Bispectral index in predicting the prognosis of patients with coma in intensive care unit
Intensive Care Unit, Tianjin First Central Hospital, Tianjin 300192, China
Corresponding Author:Hong-mei Gao, Email: gao_hongmei@hotmail.com
Bispectral index in predicting the prognosis of patients with coma in intensive care unit
Lin Dou, Hong-mei Gao, Ling Lu, Wen-xiu Chang
Intensive Care Unit, Tianjin First Central Hospital, Tianjin 300192, China
Corresponding Author:Hong-mei Gao, Email: gao_hongmei@hotmail.com
BACKGROUND:The bispectral (BIS) index is a processed electroencephalogram (EEG) parameter with extensive validation and demonstrated clinical utility. The study aimed to investigate the correlation between the BIS index and the prognosis of patients with coma in the ICU.
METHODS:A total of 208 patients with coma in the ICU were enrolled in this study. According to the BIS value, the patients were divided into four groups: group I, BIS value 0 to 20; group II, BIS value 21 to 40; group III, BIS value 41 to 60; and group IV, BIS value greater than 60. The difference in BIS values with the differences in prognosis of patients with coma was compared between the four groups, and the prognosis of patients with coma was stratified into consciousness, coma, vegetative state, and brain death. Subsequently, the best cut-off score of BIS values calculated for determining the correlation between BIS value and mental state was proposed.
RESULTS:There are no significant differences in the age and APACHE II scores between the four groups (P>0.05). An inverse correlation was observed between BIS value and mental state (r=–0.749, P=0.00). According to the ROC curve, as BIS value was greater than 42.5, there were higher sensitivity and specificity in conscious-coma patients.
CONCLUSION:BIS value is correlated with the prognosis of patients with coma in ICU, and BIS value can be a useful marker for estimating the prognosis of comatose patients.
Bispectral index; Coma; Prognosis
INTROUCTION
The bispectral (BIS) index is a processed electroencephalogram (EEG) parameter with extensive validation and demonstrated clinical utility. It is derived utilizing a composite of measures from EEG signal processing techniques including bispectral analysis, power spectral analysis, and time domain analysis. These measures were combined via an algorithm to optimize the correlation between the EEG and the clinical effects of anesthesia.
It is considered that the index can reflect the functional status of the cerebral cortex.[1]The purpose of this study is to observe the relationship between early BIS monitoring and consciousness recovery in coma patients in the intensive care units (ICU), in order tofind out the role of BIS in predicting the prognosis of patients.
METHODS
Patients
The patients were selected from the ICU of Tianjin First Center Hospital from March 2009 to March 2012. The informed consent was obtained from family members. A total of 208 coma patients, 124 males and 84 females, were enrolled in this study. Primary diseases included cerebrovascular disease, traumatic brain injury, cardiopulmonary resuscitation (CPR), and viral encephalitis. Patients with sedation, hepatic encephalopathy, pulmonary encephalopathy, and poisoning were excluded.
APACHE II score and Glasgow coma score (GCS) and EEG monitoring index of double frequency (BIS) were determined in each patient. According to the BISresults, patients were divided into four groups: group I, BIS value 0 to 20; group II, BIS value 21 to 40; group III, BIS value 41 to 60; and group IV, BIS value greater than 60.
Patients were monitored using BIS for 14 consective days, and the BIS value within the first 72 hours were recorded. The patients were followed up for 28 days. The prognosis was divided into brain death, vegetative state, and conscious coma.
Diagnostic criteria
Vegetative state was diagnosed according to the standard reported by Jennett et al.[2]The diagnosis of brain death was made according to the criteria reported by Tawil et al.[3]BIS module and the electrode (ASPECT Company, USA) were used to continuously monitor the brain electric signal in patients.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software, and the measurement data were expressed as mean±standard deviation. Single factor variance analysis was used to test the age, APACHE II score, and Glasgow coma score (GCS) of each patient. If the variance was homogeneity, LSD was used for comparison, and if not, Dunnett C was used. For the calculation of the data of 28-day prognosis, the Chi-square test and the Kruskal-Wallis test were used. The specificity and sensitivity were calculated in the fou groups, and the receiver operating characteristic curve (ROC) was drawn. The Spearman's rank-order correlation coefficient was used to analyze the correlation between BIS value and the prognosis. P<0.05 was considered statistically significant.
RESULTS
Comparison of general data
The distribution of primary diseases in the patients was shown in Table 1. There were no significant differences in age and APACHE II score between the two groups (P>0.05) (Table 2). In contrast, significant differences were observed in GCS between groups I and IV, and between groups II and IV (P<0.05) (Table 2).
Comparison of prognosis
During the period of follow-up, 45 patients were at the status of brain death. There were significant differences in conscious coma between groups I and IV, and between groups II and III (P<0.05) respectively. Moreover, significant differences were seen in vegetative state between groups I and II, between groups I and III, and between groups III and IV (P<0.05) respectively. Also there were significant differences in brain death between groups I and II, between groups I and III, and between groups I and IV respectively (P<0.05) (Table 3).
The specificity, sensitivity and accuracy of BIS
The specificity, sensitivity and accuracy were calculated when BIS was 0–20, 21–40, 41–60 respectively (Table 4). The ROC curve was drawn for BIS in predictingconscious coma, vegetative state, and brain death (Figure 1). When BIS >42.5, there was significant difference in predicting conscious-coma, with a ROC area of 0.94 (>0.5), a specificity of 82%, and a sensitivity of 90%. When BIS <42.5, there was no significant difference in predicting the vegetative state and brain death, with a ROC area of <0.5. There was an inverse correlation between BIS value and mental state (r =–0.749, P=0.00).
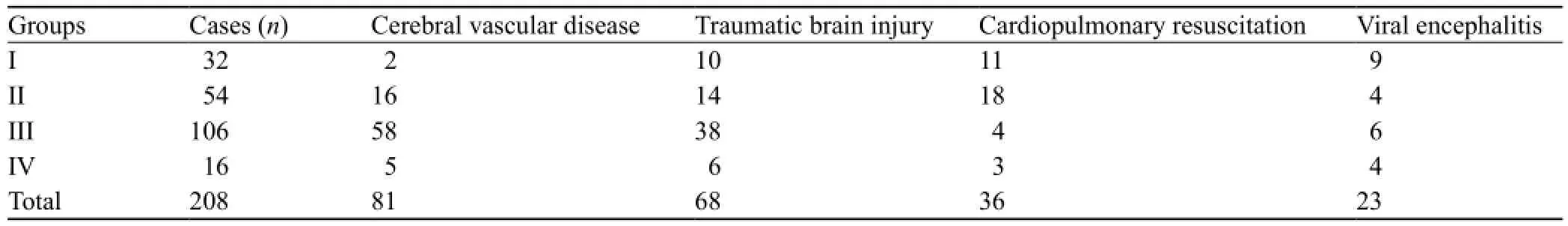
Table 1. The distribution of primary diseases in the patients

Table 2. Baseline demographic data of the patients

Table 3. Comparison of 28-day consciousness of the patients

Table 4. ROC curve analysis of BIS evaluation on conscious coma, vegetative state, and brain death

Figure 1. ROC curve analysis of BIS values on conscious coma, vegetative state, and brain death.
DISCUSSION
BIS index was introduced by Aspect Medical Systems, Inc. in 1994[4]as a novel measure of the level of consciousness by algorithmic analysis of a patient's electroencephalogram during general anesthesia. This is used in conjunction with other physiologic monitoring such as electromyography to estimate the depth of anesthesia in order to minimize the possibility of intraoperative awareness. The US Food and Drug Administration (FDA) cleared BIS monitoring in 1996 for assessing the hypnotic effects of general anesthetics and sedatives. The FDA further stated in 2003 that "A reduction in awareness provides a public health benefit, in that BIS technology can now provide anesthesiologists with a way to reduce this often debilitating, yet preventable medical error".[5,6]
The BIS index is a statistically based, empirically derived complex parameter. It is a weighted sum of several electroencephalographic subparameters, including a time domain, frequency domain, and high order spectral subparameters.[7]The BIS monitor provides a single dimensionless number, which ranges from 0 (equivalent to EEG silence) to 100. BIS values of 65–85 are recommended for sedation, whereas values of 40–60 are recommended for general anesthesia. At BIS values of less than 40, cortical suppression is discernible.[8]Fàbregas et al[9,10]observed the relation between the prognosis and BIS value in 56 patients with severe coma, and found that BIS monitoring can be used to evaluate the brain death patients with severe coma.
The coma patients in ICU always have complex and critical primary diseases, such as cerebrovascular disease, traumatic brain injury, and diseases that need cardiopulmonary resuscitation, and also have a high morbidity and mortality.[11–13]In this study, the prognosis of patients with a vegetative state and brain death was the observation target, and the rest of mental state was considered as concious coma. Moreover, we explored the specificity, sensitivity and accuracy of BIS in predicting the prognosis of patients with coma.
In this study, almost all of the enrolled patients had severe primary diseases, and their complications were complex. Their mean APACHE II score was <25. Hence, there were 16 patients with complications in the BIS>60 group and 32 patients in the BIS 0–20 group, and 27 patients were at the state of brain death. In the BIS 21–40 group of 54 patients, 38 were at the vegetative state, and the rest were at state of brain death. When BIS>42.5, there was a significant difference in predicting the conscious coma, with an ROC area of 0.94 (>0.5), a specificity of 82%, and a sensitivity of 90%. When BIS was <42.5, there was no significant difference in predicting the vegetative state and brain death, with an ROC area of <0.5. There was an inverse correlation between BIS value and mental state (r =–0.749, P=0.00). Therefore, there was a good correlation between BIS value and the prognosis in coma patients in the ICU.
In conclusion, BIS index is an objective tool to evaluate the state of consciousness, and can overcome the error of subjective assessment. The data are intuitive and digital, which can avoid the repeated stimulation to the patients. Its advantage lies in the quantized values, noninvasive, bedside operation, and dynamic monitoring without the need of transporting patients, so it is a safetool for consciousness monitoring in ICU. There are some limitations in this study. The diseases we observed were limited and the sample was small. Except the vegetative state and brain death, the other values of consciousness were not identified . The follow-up period of the patients was as short as 28 days.
Funding:The study was supported by a grant from National Clinical Key Subject Construction Funds.
Ethical approval:This study was approved by the institutional ethics committees.
Conflicts of interest:We have no conflicts of interest to report.
Contributors:Dou L proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
1 Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the Sedation-Agitation Scale with the Bispectral Index and Visual Analog Scale in adult ICU patients after cardiac surgery. Intensive Care Med 2001; 27: 853–858.
2 Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1 (7753): 734–737.
3 Tawil I, Marinaro J, Brown LH. Development and validation of a tool for assessing understanding of brain death. Prog Transplant 2009; 19: 272–276.
4 Sigl JC, Chamoun NG. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit 1994; 10: 392–404.
5 Zhang HY, Wu CJ, Li CS. Glycated hemoglobin A1C and diabetes mellitus in critically ill patients. World J Emerg Med 2013; 4: 201–204.
6 Romanov A, Moon RS, Wang M, Joshi S. Paradoxical increase in the bispectral index during deep anesthesia in new zealand white rabbits. J Am Assoc Lab Anim Sci 2014; 53: 74–80.
7 Yang N, Ge MF, Wang TL, Wu XG. Feasibility analysis of wavelet index for monitoring the depth of anesthesia in patients undergoing general anesthesia. Zhonghua Yi Xue Za Zhi 2011; 91: 2849–2852.
8 Vivien B, Paqueron X, Le Cosquer P, Langeron O, Coriat P, Riou B. Detection of brain death onset using the bispectral index in severely comatose patients. Intensive Care Med 2002; 28: 419–425. Epub 2002 Mar 19.
9 Fàbregas N, Gambús PL, Valero R, Carrero EJ, Salvador L, Zavala E, et al. Can bispectral index monitoring predict recovery of consciousness in patients with severe brain injury? Anesthesiology 2004; 101: 43–51.
10 Kulstad EB, Courtney DM, Waller D. Induction of therapeutic hypothermia via the esophagus: a proof of concept study. World J Emerg Med 2012; 3: 118–122.
11 Ma MY, Xu J, Yu N, Huang GM. Analysis of drug resistance of Acinetobacter baumannii and its related factors in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013; 25: 686–689.
12 Kashiwagi M, Ishigami A, Hara K, Matsusue A, Waters B, Takayama M, et al. Immunohistochemical investigation of the coma blister and its pathogenesis. J Med Invest 2013; 60: 256–261.
13 Gupta A, Taly AB. Functional outcome following rehabilitation in chronic severe traumatic brain injury patients: A prospective study. Ann Indian Acad Neurol 2012; 15: 120–124.
Received May 16, 2013
Accepted after revision November 17, 2013
World J Emerg Med 2014;5(1):53–56
10.5847/ wjem.j.issn.1920–8642.2014.01.009
 World journal of emergency medicine2014年1期
World journal of emergency medicine2014年1期
- World journal of emergency medicine的其它文章
- Wilderness medicine
- Lean techniques for the improvement of patients'flow in emergency department
- The knowledge level offinal year undergraduate health science students and medical interns about cardiopulmonary resuscitation at a university teaching hospital of Northwest Ethiopia
- Strategic planning and designing of a hospital disaster manual in a tertiary care, teaching, research and referral institute in India
- Effects of early rehabilitation therapy on patients with mechanical ventilation
- Significance of blood pressure variability in patients with sepsis
