducive to water and nitrogen uptake [3,28].Because of root plasticity,lower nutrient concentrations in nutritional absorption of overlapped areas may limit the horizontal distribution of root systems[29].In the present study,dry root weight in the 0–20 cm soil layer under narrow spacing was significantly decreased,and root reductive activity in all soil layers was clearly lower during the active grain-filling stage relative to normal spacing.Root size plays a leading role in nitrogen uptake,and roots in the upper soil layer have advantages in nutrient uptake [18]; however,reductions in root biomass,percentage of root in shallow soil layer and root reductive activity all circumvent nitrogen uptake.
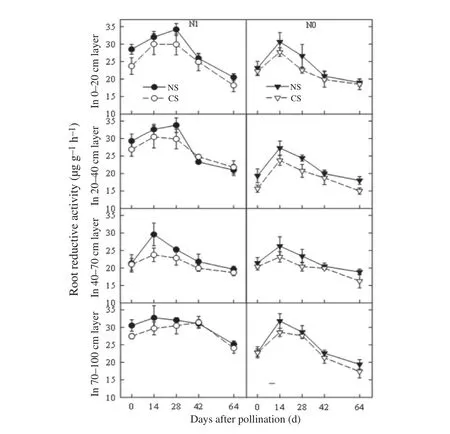
Fig.3-Dynamics of root reductive activity in each soil layer for different plant spacing treatments.
5.Conclusions
Dry root weights of narrow spaced plants were significantly lower in the shallow soil layer,and root reductive activity in each soil layer was markedly reduced,along with lower root biomass and plant nitrogen uptake.Narrow spacing led to higher nitrogen use efficiency in grain,harvest index and dry matter production capacity.The nitrogen translocation rates of roots,leaves and stem-sheaths were higher during grain formation.However,these increases did not compensate for the impact of decreased nitrogen accumulation on production.Thus grain yield increases in summer maize could be achieved with modest increases in plant density.
This research was supported by the National Natural Science Fund (No.31271662),Shandong Province Maize Industry Technology System,Special Fund for Agro-scientific Research in the Public Interest (No.201103003),and State Programs of Science and Technology Development(No.2011BAD16B09).
[1] S.L.Zhao,F.M.Li,D.Y.Zhang,S.S.Duan,Crop production is a population progress,Acta Ecol.Sin.17(1997) 100–104,(in Chinese with English abstract).
[2] W.D.Widdicombe,K.D.Thelen,Row width and plant density effects on corn grain production in the northern corn belt,Agron.J.94 (2002) 1020–1023.
[3] B.S.Sharratt,D.A.Mcwilliams,Microclimatic and rooting characteristics of narrow-row versus conventional-row com,Agron.J.97 (2005) 1129–1135.
[4] H.Gozubenli,M.Kilinc,O.Sener,O.Konuskan,Effects of single and twin row planting on yield and yield components in maize,Asian J.Plant Sci.3(2004) 203–206.
[5] J.Q.Xue,G.S.Ma,H.D.Lu,Studies of compact and big ear type maize Shendan 902 on population characters of sink-source and yield formation,Acta Boreal-Occident.Sin.22(2002)1336–1342,(in Chinese with English abstract).
[6] F.H.Andrade,P.Calvino,A.Cirilo,P.Barbieri,Yield responses to narrow rows depend on increased radiation interception,Agron.J.94(2002) 975–980.
[7] J.M.Xu,H.Q.Gao,S.G.Mao,X.Wang,C.S.Li,Y.Q.Ji,W.P.Lu,Effects of wide row space double plant cultivation on the characteristic of photosynthesis at later growth stage in maize (Zea mays L.),Yangzhou Univ.J.1 (2008) 66–70,(in Chinese with English abstract).
[8] B.B.Casper,R.B.Jackson,Plant competition underground,Annu.Rev.Ecol.Syst.28(1997) 545–570.
[9] R.Song,C.S.Wu,Y.L.Ma,J.X.Guo,F.Xing,Comparison of roots distribution in different maize plant type cultivars in the Songnen Plain,Chin.J.Appl.Ecol.11(2003) 1911–1913,(in Chinese with English abstract).
[10] G.Rubio,T.Walk,Z.Y.Ge,X.Yan,H.Liao,J.P.Lynch,Root gravitropism and below-ground competition among neighboring plants:a modelling approach,Ann.Bot.88(2001)929–940.
[11] M.Gersani,J.S.Brown,E.E.O'Brien,Tragedy of the commons as a result of root competition,Ecol.J.89 (2001)660–669.
[12] L.Hess,H.de Kroon,Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination,Ecol J 95 (2007) 241–251.
[13] H.J.Schenk,R.M.Callaway,B.E.Mahall,Spatial root segregation: are plants territorial? Adv.Ecol.Res.28(1999)145–165.
[14] E.E.O'Brien,M.Gersani,J.S.Brown,Root proliferation and seed yield in response to spatial heterogeneity of below-ground competition,New Phytol.168 (2005) 401–412.
[15] G.Hardin,The tragedy of the commons,Science 162 (1968)1243–1248.
[16] K.D.M.McConnaughay,F.A.Bazzaz,Is physical space a soil resource?Ecology 72(1991) 94–103.
[17] F.C.Loh,J.C.Grabosky,N.L.Bassuk,Growth response of Ficus benjamina to limited soil volume and soil dilution in a skeletal soil container study,Urban For.Urban Green.2 (2003) 53–62.
[18] W.S.Jiang,K.J.Wang,Q.P.Wu,Y.J.Wang,S.T.Dong,P.Liu,J.W.Zhang,Root distribution and competitive ability of summer corn Denghai 3719,Acta Agron.Sin.34 (2008)1650–1655,(in Chinese with English abstract).
[19] D.R.Duncan,J.M.Widholm,Osmotic induced stimulation of the reduction of the viability dye 2,3,5-triphenyltetrazolium chloride by maize roots and callus cultures,J.Plant Physiol.161 (2004) 397–403.
[20] Z.L.Zhang,Laboratory Guidance for Plant Physiology Experiments,Higher Education Press,Beijing,1992.88–93,(in Chinese).
[21] R.H.Moll,E.J.Kamprath,W.A.Jackson,Analysis and interpretation of factor which contribute to efficiency of nitrogen utilization,Agron.J.74(1982) 562–564.
[22] Q.Y.Tang,M.G.Feng,DPS Data Processing System:Experimental Design,Statistical and Data Mining,Science Press,Beijing,2007.85–105.
[23] D.E.Farnham,Row spacing,plant density,and hybrid effects on corn grain yield and moisture,Agron.J.93(2001)1049–1053.
[24] P.Pedersen,J.G.Lauer,Corn and soybean responses to rotation sequence,row spacing,and tillage system,Agron.J.95(2003) 965–971.
[25] Z.X.Yi,P.Wang,L.X.Shen,H.F.Zhang,M.Liu,M.H.Dai,Effects of different types of nitrogen fertilizer on nitrogen accumulation,translocation and nitrogen fertilizer utilization in summer maize,Acta Agron.Sin.32(2006)772–778,(in Chinese with English abstract).
[26] Y.N.Fan,S.Q.Li,S.X.Li,Effect of weed and nitrogen application on grain yield of summer maize and nitrogen use in semi-moist field,Plant Nutr.Fertil.Sci.14(2008) 252–257.
[27] B.Eghball,J.W.Maranville,Root development and nitrogen influx of corn genotypes grown under combined drought and N stress,Agron.J.85 (1993) 147–152.
[28] P.A.Barbieri,H.E.Echeverria,H.R.Sainz Rozas,F.H.Andrade,Nitrogen use efficiency in maize as affected by nitrogen availability and row spacing,Agron.J.100 (2008) 1094–1100.
[29] A.Hodge,Plastic plants and patchy soils,J.Exp.Bot.57(2006)401–411.
- The Crop Journal的其它文章
- Identification and fine mapping of two blast resistance genes in rice cultivar 93-11
- Zea mays(L.) P1 locus for cob glume color identified as a post-domestication selection target with an effect ontemperate maize genomes
- Identification of unconditional and conditional QTL for oil,protein and starch content in maize
- Variation of high-molecular-weight glutenin subunits and glutenin macropolymer particle distribution in wheat grains produced under different water regimes
- Dissection of two quantitative trait loci for grain weight linked in repulsion on the long arm of chromosome 1 of rice(Oryza sativa L.)
- Genome-wide association of 10 horticultural traits with expressed sequence tag-derived SNP markers in a collection of lettuce lines

