Synthesis,characterization and magnetic properties of a novel three dimensional metal-organic framework
LIU Liu,LIU Bing-bing,WU Lan-zhi,YANG Li-rong
(Henan Key Laboratory of Polyoxometalate,Henan University,Kaifeng475004,Henan,China)
Recently,the coordination chemistry of organics with lanthanide ions has drawn much attention,owing to various potential applications of the organics in gas storage and separation,storage of molecules,guest exchange,ion exchange,molecular recognition,sensor,molecular magnetism,optoelectronics,chemical separation, microelectronics,nonlinear optics,heterogeneous catalysis and luminescent probes[1-12].Here we report the synthesis,structure characterization,thermal analysis and evaluation of magnetic properties of[Pr(NTA)(H2O)]n(CCDC:920899)obtained from the self-assembly of bridging ligand nitrilotriacetic acid.In as-synthesized metal-organic coordination polymer,the formation of rhombic tetrahedral grids robust inorganic building blocks play a key role of in the construction of the metal-organic framework(denoted as MOF).
1 Experimental
1.1 Reagents and general techniques
All starting chemicals are analytical grade and used without further purification.Elemental analysis was performed with a Perkin-Elmer 240Celemental analyzer.Fourier transform infrared(FT-IR)spectra were recorded with an AVATAR 360FT-IR spectrometer(KBr pellets,in the region of 4 000-400 cm-1).The crystal structure of target product was determined with a Bruker Smart CCD X-ray singlecrystal diffractometer(XRD).Thermogravimetric(TG)and differential thermogravimetric(DTG)analyses were conducted with a Perkin-Elmer TGA7system under flowing N2stream (flow rate 40mL/min)from room temperature to 1 000℃at a heating rate of 10℃/min.Magnetic susceptibility measurements were carried out with a Quantum Design MPMS-5magnetometer in the temperature range of 2-300K.
1.2 Synthesis of complex[Pr(NTA)(H2O)]n
Complex[Pr(NTA)(H2O)]nwas synthesized with nitrilotriacetic acid and neodymium nitrate as the starting materials.Briefly,nitrilotriacetic acid and neodymium nitrate were mixed in 10mL distilled water at a molar ratio of 1∶3(0.2mmol∶0.6mmol)and adjusted to pH =5with 1mol·L-1NaOH solution.Resultant mixed solution was homogenized by stirring at ambient temperature for 20min.Upon completion of homogenization,the mixed solution was transferred into 20mL Teflon-lined stainless steel autoclave and kept at 160℃for 4dunder autogenous pressure,followed by cooling to room temperature at a rate of 5℃/h.After filtration,the product was washed with distilled water and dried to provide palegreen transparent block crystals suitable for XRD analysis.Elemental analysis calculated(calcd in short;%)for C6H8NO7Pr:C,20.77;H,2.32;N,4.04.Found:C,20.33;H,2.13;N,3.84.FT-IR(KBr,cm-1)σ:3 423(br),2 968(w),1 676(s),1 612(s),1 464(w),1 429(s),1 386(m),1 334(m),1 336(w),1 282(w),256(w),1 145(w),1 072(w),987(w),968(w),956(w),929(m),917(w),758(w),746(w),691(m),652(w),673(w),592(w),443(w).
1.3 X-ray crystallographic determination
XRD measurements of complex[Pr(NTA)(H2O)]nwas carried out with a Bruker Smart CCD X-ray single-crystal diffractometer.Reflection data were measured at 296(2)K using graphite monochromated MoKα-radiation(λ=0.071 073nm)andω-scan technique.All independent reflections were collected in a range of 2.63°to 25.00°and determined in subsequent refinement.SADABS multi-scan empirical absorption corrections were applied to the data processing[13].The crystal structures were solved by direct methods and Fourier synthesis.Positional and thermal parameters were refined by the full-matrix leastsquares method onF2using the SHELXTL software package[14].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.Analytical expressions of neutral-atom scattering factors were employed,and anomalous dispersion corrections were incorporated.The crystallographic data,selected bond lengths and angles for complex[Pr(NTA)(H2O)]nare listed in Table 1and Table 2.

Table 1 Crystal data and structure refinement parameters for title MOF

Continued to Table 1
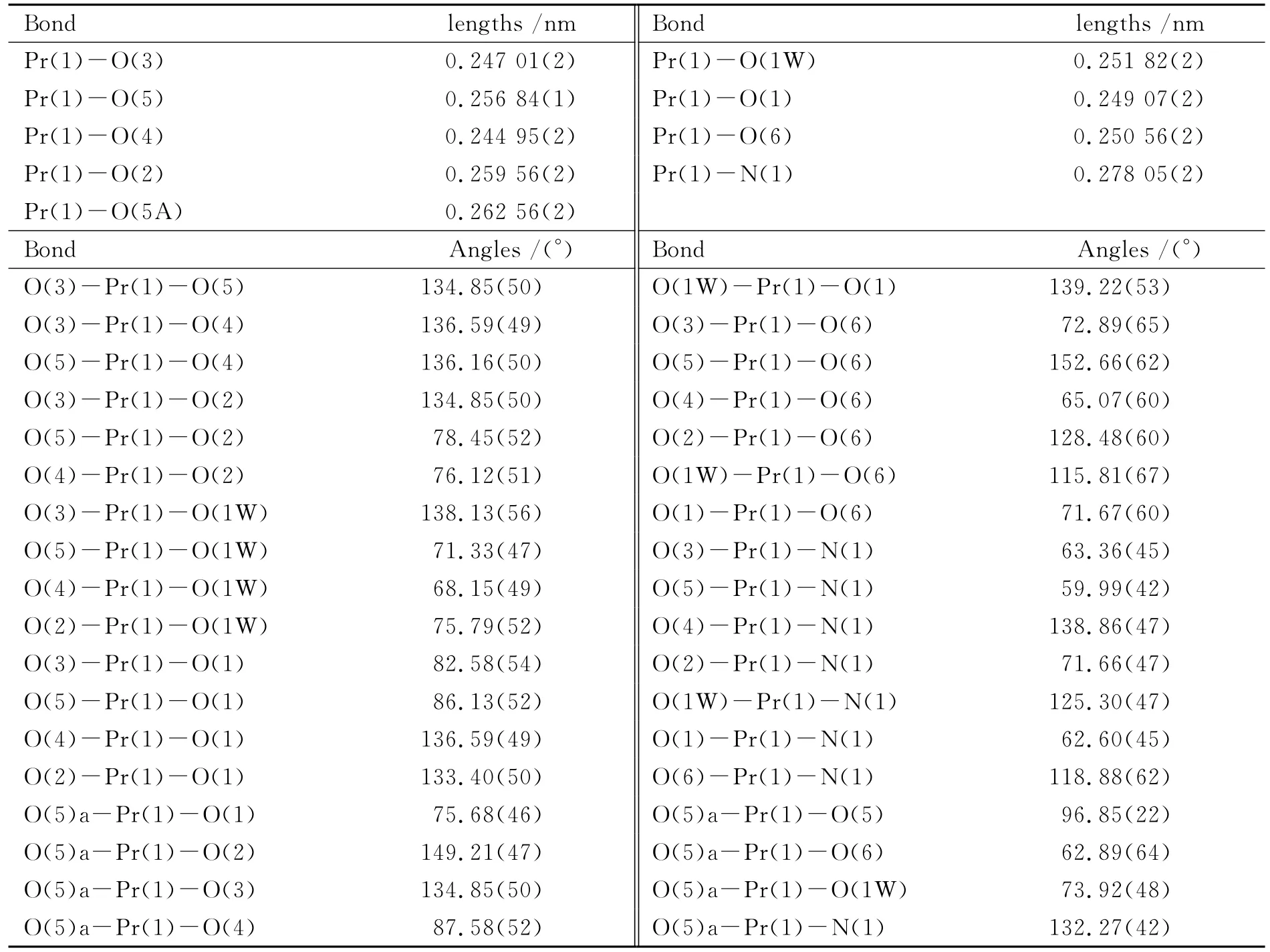
Table 2 Selected bond lengths and bond angles for title complex
2 Results and discussion
2.1 FT-IR spectra of complex[Pr(NTA)(H2O)]n
Complex[Pr(NTA)(H2O)]nis insoluble in common solvents such as CH3COCH3,CH3CH2OH,CH3OH-CH3CN and tetrahydrofuran(THF),and it is slightly soluble in dimethyl sulphoxide(DMSO)or dimethylformamide(DMF).The structure of the complex is identified by FT-IR,elemental analysis and XRD.High yield of the product indicates that the title complex is thermodynamically stable under the reaction conditions.The strong and broad absorption bands of[Pr(NTA)(H2O)]nat 3 423cm-1and in the range of 929-917cm-1are assigned to the characteristic peaks of water molecules in coordination and lattice forms[15-16].The strong vibrations around 1 650cm-1and 1 425cm-1are ascribed to the coordinated carboxylates.The absorption bands at 1 145cm-1is attributed to anti-symmetric stretching of the C-OC bridge[17].The sharp peaks in the range of 680-770cm-1are assigned toδO-C-Oplane vibration.The absorption around 1 600cm-1is related to the N-C stretching vibration[18-19].The absence of the characteristic bands ranging from 1 690cm-1to 1 730cm-1indicates that the H3NTA ligands are completely deprotonated in the form of NTA3-anions upon reaction with the metal ions[20-21].These conclusions are also supported by relevant XRD measurements.
2.2 Structural description of complex[Pr(NTA)(H2O)]n
The PrN1O8unit forms a nine-coordinated structure in which one N and three O atoms are from one NTA ligand,four O atoms from other NTA ligands and one O atom from one coordinate water molecule(see Fig.1a).The coordination environment of NTA is shown in Fig.1b.The carboxylic O atoms of NTA chelate with the central Pr(Ⅲ),and two Pr(Ⅲ)centers are bridged by one O atom from NTA ligand(see Fig.1c).The Pr-ONTAdistances range from 0.249 07(2)nm to 0.256 84(1)nm,the distance of Pr-OWis 0.251 82(2)nm,and the average Pr-ONTAbond length is significantly shorter than that of Pr-OW.The Pr-N distance is 0.278 05(2)nm.The bond length data in the present work are consistent with those in previous work covering lanthanide coordination polymers[22-23].
The most striking feature of as-synthesized complex[Pr(NTA)(H2O)]nis the motif of building block of Pr8C14O28rhombic tetrahedron.Better insight into the nature of this intricate skeleton frame of rhombic tetrahedron networks can be achieved by topological approach,that is,by reducing the multidimensional structures to simple node and connection nets,as depicted in Fig.2a.It is noteworthy that the rhombic tetrahedral Pr8C14O28unit has a dimension of 0.699 70nm×0.690 19nm×0.650 26nm,and corresponding angles are 149.210°and 59.990°.Remarkably,the rhombic tetrahedral Pr8C14O28unit generate a large nanoscale cage which can be considered as consisting of eight Pr(Ⅲ)vertices and fourteen bended O-CO edges,leading to a cage building unit(CBU)thereby allowing wrapping into a dummy ball with a radius of ca.0.315 0nm (see Fig.2b).There are four large quadrilateral windows on the surface of each cage with a dimension of 0.690 19nm×0.650 26nm.As a result,a novel 3Dframework is prolonged alonga,bandcaxes through the above cage-to-cage connections(see Fig.2c).

Fig.1 (a)Coordination environment of Pr(III).(b)Coordination environment of NTA.(c)Special O atom coordinate with two Pr(III).In(a),(b)and(c),the asymmetric unit and related coordination atoms are labeled;lattice water and hydrogen atoms are omitted for clarity

Fig.2 (a)Change of Pr-O-C-O-Pr to Pr-Pr through reducing the multidimensional structures.(b)Pr8C14O28building block of complex.(c)Complex presenting 3Dnetwork viewed froma-axis direction
2.3 Magnetic properties
The magnetic properties of[Pr(NTA)(H2O)]nwere investigated over the temperature range 2.0-300.0K (see Fig.3).TheχMTvalue of[Pr(NTA)(H2O)]nat 300Kis 1.489cm3·K·mol-1(3.451μB),and it is close to the expected value(0.160 0cm3·K·mol-1,1.131μB)of an isolated spin-only Pr(Ⅲ)ions(S=1,g=4/5).Besides,theχMTvalue of[Pr(NTA)(H2O)]nremains almost constant within 300-230K,and then it decreases with further cooling and reaches a value of 0.047cm3·K·mol-1at 2K.This indicates that there exists a dominant antiferromagnetic interaction between the Pr(Ⅲ)ions in the structure.In general,the 1/χMversusTplot of[Pr(NTA)(H2O)]nwell conforms to Curie-Weiss constant determined in the range of 2-300K(C=1.659cm3·K·mol-1,θ= -31.242K).

Fig.3 Thermal variation ofχMandχMTfor the complex Insert:Plot of thermal variation of 1/χMfor the complex

Fig.4 TG and DTG curves of the complex
2.4 Thermogravimetric analysis
Thermogravimetric analysis of complex [Pr(NTA)(H2O)]nwas performed in the N2stream from room temperature to 1 000℃.The TG and DTG analyses are particularly informative regarding the water content of as-synthesized product and are of great importance to help quantifying water content in solvent accessible area of the 3Dframeworks.TG and DTG curves show that the complex decomposes in two steps,as displayed in Fig.4.The first stage weight loss of as-synthesized complex (5.13%)in the temperature range of 205-341℃corresponds to the destruction of the coordinated water molecules,which is close to relevant calculated weight loss of 6.55%and consistent with the crystal structure analysis.The second stage weight loss at 368℃is due to the ligand decomposition giving the final residual product Pr6O11.The remnant of the complex at the second stage weight loss amounts to 38.86%,which suggests that it can be decomposed completely under the tested temperature.
3 Conclusions
A novel coordination polymer containing NTA ligand and Pr(Ⅲ)with nine-coordinated environment has been successfully synthesized under hydrothermal condition.As-synthesized 3Dsupramolecular network is assembled based on the building block of octanuclear homometallic Pr8C14O28through O-C-O linkers.Besides,the title Pr(Ⅲ)coordination polymer possesses good thermal stability and exhibits antiferromagnetism,showing potential as a magnetic material.
[1]SHI F N,CUNHA-SILVA L,FERREIRA R A S,et al.Interconvertable modular framework and layered lanthanide(III)-etidronic acid coordination polymers[J].J Am Chem Soc,2008,130(1):150-167.
[2]GHOSH S K,BHARADWAJ P K.Self-assembly of lanthanide helicate coordination polymers into 3Dmetal-organic framework structures[J].Inorg chem,2004,43(7):2293-2298.
[3]HILL R J,LONG D L,HUBBERSTEY P,et al.Lanthanide coordination frameworks:opportunities and diversity[J].J Solid State Chem,2005,178(8):2414-2419.
[4]SUBHAN M A,NAKATA H,SUZUKI T,et al.Simultaneous observation of low temperature 4f-4fand 3d-3demission spectra in a series of Cr(III)(ox)Ln(III)assembly[J].J Lumin,2003,101(4):307-315.
[5]D'VRIES R F,IGLELIAS M,SNEJKO N,et al.Lanthanide metal organic frameworks:searching for efficient solvent-free catalysts[J].Inorg Chem,2012,51(21):11349-11355.
[6]LI H,SHI W,ZHAO K,et al.Highly selective sorption and luminescent sensing of small molecules demonstrated in a multifunctional lanthanide microporous metal organic framework containing 1Dhoneycomb-type channels[J].Chem Eur J,2013,19(10):3358-3365.
[7]NOUAR F,ECKERT J,EUBANK J F,et al.Zeolite-like metal organic frameworks(ZMOFs)as hydrogen storage platform:lithium and magnesium ion-exchange and H2-(rho-ZMOF)interaction studies[J].J Am Chem Soc,2009,131(8):2864-2870.
[8]CHEN B,XIANG S,QIAN G.Metal-organic frameworks with functional pores for recognition of small molecules[J].Accounts Chem Res,2010,43(8):1115-1124.
[9]KRENO L E,LEONG K,FARHA O K,et al.Metal-organic framework materials as chemical sensors[J].Chem Rev,2012,112(2):1105-1125.
[10]SHULTZ A M,FARHA O K,HUPP J T,et al.A catalytically active,permanently microporous MOF with metalloporphyrin struts[J].J Am Chem Soc,2009,131(12):4204-4205.
[11]LUZ I,LLABRÉS I XAMENA F X,CORMA A.Bridging homogeneous and heterogeneous catalysis with MOFs:“Click”reactions with Cu-MOF catalysts[J].J Catal,2010,276(1):134-140.
[12]ALLENDORF M D,BAUER C A,BHAKTA R K,et al.Luminescent metal organic frameworks[J].Chem Rev,2009,38(5):1330-1352.
[13]SHELDRICK G M.SADABS:Empirical absorption and correction software[CP].University of Göttingen,Institut fur Anorganische Chemieder Universitat,Tammanstrasse,1999,4:1999-2003.
[14]SHELDRICK G M.SHELXTL V5.1software reference manual[CP].Bruker AXS Inc,Madison,1997.
[15]TANG R R,GU G L,ZHAO Q.Synthesis of Eu(III)and Tb(III)complexes with novel pyridine dicarboxamide derivatives and their luminescence properties[J].Spectrochim Acta A:Mol Biomol Spectrosc,2008,71(2):371-376.
[16]YANG L R,SONG S,ZHANG H M,et al.Synthesis,structure of 3Dlanthanide(La(III),Pr(III))nanoporous coordination polymers containing 1Dchannels as selective luminescent probes of Pb2+,Ca2+and Cd2+ions[J].Synthetic Met,2011,161(21):2230-2240.
[17]DE VASCONCELOS C L,BEZERRIL P M,DOS SANTOS D E S,et al.Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly(methacrylic acid)and chitosan[J].Biomacromolecules,2006,7(4):1245-1252.
[18]KOVACIC J E.The C-N stretching frequency in the infrared spectra of Schiff's base complexes-I.Copper complexes of salicylidene anilines[J].Spectrochim Acta A:Mol Biomol Spectrosc,1967,23(1):183-187.
[19]AGHABOZORG H,MOGHIMI A,MANTEGHI F,et al.A nine-coordinated ZrIV complex and a self-assembling system obtained from a proton transfer compound containing 2,6-pyridinedicarboxylate and 2,6-pyridinediammonium;synthesis and X-ray crystal structure[J].Z Anorg Allg Chem,2005,631(5):909-913.
[20]LIU M S,YU Q Y,CAI Y P,et al.One-,two-,and three-dimensional lanthanide complexes constructed from pyridine-2,6-dicarboxylic acid and oxalic acid ligands[J].Cryst Growth Des,2008,8(11):4083-4091.
[21]SHI F N,CUNHA-SILVA L,TRINDADE T,et al.Three-dimensional lanthanide-organic frameworks based on di-,tetra-,and hexameric clusters[J].Cryst Growth Des,2009,9(5):2098-2109.
[22]ZHANG H,YANG H,WU L,et al.A series of 3Dlanthanide complexes containing(La(III),Sm(III)and Gd(III))metal-organic frameworks:synthesis,structure,characterization and their luminescent properties[J].Bull Korean Chem Soc,2012,33(11):3777.
[23]LI J R,BU X H,ZHANG R H.Novel lanthanide coordination polymers with a flexible disulfoxide ligand,1,2-bis(ethylsulfinyl)ethane:structures,stereochemistry,and the influences of counteranions on the framework formations[J].Inorg Chim Acta,2004,43(1):237-244.
- 化学研究的其它文章
- CO2加氢制甲醇用高活性和高选择性催化剂的研制
- 淀粉功能化Fe3O4纳米簇球的制备与表征
- Synthesis and characterization of a metal-organic framework of grid networks based on nitrilotriacetic acid and neodymium
- Sandwich-type polyoxomolybdate constructed from tetra-nuclear Fe(Ⅱ)cluster and trivacant Keggin tungstophosphate fragment
- 量子点的制备及应用研究进展
- 不饱和聚酯型人造大理石研究进展

