Application of Discrete Lumped Kinetic Modeling on Vacuum Gas Oil Hydrocracking
Han Longnian; Fang Xiangchen; Peng Chong; Zhao Tao
(1. Liaoning Shihua University, Fushun, Liaoning 113001; 2. SINOPEC Fushun Research Institute of Petroleum and Petrochemicals)
Application of Discrete Lumped Kinetic Modeling on Vacuum Gas Oil Hydrocracking
Han Longnian1; Fang Xiangchen2; Peng Chong2; Zhao Tao1
(1. Liaoning Shihua University, Fushun, Liaoning 113001; 2. SINOPEC Fushun Research Institute of Petroleum and Petrochemicals)
The kinetic model of vacuum gas oil (VGO) hydrocracking based on discrete lumped approach was investigated, and some improvement was put forward at the same time in this article. A parallel reaction scheme to describe the conversion of VGO into products (gases, gasoline, and diesel) proposed by Orochko was used. The different experimental data were analyzed statistically and then the product distribution and kinetic parameters were simulated by available data. Furthermore, the kinetic parameters were correlated based on the feed property, reaction temperature, and catalyst activity. An optimization code in Matlab 2011b was written to fine-tune these parameters. The model had a favorable ability to predict the product distribution and there was a good agreement between the model predictions and experiment data. Hence, the kinetic parameters indeed had something to do with feed properties, reaction temperature and catalyst activity.
hydrocracking; kinetic modeling; vacuum gas oil (VGO); optimization code; parallel reaction scheme
1 Introduction
Faced with a growing demand for middle distillate and an increasing production of heavy crude oils, the hydrocracking process has become one of the most important secondary petroleum refining processes. This process is versatile, flexible and can be strongly adapted to inferior feed oil with a strong capability to convert the heavy, high-boiling, and high EBP (end boiling point) feedstock to smaller, lower-boiling ones like qualified jet fuel, diesel, lubrication base oil, and naphtha for chemical use[1]. Hydrocracking takes place over a dual-functional catalyst in a hydrogen-rich, high temperature atmosphere, with other reactions, including hydrodesulfurization, hydrodenitrogenation and other hydrotreating reactions, occurring simultaneously[2]. Despite the successful application of hydrocracking technology in commercial scale, the research on its reaction mechanism and reaction kinetics still lags behind the customer needs.
There are various kinetic models for the VGO hydrocracking reported in the literature, and the lumped model and detailed molecular model[3]are regarded as two main approaches being studied for a long time. Moreover, all of the hydrocracking kinetic models could be included in the two approaches. Among them, the lumped models incorporate the fixed lumped models, discrete lumped models[4-7]and continuous mixtures involved lumped models[8-10]. For the hydrocracking models based on the lumping technique, these physical properties such as true boiling point (TBP), carbon number (CN), and molecular weight[11]are usually adopted to divide discrete pseudocomponents (lumps). For the fixed lumped model and discrete lumped model, the major disadvantage is that a change in the cutting scheme of the hydrocracker products or in the number of products requires reformulating the model parameters to refit the data. The so-called continuous lumped model is a general case developed from the discrete lumped model, which allows for prediction of the entire distillation curve, but the dependency of model parameters on feed properties still exists. Furthermore, the distillation curves of heavy oils are not accurate when the feedstock is a mixed oil consisting of VGO, CGO, DAO and other fractions. Detailed approaches include the structure oriented lumped models[12]and the single event models[13-14], which express the chemical transformations in terms of typical molecular structures and elementarysteps of cation chemistry, respectively. For a relatively large number of pseudo-components the analytical information and experimental data are required, which impose restrictions on their applications to hydrocracking of real feedstocks.
The complexity of real feedstocks suggests that lumped model will continue to be used for the research on VGO hydrocracking kinetics. However, detailed approaches need to be studied more precisely in order to obtain a better understanding of hydrocracking kinetics and provide an idea to optimize lumped model for prediction of hydrocracking product properties. A good kinetic model is a useful tool for reactor design, simulation, and optimization of oil refining processes.
To have a better understanding of the VGO hydrocracking, in this work we conducted some experiments in a pilot plant equipped with two downflow fixed-bed reactors and developed a discrete lumped kinetic model, which will be presented in further papers.
2 Experimental Data and Kinetic Model
2.1 Experimental setup and feedstock
The experiments were conducted in a single-stage series fixed-bed high-pressure pilot plant, which is shown schematically in Figure 1. Hydrocracking was conducted in a downflow mode of operation. The hydrotreating catalyst was loaded in a hydrotreating reactor and the hydrocracking catalyst (A) was loaded in a hydrocracking reactor. The reactor temperature was controlled at the desired level by using a three-zone electric furnace providing an isothermal temperature condition along the reaction section. The temperature inside the reactor was measured by a movable axial thermocouple located inside the reactor. Three typical heavy VGO samples, including Iranian VGO, Saudi VGO and mixed oil (Iranian VGO: CGO=8:2 sampled from Zhenhai refinery), were chosen as the feed. Main properties of these feedstocks are listed in Table 1. These feedstocks had high density, EBP, BMCI and sulfur content so that severe hydrogenation operating conditions were needed in order to achieve the expected conversion degree and index requirements of product properties. Hydrogen gas was provided from an electrolysis hydrogen source, with the hydrogen purity exceeding 99.9%.
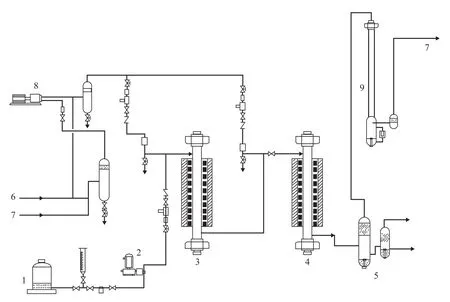
Figure 1 Experimental setup for high-pressure hydrocracking experiment
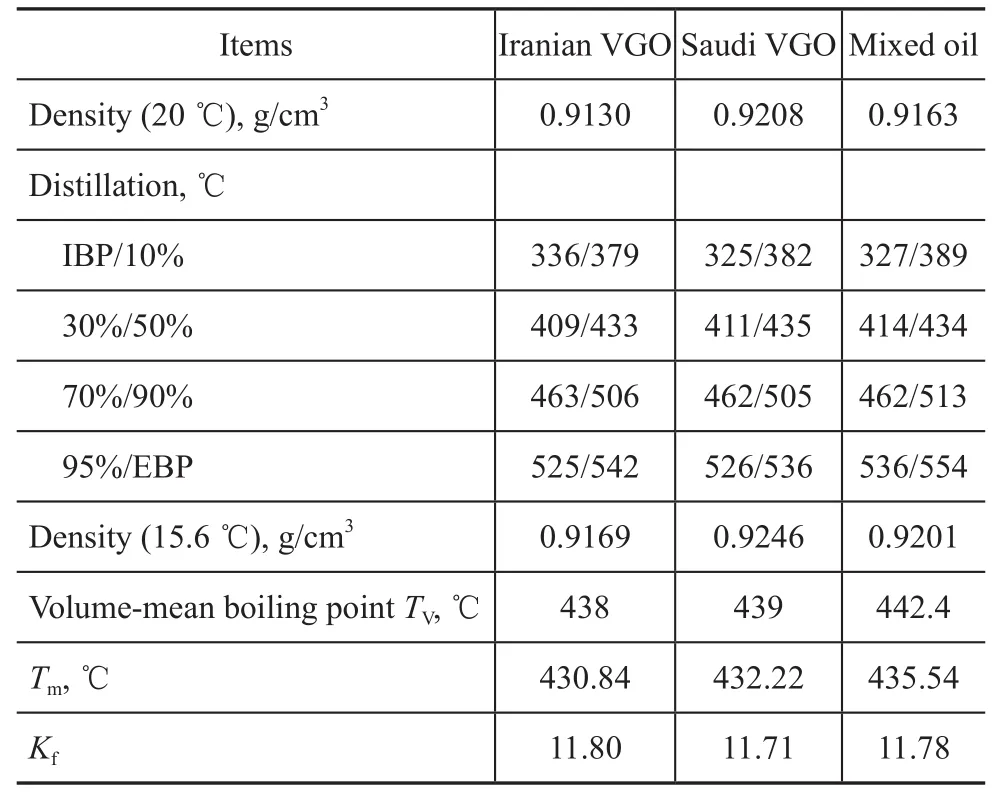
Table 1 Main properties of feedstock
2.2 Some properties of hydrocracking catalyst
There were several advanced hydrocracking catalysts with high middle distillate yield, and the hydrocracking catalyst A was a new generation catalyst developed on the base of reference catalyst by modifying the support (Si/Al) and Y zeolite, which was a W/Ni commercial catalyst employed in the experiments (with a specific surface area of 200 m2/g, a pore volume of 0.28 cm3/g, and a mean pore diameter of 1.5—1.7 mm). The catalyst A was loaded into the hydrocracker and activated in situ by sulfiding with kerosene containing CS2. This catalyst had high hydrogenation performance, high middle distillate yield, and suitable activity and stability, and demonstrated strong adaptability to inferior feedstock. It had been used in many hydrocrackers to deliver qualified middle distillates and hydrocracked residue with low BMCI value.
2.3 Experimental data of hydrocracking
Experiments on hydrocracking of Iranian VGO at different conversion rates were carried out under the operating condition specifying a partial hydrogen pressure of 15.7 MPa, a total LHSV of 1.5 h-1and a total H2/oil ratio of 1 500:1. Moreover, the nitrogen content of hydrotreated oil was controlled at around 10 µg/g. The products were analyzed by true boiling point distillation, with the product distribution presented in Table 2. It can be seen from Table 2 that the VGO conversion increased following the increase of reaction temperature, which was accompanied by an increase of kerosene yield but a decrease of diesel yield. Meanwhile, it is obvious that the middle distillate yield increased when the hydrocracking temperature increased from 381 ℃ to 390 ℃ and these results were consistent with the catalytic activity of the hydrocracking catalyst A.
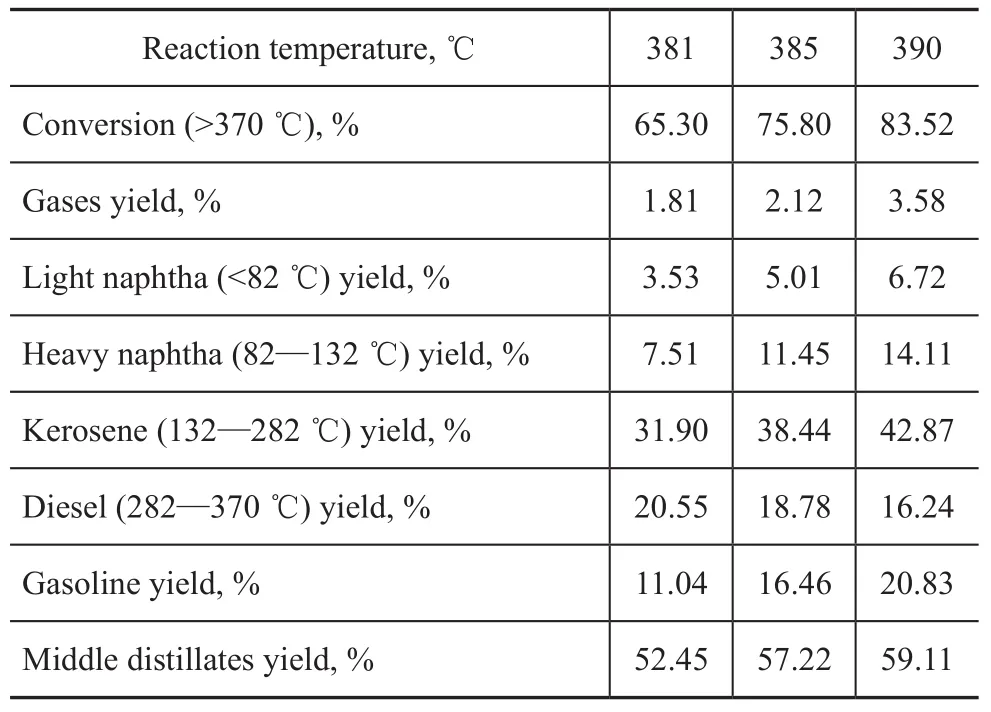
Table 2 Product yield distribution of Iran VGO hydrocracking
2.4 Kinetic model of VGO hydrocracking
In this study, the kinetic model developed by Orochko[15]was used to simulate the product distribution in hydrocracking process. The hydrocracking kinetics of vacuum gas oil from Iranian crude oils was studied in a fixed-bed reactor over the hydrocracking catalyst A using a firstorder kinetic scheme involving four lumps. The reaction scheme is shown in Figure 2. Theoretically speaking, the four lumped kinetic model schemes should include a series of reactions and parallel reactions. But the hydrocracking catalyst A has low cracking activity and rarely takes part in secondary cracking reaction. So only the parallel reaction of heavy oil cracking was considered in the model scheme, while ignoring the secondary reactions, namely the formation of diesel lump to gasoline, the gas lump, and gasoline lump to gas lump.
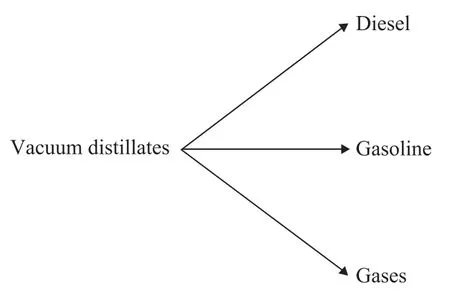
Figure 2 Reaction scheme for hydrocracking lump-kinetic model
In this study, gases lump (include LPG and off-test),gasoline lump (light and heavy naphtha), and diesel lump (kerosene and diesel) were considered as three of four lumps. Moreover, the hydrocracker products and yield are shown in Table 2. The experimental data of hydrocracking process were used to model the kinetic parameters of hydrocracking reactions.
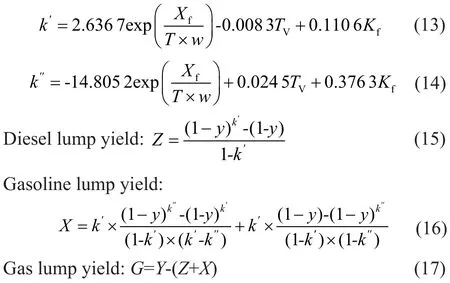
The hydrocracking process took place according to a retarded first-order reaction, which was the equation developed by Orochko[1]. Based on this method, the yields of diesel lump and gasoline lump can be expressed by the total conversionyand macro-kinetic parameters.
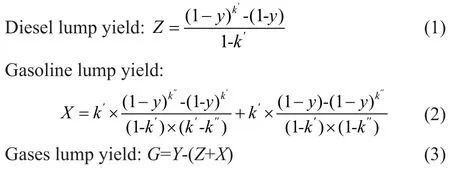
in whichZ,X, andGare experimental yield of diesel lump, gasoline lump and gases lump, respectively, andyis the conversion rate of VGO.
Among them, the macrokinetic parameters (k′andk″) depend on feed properties, process temperature and catalyst characteristics. All kinetic model parameters are determined by experimental data and simulated by an optimization procedure written in the Matlab 2011b. Furthermore, the objective function which determines the optimized model parameters is defined as follows.
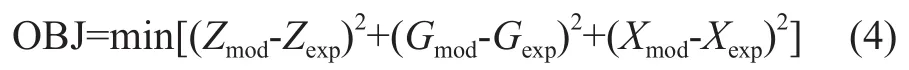
3 Results and Discussion
The operating conditions and experimental data of a pilotplant scale hydrocracker were used to calculate and validate the model parameters. The results of fine-tuning are shown in Table 3. By using these parameters, the product distribution can be calculated. A comparison between the model results for predicting the product yields is also shown in Table 3. It can be seen from Table 3 that there was an extraordinarily good agreement between model prediction results and experimental data. The absolute relative deviation (ARD) and mean square error (OBJ) of the model for predicting the product yields are defined as follows:
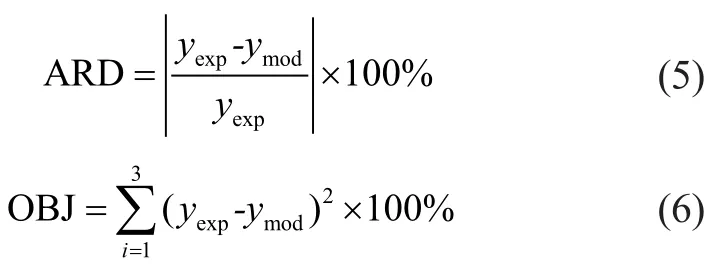
in which yexpand ymodare experimental and model yield of product, respectively. The ARD and OBJ of model predictions are shown in Table 3. It can be seen from Table 3 that the calculation error was small enough to meet the requirements of the model.
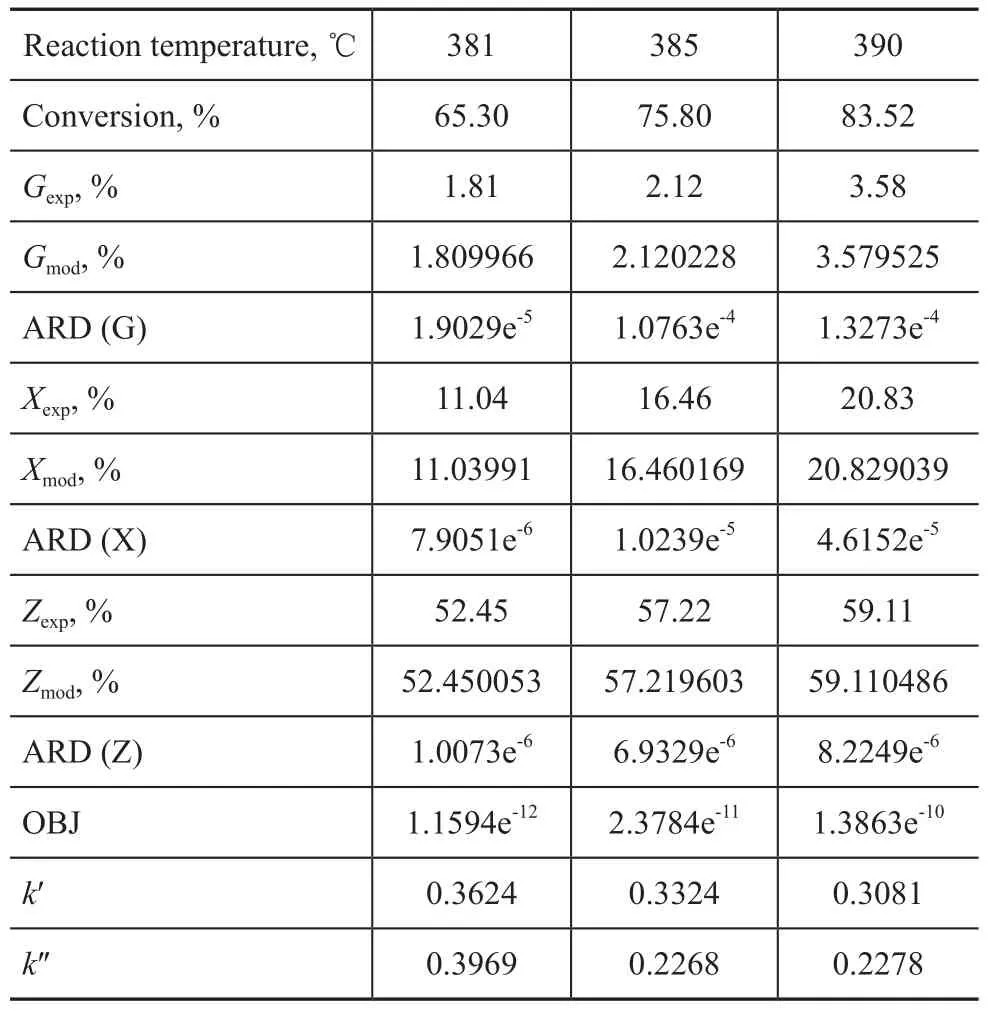
Table 3 Model macrokinetic parameters and standard deviation
Sincek′andk″are kinetic factors with similar meaning to the rate constants, the relation between macrokinetic parameters and reaction temperature based on the Arrhenius equation should be studied. The correlation between -ln(k′,k″) and 1/Tis presented in Figure 3. It can be obviously seen that the macrokinetic parameterk′could satisfy the Arrhenius equation along with high related coefficient (R2=0.9905), but there was not a close relationship between macrokinetic parameterk″and reaction temperature because of its relatively low coefficient (R2=0.6899). It might occur because the model parameter (k″) was not only a function of reaction temperature, but was also related with other process conditions. This experiment studied only the process conditions of different reaction temperatures so that the complete correlation could not be fully reflected. Perhaps, a further research work needs tobe accomplished.
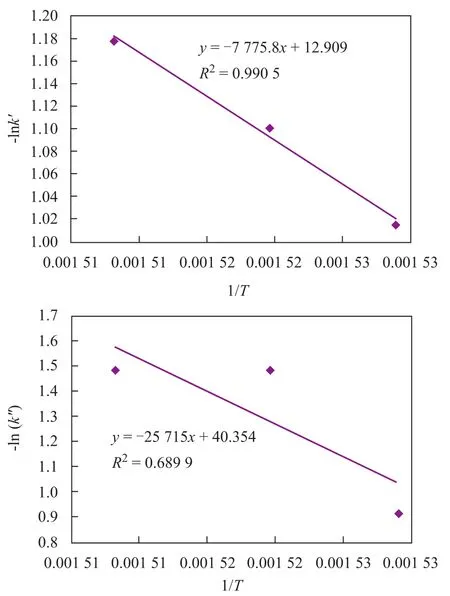
Figure 3 Relationship between -ln(k′, k″) and 1/T
3.2 Correlation of kinetic modeling parameters
It has been referred to in the previous research[7]that the macrokinetic parameters (k′andk″) depend upon the feedstock property, the reaction temperature, and the nature of catalyst. Furthermore,k′andk″are kinetic factors with similar meaning to the rate constants.
So a correlation function was put forward to calculate the macrokinetic parameters. Among them, the characteristic factor (Kf) and the volume-mean boiling point (Tv) functioned as factors which could mainly represent the feedstock property. The weight percent conversion of VGO (Xf), reaction temperature (T), and zeolite content of catalyst are also included in this empirical correlation.KfandTVare calculated by the following formulas and the calculated results are listed in Table 1.
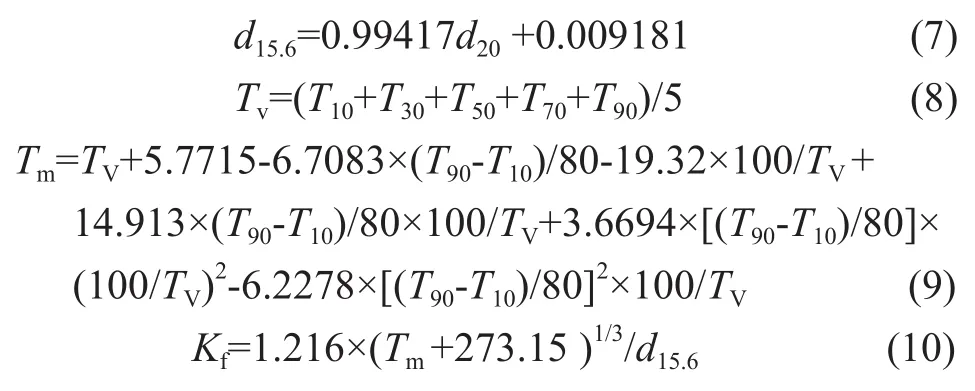
Through correlation and selection of various methods, the two empirical model correlations were chosen, which was an innovative idea adopted in this paper. The empirical correlations aboutk′andk″are listed as follows:
据了解,我国保健食品注册管理制度已经实行了20年,约1.6万个产品获得了批准。现行注册与备案的双轨管理制度,也为产品准入、市场发展提供了强大活力。此外,婴幼儿配方乳粉、特殊医学用途配方食品注册形成了科学完善的管理制度,实现了注册管理的平稳过渡。
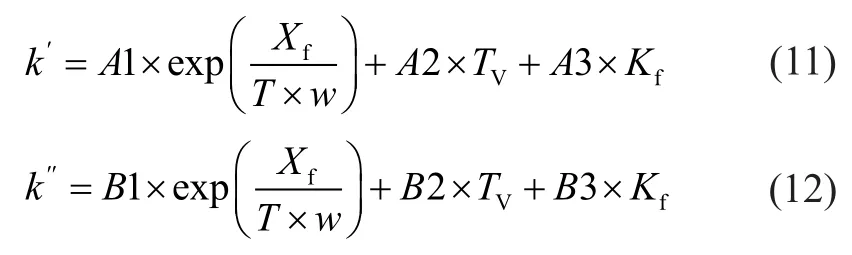
whereA1,A2,A3,B1,B2, andB3 are model parameters, which are calculated from the experimental data;wis the zeolite content in catalyst.
The hydrocracking experiments were carried out on different feedstocks, with the reaction temperature and experimental data shown in Table 4. Firstly, the macrokinetic parameters (k′andk″) were calculated from experimental data listed in Table 4 and then the model parameters were correlated by the nonlinear least square method described in the Matlab 2011b. According to the calculated value listed in Table 4, the macrokinetic parameters (k′andk″) optimized from experimental data of three typical feedstocks were suited quite well to implementing the model calculation because of its low OBJ.
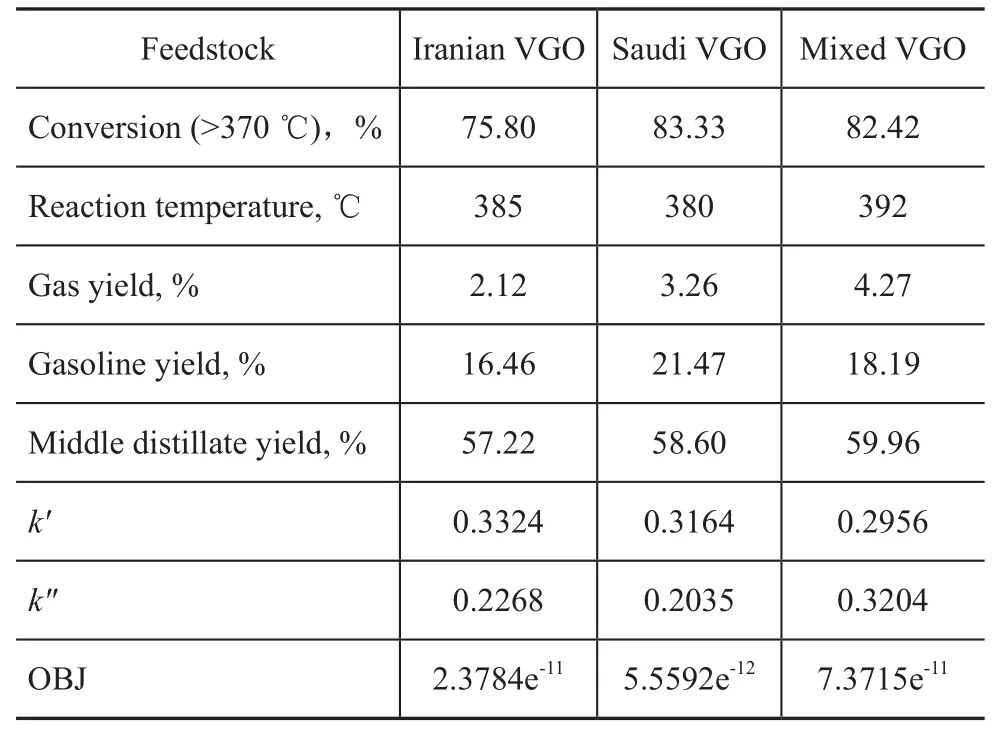
Table 4 Hydrocracking experiments on different feedstocks and kinetic parameters
And then the model parameters describing the relationship between macrokinetic parameters and feedstock properties, catalyst activity, and reaction temperature were simulated by an optimization procedure written in the Matlab 2011b. The objective function which determines the optimized model parameters is defined as mentioned previously. Judging from the optimization results listed in Table 5, it has good prediction accuracy which is appro-priate enough for meeting the model not only in terms of the ARD value but also the OBJ value.
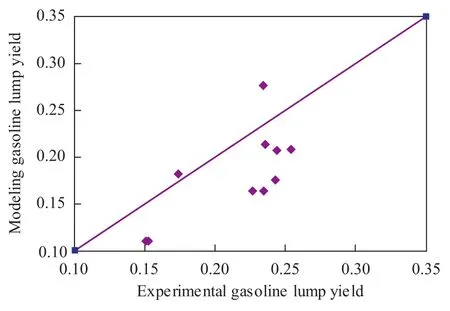
Figure 5 Model gasoline yield versus experimental gasoline yield
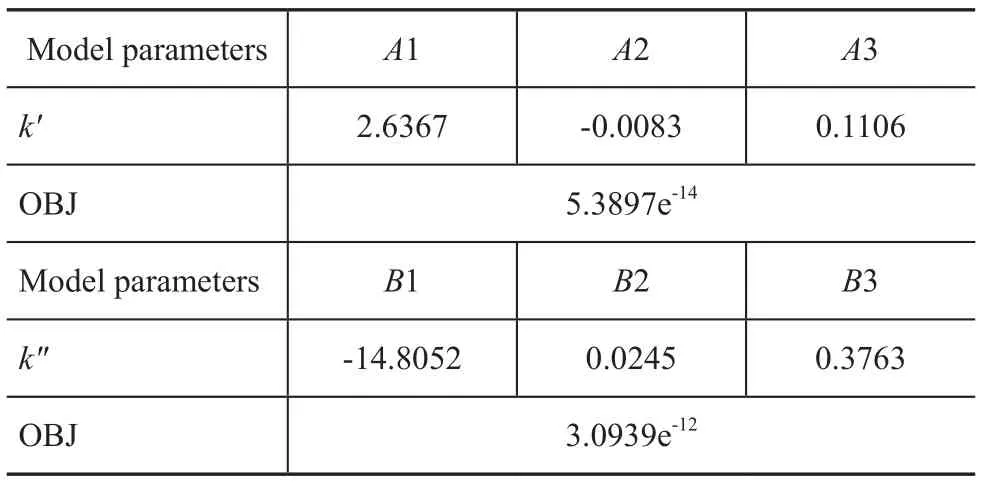
Table 5 Model parameters of macrokinetic parameters (k′, k″)
3.3 Validation of kinetic modeling
After the kinetic modeling parameters were calculated from the above-mentioned experimental data, the kinetic model could be written as follows:
By using these modeling parameters, the hydrocracking product distribution can be calculated. The comparison between the calculated results and the experimental data are shown in Figures 4—5. It can be seen from the data depicted in Figures 4—5 that there was a good agreement between model diesel predictions and experimental data.
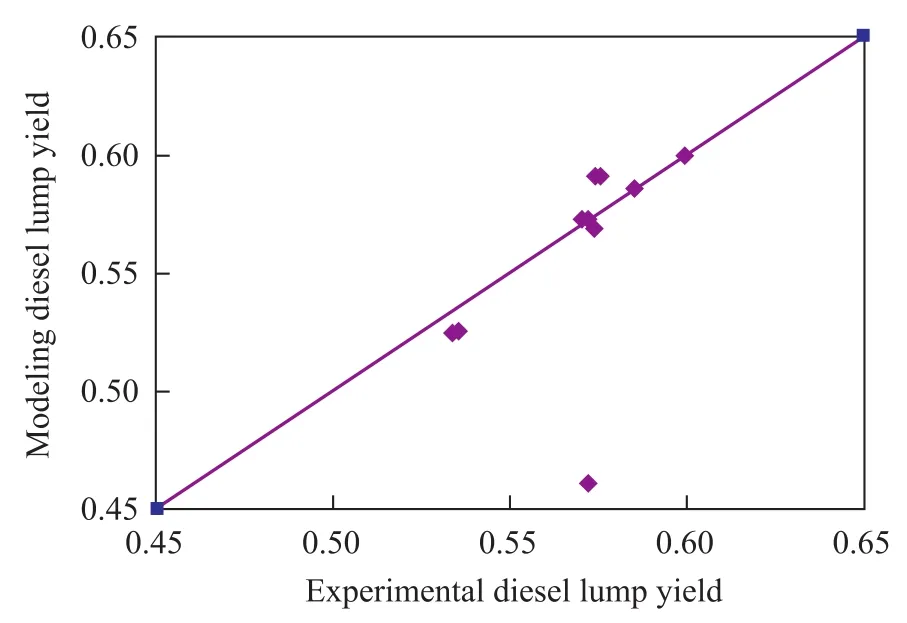
Figure 4 Model diesel yield versus experimental diesel yield
But the model estimation relating to the gasoline lump and the gas lump was less reliable than the diesel lump especially with respect to the gas lump. Furthermore, the kinetic modeling cannot realize prediction when the products cutting scheme was changed.
So far there would not be a model that can realize the prediction on different catalysts, feedstocks, and other factors. Despite the vast commercial application prospects of hydrocracking catalyst A, this model also does not have practicability for industrial hydrocrackers because of constraints related with internal diffusion, external diffusion and other factors.
In general, this model can be only suitable for prediction of the hydrocracking catalyst at some extent. There is still a long way to establish a perfect kinetic model of hydrocracking.
4 Conclusions
(1) In this article, a discrete lumped kinetic approach was used to simulate the kinetics of hydrocracking reactions in a pilot-plant hydrocracker. The data sets were statistically analyzed and then an optimization code was applied to determine the model parameters. The model prediction was validated by experimental data and calculated data based on the model parameters.
(2) Furthermore, the effect of feedstock properties, such as characteristic factor (K), volume-mean boiling point (TV), reaction temperature (T) and other parameters were also correlated in the model. The ARD and OBJ of the model prediction were calculated and the results verified the accuracy of the model parameters and model suitability for predicting the product distribution.
Acknowledgements:Thanks to the fund of “National ‘Twelfth Five-Year’ Plan for Science & Technology Support” (No. 2012BAE05B04) and “Research on Hydrocracking Catalysts Grading Technology” undertaken by Fushun Research Institute of Petroleum and Petrochemicals (FRIPP) supported by SINOPEC (No. 101102).
[1] Kumar H, Froment G F. Mechanistic kinetic modeling of the hydrocracking of complex feedstocks, such as vacuum gas oils[J]. Ind Eng Chem Res, 2007, 46(18): 5881-5897
[2] Ancheyta J, Sanchez S, Rodriguez M A. Kinetic modeling of hydrocracking of heavy oil fractions: A review[J]. Catalysis Today, 2005, 109(1-4): 76-92
[3] Schweitzer J M, Galtier P, Schweich D. A single event kinetic model for the hydrocracking of paraffins in a three-phase reactor [J]. Chem Engng Sci, 1999, 54(13/14): 2441-2452
[4] Sadighi S, Ahmad A, Mohaddecy S R S. 6-Lump kinetic model for a commercial vacuum gas oil hydrocracker[J]. International Journal of Chemical Reactor Engineering, 2010, 8(A1): 1-24
[5] Sadighi S, Ahmad A, Rashidzadeh M. 4-Lump kinetic model for vacuum gas oil hydrocracker involving hydrogen consumption[J]. Korean J Chem Eng, 2010, 27(4): 1099-1108
[6] Moghadassi A R, Amini N, Fadavi O, et al. The application of the discrete lumped kinetic approach for the modeling of a vacuum gas oil hydrocracker unit[J]. Petroleum Science and Technology, 2011, 29(23): 2416-2424
[7] Kumar A, Sinha S. Steady state modeling and simulation of hydrocracking reactor[J]. Petroleum & Coal, 2012, 54(1): 59-64
[8] Elizalde I, Rodriguez M A, Ancheyta J. Application of continuous kinetic lumping modeling to moderate hydrocracking of heavy oil[J]. Applied Catalysis A: General, 2009, 365(2): 237-242
[9] Sadeghi M T, Shahhosseini S, Behroozshad F. Continuous lumping model of an industrial refinery isomax reactor[J]. Iranian Journal of Chemical Engineering, 2010, 7(2): 39-50
[10] Elizaldea I, Rodríguezb M A, Ancheyta J. Modeling the effect of pressure and temperature on the hydrocracking of heavy crude oil by the continuous kinetic lumping approach[J]. Applied Catalysis A: General, 2010, 382(2): 205-212
[11] Sadighi S, Ahmad A, Irandoukht A. Kinetic study on a commercial amorphous hydrocracking catalyst by weighted lumping strategy[J]. International Journal of Chemical Reactor Engineering, 2010, 8(1): 1-24
[12] Jaffe S B. Extension of structure-oriented lumping to vacuum residue[J]. Ind Eng Chem Res, 2005, 44(26): 9840-9852
[13] Mitsios M, Guillaume D, Galtier P, et al. Single-event microkinetic model for long-chain paraffin hydrocracking and hydroisomerization on an amorphous Pt/SiO2·Al2O3catalyst[J]. Ind Eng Chem Res, 2009, 48(7): 3284-3292
[14] Guillaume D, Valery E, Verstraete J J, et al. Single event kinetic modeling without explicit generation of large networks: Application to hydrocracking of long paraffins[J]. Oil & Gas Science and Technology, 2011, 66(3): 399-422
[15] Orochko D I, Perezhigina I Y, Rogov S P, et al. Applied over-all kinetics of hydrocracking of heavy petroleum distillates[J]. Khimiya I Tekhnologiya Toplivi Masel, 1970, 8(6): 561-565 (in Russian)
Recieved date: 2012-12-27; Accepted date: 2013-02-05.
Professor Fang Xiangchen, E-mail: fangxiangchen.fshy@sinopec.com.
- 中国炼油与石油化工的其它文章
- Influence of Carbon Content on S Zorb Sorbent Activity
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
- Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil

