Recovery of the corticospinal tracts injured by subfalcine herniation: a diffusion tensor tractography study
Recovery of the corticospinal tracts injured by subfalcine herniation: a diffusion tensor tractography study
Motor weakness is one of the most serious disabling sequelae of stroke. For successful rehabilitation, thorough estimation of the state of injured neural tracts for motor function is mandatory. After development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), three-dimensional reconstruction and estimation for three motor tracts, such as the corticospinal tract, the rubrospinal tract, and the corticoreticular pathway became possible (Kunimatsu et al., 2004; Puig et al., 2010; Yang et al., 2011). The corticospinal tract is known to be a major neural tract for motor function in the human brain (Binkofski et al., 1996). Many studies using DTI have reported on recovery of an injured corticospinal tract in various brain pathologies, including cerebral infarct, intracerebral hemorrhage, and traumatic diffuse axonal injury (Skoglund et al., 2008; Jang, 2011a).
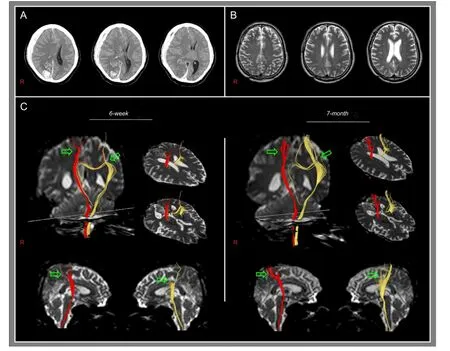
Figure 1 Brain CT, T2-weighted MRI, and diffusion tensor tractography images of a 53-year-old male patient with subdural hematoma in the right fronto-parietal-temporal lobe and intracerebral hemorrhage in the right occipital lobe exhibiting mental deterioration and quadriparasis.
Brain herniation occurs when the brain shifts across structures such as the falx cerebri or the tentorium cerebelli within the skull (Johnson et al., 2002). Brain herniationusually accompanies severe neurological sequelae, therefore, accurate evaluation of the state of an injured neural tract in patients with brain herniation is necessary to elucidate the causes of neurological manifestations, and for establishment of scientific rehabilitative strategies, and in prediction of prognosis (Johnson et al., 2002; Yoo et al., 2008; Cho et al., 2011; Hong et al., 2012). Several studies have reported on injury of the corticospinal tract by transtentorial herniation (Yoo et al., 2008; Cho et al., 2011; Choi et al., 2012; Hong et al., 2012). In addition, some studies have demonstrated recovery of a corticospinal tract injured by transtentorial herniation (Kwon et al., 2011; Yeo and Jang, 2013). However, very little is known about injury and recovery of the corticospinal tract related to subfalcine herniation.
In the current study, we report on a patient who showed recovery of the corticospinal tract, which was injured by the effect of a subfalcine herniation, using DTT.
A 53-year-old, right-handed male presented with mental deterioration and quadriparasis, which occurred at the onset of subdural hematoma in the right fronto-parietal-temporal lobe and intracerebral hemorrhage in the right occipital lobe (Figure 1A). Brain CT images showed midline shifting under the falx cerebri toward the left hemisphere. He underwent decompressive craniectomy and removal of a hematoma at the department of neurosurgery of a university hospital. At 6 weeks after onset, he was transferred to the rehabilitation department of the same university hospital for rehabilitation.
The Motricity Index (MI) and Medical Research Council (MRC) were used for evaluation of motor function of the affected extremities. The reliability and validity of the MI is well-established (maximum score: 100). He presented with quadriparesis of all four extremities at onset (MI: 0/0) and at the start of rehabilitation (6 weeks after onset, [MI]: 55/55) (Table 1). From 6 to 12 weeks after onset, he received comprehensive rehabilitative management, including administration of neurotrophic drugs (ropinirole, levodopa, and amantadine), movement therapy, and neuromuscular electrical stimulation of the affected finger extensors and ankle dorsiflexors (Scheidtmann et al., 2001). Movement therapy focused on improvement of motor weakness and was performed at the physical and occupational therapy sessions fi ve times per week. His quadriparesis was improved from the MI score of 55/55 points at 6 weeks to 79/75 points (12 weeks) during a 6-week period of rehabilitation. After discharge, he was prescribed the same medication and performed a home exercise program, which focused on walking and upper extremity mobility. As a result, his weakness was recovered to a nearly normal state at 7 months after onset (96/96 points). The patient provided signed, informed consent and our institutional review board approved the study protocol.
DTI data were acquired twice (6 weeks and 7 months after onset) using a six-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Ltd., Best, the Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients, we acquired 70 contiguous slices parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192 matrix, field of view = 240 × 240 mm2, repetition time = 10,398 ms, echo time = 72 ms, parallel imaging reduction factor (SENSE factor) = 2, echo planar imaging factor = 59 and b = 1,000 s/mm2, number of excitations = 1, slice gap = 0, and a slice thickness of 2.5 mm. Fiber tracking was performed using the fi ber assignment continuous tracking (FACT) algorithm implemented within the DTI task card software. Each of the DTI replications was intra-registered to the baseline “b0” images to correct for residual eddy-current image distortions and head motion effect, using a diffusion registration package (Philips Medical Systems). Corticospinal tracts were determined by selection of fi bers passing through two regions of interest (ROIs) at the upper and lower pons (portion of anterior blue color) (Jang, 2011b). Fiber tracking was performed with a fractional anisotropy (FA) threshold of > 0.15 and a direction threshold of < 27°.
Both corticospinal tracts originated from the cerebral cortex, including the primary motor cortex, which passed along the known corticospinal tract pathway (Figure 1C). However, both corticospinal tracts were narrowed from the cerebral cortex to the subcorical white matter (right: between the cerebral cortex and the subcortical white matter just below the cerebral cortex, left: between the cerebral cortex and the subcortical white matter at the level of the corpus callosum). However, thickenings of these narrow portions were observed on 7-month DTT.
The subfalcine herniation occurs when one hemisphere swells and shifts the cingulate gyrus beneath the falx cerebri, consequently causing injury of the cingulate gyrus (Johnson et al., 2002). In the current study, on 6-week DTT, we found that both corticospinal tracts were narrowed at the subcortical white matter in both hemispheres, suggesting injury of the corticospinal tract. Because the subfalcine herniation indicates injury of the cingulate gyrus by herniation below the falx cerebri, it appears that the right corticospinal tract was injured mainly by compression of the right subdural hematoma and the left corticospinal tract was injured by the effect of the subfalcine herniation. The quadriparesis of the patient was compatible with the corticospinal tract fi ndings on 6-week DTT. Thickenings of narrowed portions of both corticospinal tracts on 7-month DTT appear to indicate recovery of the injured corticospinal tracts. The good recovery of quadriparesis in this patient appears to coincide with changes of the injured corticospinal tracts in both hemispheres.
Since the introduction of DTI, many studies using DTI have reported on injury of the corticospinal tract by transtentorial herniation in patients with brain injury (Yoo et al., 2008; Cho et al., 2011; Choi et al., 2012; Hong et al., 2012). As for recovery of the corticospinal tract injured by brain herniation, two studies have reported on recovery of a corticospinal tract injured by transtentorial herniation in patients with intracerebral hemorrhage and traumatic braininjury, respectively (Kwon et al., 2011; Yeo and Jang, 2013). With regard to the subfalcine herniation, Hong et al. (2012) reported on injury of the cingulum and fornix in two patients with traumatic brain injury. As a result, to the best of our knowledge, this is the fi rst study to demonstrate injury and recovery of the corticospinal tract related to a subfalcine herniation.
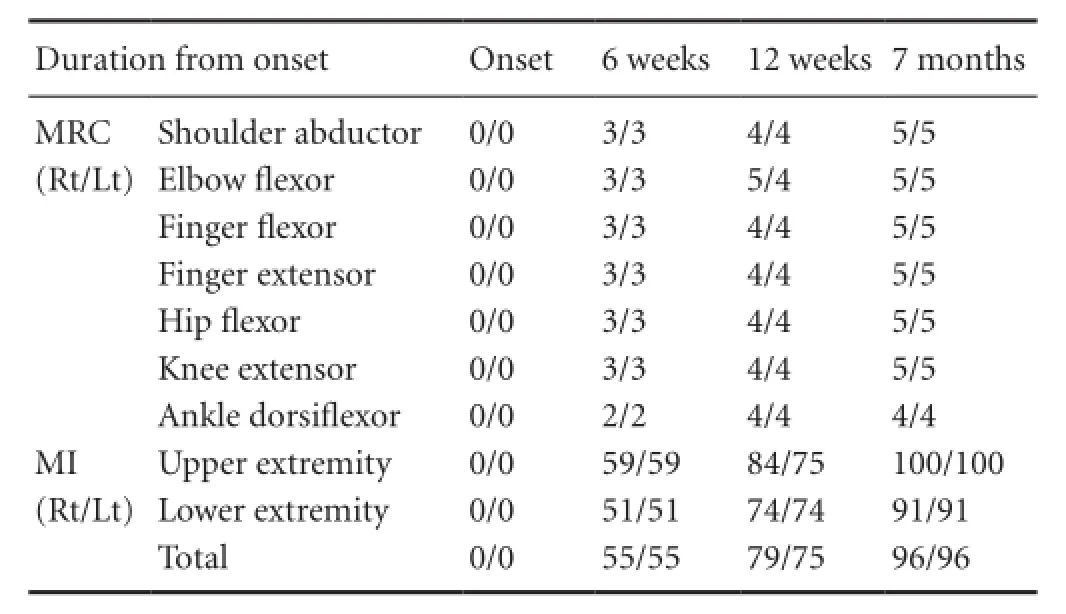
Table 1 Changes in motor function of the patient
In conclusion, we described a patient who showed recovery of the corticospinal tracts in both hemispheres, which appeared to be injured by the effect of a subfalcine herniation. This study demonstrated injury of the corticospinal tract and the process of recovery of an injured corticospinal tract in a patient with a subfalcine herniation, using DTT. Therefore, we believe that DTT would be useful in detection of an injury of a neural tract and in demonstration of recovery of an injured corticospinal tract in patients with a subfalcine herniation. However, further studies involving large numbers of patients need to be performed.
Jeong Pyo Seo, Sung Ho Jang Department of Radiology, Second Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Namku, Daegu, Republic of Korea
Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ (1996) Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol 39:460-470.
Cho HK, Hong JH, Kim SH, Kim OL, Ahn SH, Jang SH (2011) Clinical usefulness of diffusion tensor imaging in patients with transtentorial herniation following traumatic brain injury. Brain Inj 25:1005-1009.
Choi GS, Kim OL, Kim SH, Ahn SH, Cho YW, Son SM, Jang SH (2012) Classi fi cation of cause of motor weakness in traumatic brain injury using diffusion tensor imaging. Arch Neurol 69:363-367.
Hong JH, Kim SH, Kim OL, Byun WM, Jang SH (2012) Neural tract injuries by brain herniations after head trauma. J Head Trauma Rehabil 27:154-158.
Jang SH (2011a) A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation 28:345-352.
Jang SH (2011b) Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J 52:553-557.
Johnson PL, Eckard DA, Chason DP, Brecheisen MA, Batnitzky S (2002) Imaging of acquired cerebral herniations. Neuroimaging Clin N Am 12:217-228.
Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori H, Masumoto T, Ohtomo K (2004) The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 3:11-17.
Kwon HG, Lee DG, Choi BY, Chang CH, Kim SH, Jang SH (2011) Recovery of the corticospinal tract after injury by transtentorial herniation: a case report. NeuroRehabilitation 29:243-246.
Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prats A, Prados F, Boada I, Castellanos M, Sánchez-González J, Remollo S, Laguillo G, Quiles AM, Gómez E, Serena J (2010) Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor de fi cit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 31:1324-1330.
Scheidtmann K, Fries W, Müller F, Koenig E (2001) Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet 358:787-790.
Skoglund TS, Nilsson D, Ljungberg M, Jönsson L, Rydenhag B (2008) Long-term follow-up of a patient with traumatic brain injury using diffusion tensor imaging. Acta Radiol 49:98-100.
Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH (2011) The rubrospinal tract in the human brain: diffusion tensor imaging study. Neurosci Lett 504:45-48.
Yeo SS, Jang SH (2013) Corticospinal tract recovery in a patient with traumatic transtentorial herniation. Neural Regen Res 8:469-473.
Yoo WK, Kim DS, Kwon YH, Jang SH (2008) Kernohan’s notch phenomenon demonstrated by diffusion tensor imaging and transcranial magnetic stimulation. J Neurol Neurosurg Psychiatry 79:1295-1297.
Copyedited by Chen ZG, Zhang J, Wang TH, Jiang BG, Li CH, Song LP, Zhao M
Sung Ho Jang, M.D., Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 317-1, Daemyungdong, Namku, Daegu, 705-717, Republic of Korea, strokerehab@hanmail.net.
10.4103/1673-5374.135331 http://www.nrronline.org/
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1A4A01001873.
Author contributions: Seo JP designed this study, was responsible for data collection and analysis, and drafted the manuscript. Jang SH participated in the design of this study, was responsible for fundraising and revised the manuscript. Both of these two authors approved the fi nal manuscript.
Con fl icts of interest: None declared.
Accepted: 2014-05-14
Seo JP, Jang SH. Recovery of the corticospinal tracts injured by subfalcine herniation: a diffusion tensor tractography study. Neural Regen Res. 2014;9(12):1231-1233.
- 中国神经再生研究(英文版)的其它文章
- Assessment on self-care, mobility and social function of children with spina bi fi da in Turkey
- Prolonged electrical stimulation causes no damage to sacral nerve roots in rabbits
- Autophagy: a double-edged sword for neuronal survival after cerebral ischemia
- Diffuse axonal injury after traumatic cerebral microbleeds: an evaluation of imaging techniques
- Implanting iodine-125 seeds into rat dorsal root ganglion for neuropathic pain: neuronal microdamage without impacting hind limb motion
- Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells

