前体物对石蒜悬浮细胞生长和生物碱积累的影响
张玉琼,李勇,,周建辉,陈娜,王梅方,董召荣,高翠云,仲延龙 安徽农业大学生命科学学院,安徽 合肥 006 安徽农业大学农学院,安徽 合肥 006 广西农业科学院蔬菜研究所,广西 南宁 50007
前体物对石蒜悬浮细胞生长和生物碱积累的影响
张玉琼1,李勇1,2,周建辉3,陈娜1,王梅方1,董召荣2,高翠云1,仲延龙1
1 安徽农业大学生命科学学院,安徽 合肥 230036 2 安徽农业大学农学院,安徽 合肥 230036 3 广西农业科学院蔬菜研究所,广西 南宁 530007
为探究苯丙氨酸、酪氨酸和酪胺3种前体物对石蒜悬浮细胞系生长和生物碱积累的影响。通过向培养基添加不同浓度的3种前体物,以及同时添加苯丙氨酸和酪氨酸,考察其对细胞生长量及细胞中生物碱累积的影响。结果表明:苯丙氨酸对细胞的生长和生物碱的积累影响不明显;酪氨酸和酪胺作用显著:添加200 μmol/L酪氨酸,细胞中生物碱的含量是对照组的2.56倍,其中力可拉敏和加兰他敏含量为3.77 mg/g和4.46 mg/g,分别是对照组的6.61倍和6.97倍;添加200 μmol/L酪胺,细胞中生物碱含量是对照组的2.63倍,力可拉敏和加兰他敏含量为4.45 mg/g和5.14 mg/g分别是对照组的9.08倍和9.18倍;在200 μmol/L酪氨酸的基础上添加苯丙氨酸没有明显的增效作用。表明添加酪氨酸和酪胺对细胞生长及生物碱生物合成具有显著的促进作用
石蒜,悬浮细胞系,前体物,生物碱
石蒜Lycoris radiata是石蒜科Amaryllidaceae石蒜属Lycoris Herb.多年生单子叶植物,具有重要的药用价值。石蒜中含有多种具有生物活性的生物碱,如石蒜伦碱 (Lycorenine),加兰他敏(Galanthamine)、表加兰加敏 (Epigalanthamine)、普鲁维因 (Pluviine)、力可拉敏 (Lycoramine)、小星蒜碱 (Hippeastrine)、石蒜碱 (Lycorine)、漳州水仙碱 (Tazettine) 等[1-2]。其中加兰他敏是乙酰胆碱酯酶的可逆抑制剂,可以被用于治疗阿尔茨海默病 (Alzheimers disease)[3-5];石蒜碱具有抗癌活性和潜在的抗SAS-CoV病毒的活性[6]。这些药用成分主要取自石蒜鳞茎,由于石蒜的生长发育受环境影响较大,生长缓慢[7-8],且生物碱特别是加兰他敏含量较低[9]。所以,大规模生产生物碱受制于石蒜野外资源。
石蒜生物碱产业化主要有两条途径:一是化学合成方法,如加兰他敏合成方法[10-12],但步骤复杂、得率低且成本高;二是利用植物组织或细胞培养调控次生代谢进程,提高目标化合物的产量。这一途径具有生产周期较短,利于大规模培养,可人为调控细胞生长与获得高产细胞株等优点,已成为开发药用植物资源的重要途径。通过间歇浸入技术 (Temporary immersion technology) 培养雪片莲属Leucojum aestivum L.芽组织,加兰他敏和力可拉敏最高可达256 μg/RITA和1 699 μg/RITA[13]。培养基中添加蔗糖、植物生长调节因子或茉莉酸甲酯 (MeJA),可明显促进生物碱的生物合成[14-15]。此外,在培养体系中添加生物碱的前体能够提升产物合成的水平。有研究显示石蒜生物碱的合成前体为苯丙氨酸和酪胺[4-16],D-苯丙氨酸和酪氨酸对培养物的次生代谢和生长均有显著的影响[17-19]。目前还没有石蒜细胞悬浮培养体系及生物碱生物合成调控的报道,本实验在周建辉等[20]的基础上建立石蒜悬浮细胞培养体系,探究苯丙氨酸、酪氨酸和酪胺3种前体物对石蒜悬浮细胞系的生长和加兰他敏、石蒜碱和力可拉敏合成的影响,以期获得细胞生长和目标化合物较高的培养体系,同时为进一步研究石蒜生物碱的合成途径奠定基础。
1 材料与方法
1.1 材料与仪器
石蒜悬浮细胞系:将疏松、增殖力强的石蒜愈伤组织夹碎转接到液体培养基中,经过多次继代培养,待悬浮细胞系均一性较好时可作为试验研究材料。
仪器与试剂:Agilent 1 200高效液相色谱仪,Agilent 1 200系列泵,可变波长检测器,TUl8l0紫外可见分光光度计(北京普析通用仪器有限责任公司),320-S pH计(Mettler Toledo),HR-120电子天平(A&D公司)。加兰他敏、力可拉敏和石蒜碱购于株洲大有生物技术有限公司(纯度≥98%)。甲醇和乙腈 (色谱级,美国Tedia公司)。
1.2 方法
1.2.1 细胞生物量的测定
将细胞培养液于真空泵下抽滤,去离子水洗涤2–3次,滤纸吸干表面水分,即为细胞培养物的鲜重 (Fresh weight, FW)。悬浮细胞的增长率=(试验后的鲜重-初始鲜重)/初始鲜重× 100%。将细胞培养物在80 ℃烘干2 h,60 ℃干燥至恒重,冷却称重为干重 (Dry weight, DW),重复3次取平均值。
1.2.2 石蒜悬浮细胞系生物碱含量的测定
按照李明凯等[9]的提取与检测方并改进优化。将烘干的悬浮细胞研磨成粉末过筛,称取0.5 g,加入95%乙醇5 mL,320 W微波炉处理1 min,浸取1 h,减压浓缩至干,将浓缩物用5 mL 1 mol/L HCl溶解,用无水碳酸钠调pH至9.5,用三氯甲烷萃取3次合并三氯甲烷相,总生物碱在234 nm测定总生物碱含量。三氯甲烷减压浓缩至干后,溶于0.5 mL甲醇,0.22 μm微孔滤膜过滤即为待测生物碱样品。
HPLC分析方法采用文献[9]的方法。色谱柱为ZORBAX ODS-C18 (150 mm×4.6 mm,5 μm, Agilent公司);流动相:A:0.9%三乙胺水溶液(pH 8.0),B:乙腈,采用梯度洗脱。流速为1 mL/min,进样量为10 μL,检测波长为234 nm,检测温度为室温。标准曲线为:以加兰他敏(X1)、力克拉敏 (X2) 和石蒜碱 (X3) 为对照品,得线性回归方程为:加兰他敏,Y1=29.17X1+28.18,R1=0.9995;力可拉敏,Y2=21.83X2-1.105, R2=0.9995;石蒜碱,Y3=14.24X3-21.42, R3=0.9996。Y:峰面积,X:生物碱含量 (mg/L)。实验数据采用Microsoft Excel 2003软件处理分析。
2 结果与分析

图1 苯丙氨酸对细胞生长和生物碱含量的影响Fig. 1 Effect of phenylalanine on the growth of Lycoris radiata suspension cells and the content alkaloids in the cells. The addition of phenylalanine had no significant effect the growth of the cells and the content alkaloids in the cells compared to the control group.

图2 酪氨酸对细胞生长和生物碱含量的影响Fig. 2 Effect of tyrosine on the growth of Lycoris radiata suspension cells and the content alkaloids in the cells. The low concentration of tyrosine significantly increased the growth of the cells and the content of alkaloids in the cells compared to the control group. And the best concentration of tyrosine for the cells was 200 μmol/L.

图3 酪氨酸对石蒜悬浮细胞中的3种生物碱的含量的影响Fig. 3 Effect of tyrosine on the content of three kinds alkaloids in L. radiate suspension cells. Tyrosine had no effect on the accumulation of lycorine. Tyrosine at appropriate concentration (200 μmol/L) promoted the accumulation of lycoramine and galanthamine, but the effect of higher concentration of tyrosine on the accumulation of lycoramine and galanthamine weaken gradually.
2.1 苯丙氨酸对细胞生长和生物碱含量的影响
实验结果表明,苯丙氨酸对石蒜悬浮细胞系的生长和生物碱的合成都没有明显的影响(图1)。添加不同浓度的苯丙氨酸,石蒜悬浮细胞的生长情况与对照组的差异不明显,苯丙氨酸不能够促进培养物的生长。并且,处理组的生物碱的含量与对照组也没有明显的差异。可能是在石蒜生物碱合成途径中,苯丙氨酸不是合成途径中的关键化合物,合成途径中存在着其他化合物调节着生物碱合成的进行。
2.2 酪氨酸对细胞生长和生物碱含量的影响
酪氨酸对悬浮细胞系的生长和生物碱的积累有较大的影响 (图2)。低浓度的酪氨酸明显促进细胞生长。当酪氨酸的浓度为200 μmol/L时悬浮细胞系的鲜重增加量达到最大,继续增加酪氨酸的浓度,细胞鲜重增加幅度减小。低浓度的酪氨酸对生物碱积累有明显的促进作用,随着酪氨酸的浓度增加,总生物碱含量减小,当酪氨酸浓度为200 μmol/L时生物碱的积累量达到最大。
酪氨酸对石蒜悬浮细胞的3种生物碱积累的影响不同 (图 3)。实验结果表明:酪氨酸对石蒜碱的含量没有影响,对力可拉敏和加兰他敏的含量影响比较明显。当酪氨酸浓度为200 μmol/L时,力可拉敏的含量可达3.77 mg/g,是对照组的6.61倍,加兰他敏可达4.46 mg/g,是对照组的6.97倍,力可拉敏和加兰他敏的含量都远高于对照组。
2.3 酪胺对细胞生长和生物碱含量的影响
酪胺对悬浮细胞系的生长影响较大 (图4),低浓度的酪胺对悬浮细胞的生长促进作用明显。酪胺对总生物碱的积累亦有较大的影响,随着酪胺浓度的增加,总生物碱含量增加,当酪胺浓度高于150 μmol/L,总生物碱积累增幅减小,酪胺浓度为250 μmol/L时,总生物碱含量是对照组的2.63倍。
酪胺对细胞中3种生物碱含量的影响不同,对石蒜碱的含量影响不大,对力可拉敏和加兰他敏的含量影响较大。酪胺浓度为200 μmol/L时,力可拉敏的含量可达4.45 mg/g,是对照组的9.08倍。酪胺浓度为150 μmol/L时,加兰他敏含量可达5.14 mg/g,是对照组的9.18倍,力可拉敏和加兰他敏的含量都远高于对照组 (图5)。
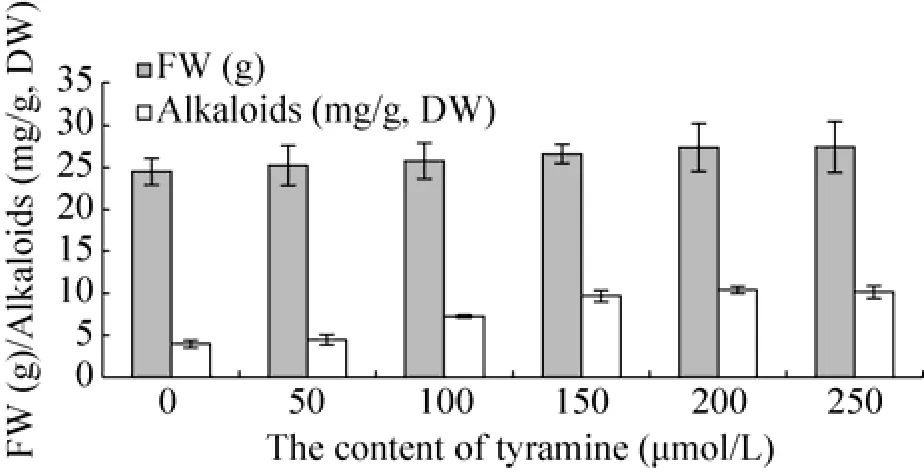
图4 酪胺对石蒜悬浮细胞系的影响Fig. 4 Effect of tyramine on the growth of Lycoris radiata suspension cells and the content alkaloids in the cells. The low concentration of tyramine significantly increased the growth of the cells compared to the control group. And the best concentration of tyramine for the cells was 200 μmol/L.
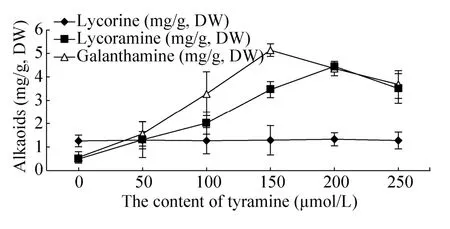
图5 酪胺对石蒜悬浮细胞中3种生物碱含量的影响Fig. 5 Effect of tyramine on the content of three kinds alkaloids in suspension cells. Tyramine also had no significant effect on accumulation of lycorine. The highest level of lycoramine and galanthamine was obtained by treated with 150 μmol/L and 200 μmol/L of tyramine, respectively.
2.4 苯丙氨酸和酪氨酸对细胞生长和生物碱含量的影响
生物碱合成有共同的前体苯丙氨酸和酪氨酸[16]。试验发现,同时添加苯丙氨酸和酪氨酸对生长和生物碱的合成影响较小,与单独添加酪氨酸组相比差异不明显 (表1)。
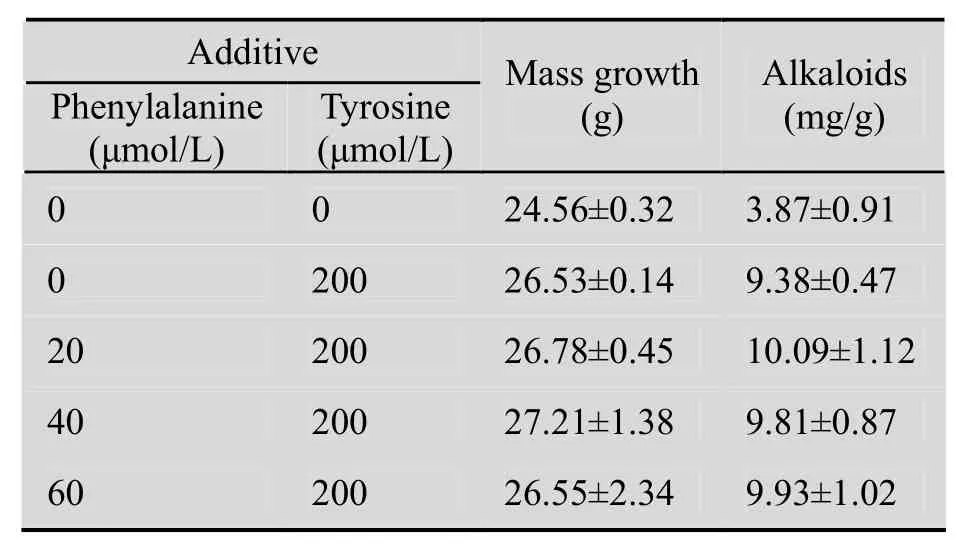
表1 苯丙氨酸和酪氨酸对细胞生长和生物碱含量的影响Table 1 Effects of phenylalanine combined with tyrosine on the growth of Lycoris radiata suspension cells and the content alkaloids in the cells
3 讨论
实验结果表明,苯丙氨酸对石蒜悬浮细胞的生长和生物碱积累影响不明显;酪氨酸和酪胺处理,细胞鲜重和3种生物碱含量要高于对照组。低浓度的酪氨酸和酪胺能够促进细胞生长和生物碱合成。当酪氨酸浓度大于200 μmol/L时,细胞鲜重增加的趋势减缓,生物碱含量降低;当酪胺浓度大于 150 μmol/L时,细胞鲜重和生物碱含量增加的趋势减缓。但在相同的浓度下,酪胺促进细胞生长的作用要强于酪氨酸。酪氨酸可在相关酶的作用下转化为其他物质进入其他途径中[16-21]。在已发表的文献中,精胺、MeJA、黄体酮、酪蛋白水解物、NO和酵母提取物均能促进石蒜科植物芽的生长,水杨酸和高浓度的茉莉酸甲酯则抑制生长[22-23]。蔗糖能增加培养物存活率,2,4-D会降低存活率,但是将2,4-D和BA同时加入会促进愈伤和根的生成[13]。
加兰他敏和力可拉敏的积累量最高时(5.14 mg/g,4.45 mg/g) ,酪胺浓度分别为150 μmol/L、200 μmol/L。同时添加苯丙氨酸(20 μmol/L) 和酪氨酸 (200 μmol/L) 时,总生物碱含量最高。Schumann等[24]和Ivanov等[13]获得的加兰他敏分别为2.40 mg/g DW和256 μg/RITA,后者获得的石蒜碱高达1 699 μg/RITA。Georgiev等[25]培养的雪片莲芽组织中加兰他敏含量1.7 mg/L,石蒜碱为8.3 mg/L。茉莉酸甲酯、NO和酵母提取物均能促进加兰他敏的合成,茉莉酸甲酯和酵母提取物对石蒜碱的合成也有促进作用,然而水杨酸、NO和酵母提取物对力可拉敏的合成均有抑制作用[22]。25 μmol/L的茉莉酸甲酯处理后,水仙细胞和培养基中的加兰他敏的含量约8.1 mg/g DW和5.0 mg/g DW[14]。在本实验中,悬浮细胞中的生物碱含量与已发表的文献中生物碱含量相比均要高出很多。因此,在本实验和前人研究的基础上可以建立用于大规模制备生物碱的培养体系。
REFERENCES
[1] Yuan JH. Research advances on the chemical constituents of Lycoris and their extraction and detection methods. J Anhui Agri Sci, 2010, 38(2): 684–686, 692 (in Chinese).袁菊红. 石蒜属化学成分及其提取、检测方法研究进展. 安徽农业科学, 2010, 38(2): 684–686, 692.
[2] Gotti R, Fiori J, Bartolini M, et al. Analysis of amaryllidaceae alkaloids from Narcissus by GC–MS and capillary electrophoresis. J Pharm Biomed, 2006, 42(1): 17–24.
[3] Marco L, do Carmo Carreiras M. Galanthamine, a natural product for the treatment of Alzheimer’s disease. Rec Pat CNS Drug Discov, 2006, 1(1): 105–111.
[4] Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci, 2013, 14(6): 11713–11741.
[5] Nair JJ, Bastida J, Viladomat F, et al. Cytotoxic agents of the crinane series of amaryllidaceae alkaloids. Nat Prod Commun, 2013, 8(5): 553–564.
[6] Li SY, Chen C, Zhang HQ. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res, 2005, 67(1): 18–23.
[7] Zhao TR, Shi YT, Cai JG, et al. The headway of research in Lycoris. Northern Horticulture, 2008, 4: 65–69 (in Chinese).赵天荣, 施永泰, 蔡建岗, 等. 石蒜属植物的研究进展. 北方园艺, 2008, (4): 65–69.
[8] Berkov S, Georgieva L, Kondakova V, et al. The geographic isolation of Leucojum aestivum populations leads to divergation of alkaloid biosynthesis. Biochem Syst Ecol, 2013, 46: 152–161.
[9] Li MK, Zhang YQ, Dong ZR, et al. Determination of galanthamine, lycoramine and lycorine in Lycoris radiata by high performance liquid chromatography. J Instrum Anal, 2012, 31(8): 957–961 (in Chinese).李明凯, 张玉琼, 董召荣, 等. 高效液相色谱法测定石蒜中加兰他敏、力可拉敏及石蒜碱3种生物碱. 分析测试学报, 2012, 31(8): 957–961.
[10] Fang L, Gou SH, Zhang YH. Progresses in total synthesis of galantamine. Chin J Org Chem, 2011, 31(3): 286–296 (in Chinese).房雷, 苟少华, 张奕华. 加兰他敏全合成研究进展. 有机化学, 2011, 31(3): 286–296.
[11] Guo T, Song Q, Qiu YH, et al. Synthesis of important intermediate bromonarwedine of galantamine. Chin J Syn Chem, 2012, 20(2): 251–253 (in Chinese).果婷, 宋琦, 邱银华, 等. 加兰他敏重要中间体溴那维定的合成. 合成化学, 2012, 20(2): 251–253.
[12] Choi J, Kim H, Park S, et al. Asymmetric total synthesis of (-)-galanthamine via intramolecular Heck reaction of conjugated diene. Synlett, 2013, 24(3): 379–382.
[13] Ivanov I, Georgiev V, Georgiev M, et al. Galanthamine and related alkaloids production by Leucojum aestivum L. shoot culture using a temporary immersion technology. Appl Biochem Biotechnol, 2011, 163(2): 268–277.
[14] Colque R, Viladomat F, Bastida J, et al. Improved production of galanthamine and related alkaloids by methyl jasmonate in Narcissus confusus shoot-clumps. Planta Med, 2004, 70(12): 1180–1188.
[15] El Tahchy A, Bordage S, Ptak A, et al. Effects of sucrose and plant growth regulators on acetylcholinesterase inhibitory activity of alkaloids accumulated in shoot cultures of Amaryllidaceae. Plant Cell Tiss Organ Cult, 2011, 106(3): 381–390.
[16] Bastida J, Lavilla R, Viladomat F. Chemical and biological aspects of Narcissus alkaloids. Alkaloids Chem Biol, 2006, 63: 87–179.
[17] Zhai XX, Li YY. Effect of amino acid precursors on callus growth and taxol content. Hubei Agri Sci, 2009, 48(10): 2944–2946 (in Chinese).翟雪霞, 李友勇. 几种氨基酸前体物对红豆杉愈伤组织的生长和紫杉醇含量的影响. 湖北农业科学, 2009, 48(10): 2944–2946.
[18] Qu JG, Yu XJ, Zhang W, et al. Significant suspension improved anthocyanins biosynthesis in cultures of Vitis vinifera by process intensification. Chin J Biotech, 2006, 22(2): 299–305 (in Chinese).曲均革, 虞星炬, 张卫, 等. 前体饲喂、诱导子和光照联合使用对葡萄细胞培养合成花青素的影响. 生物工程学报, 2006, 22(2): 299–305.
[19] Cao XB, Wang Z, Peng S, et al. Effects of lanthanum nitrate and L-phenylalanine on cailus growth and total alkaloid accumulation of Pinellia ternata. Shandong Agri Sci, 2012, 44(7): 26–28 (in Chinese).曹孝鲍, 王震, 彭爽, 等. 硝酸镧、L-苯丙氨酸对半夏愈伤组织生长和总生物碱积累的影响.山东农业科学, 2012, 44(7): 26–28.
[20] Zhou JH, Zhang YQ, Li MK, et al. Inducing callus tissues callus subculture of Lycoris radiata Herb. Plant Physiol Commun, 2010, 46(12): 1215–1218 (in Chinese).周建辉, 张玉琼, 李明凯, 等. 石蒜愈伤组织的诱导及其继代培养. 植物生理学通讯, 2010, 46(12): 1215–1218.
[21] Lee EJ, Facchini PJ. Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in Opium poppy. Plant Physiol, 2011, 157(3): 1067–1078.
[22] Zayed R, El-Shamy H, Berkov S, et al. In vitro micropropagation and alkaloids of Hippeastrum vittatum. In Vitro Cell Dev Biol Plant, 2011, 47(6):695–701.
[23] Mu HM, Wang R, Li XD, et al. Effect of abiotic and biotic elicitors on growth and alkaloid accumulation of Lycoris chinensis seedlings. Z Naturforsch C, 2009, 64(7/8): 541–550.
[24] Schumann A, Berkov S, Claus D, et al. Production of galanthamine by Leucojum aestivum shoots grown in different bioreactor systems. Appl Biochem Biotechnol, 2012, 167(7): 1907–1920.
[25] Georgiev V, Ivanov I, Berkov S, et al. Galanthamine production by Leucojum aestivum L. shoot culture in a modified bubble column bioreactor with internal sections. Eng Life Sci, 2012, 12(5): 534–543.
(本文责编 陈宏宇)
Effect of precursor on growth and accumulation of alkaloids of Lycoris radiata suspension cells
Yuqiong Zhang1, Yong Li1,2, Jianhui Zhou3, Na Chen1, Meifang Wang1,Zhaorong Dong2, Cuiyun Gao1, and Yanlong Zhong1
1 School of Life Sciences, Anhui Agricultural University, Hefei 230036, Anhui, China 2 School of Agronomy, Anhui Agricultural University, Hefei 230036, Anhui, China 3 Vegetable Research Institute, Guangxi Academy of Agricultural Sciences, Nanning 530007, Guangxi, China
In order to investigate the effects of phenylalanine, tyrosine and tyramine on the growth of Lycoris radiata suspension cells and the accumulation of alkaloids, the growth quantity of the cells as well as the content of alkaloids in cells were determined, which were treated with above three kinds of precursors alone and phenylalanine combined with tyrosine respectively. The results indicate that the addition of phenylalanine alone and addition of phenylalanine on the basis of tyrosine at high concentration (200 μmol/L) had no significant effect on the growth of Lycoris radiata suspension cells and the content of alkaloids in cells; whereas tyrosine and tyramine promoted the growth of the cells and alkaloids accumulation. Treated with tyrosine at high concentration (200 μmol/L), the content of alkaloids of the cells was 2.56-fold higher than that of the control group, the amounts of lycoramine (3.77 mg/g) and galanthamine (4.46 mg/g) were 6.61-fold and 6.97-fold higher than that of the control group, respectively. When treated with tyramine (200 μmol/L), the amount of alkaloids in Lycoris radiata suspension cells was 2.63-fold higher than that of the control group, and the amounts of lycoramine (4.45 mg/g) and galanthamine (5.14 mg/g) were 9.08-fold and 9.18-fold higher than that of the control group, respectively. The above results demonstrate that adding tyrosine and tyramine in the media significantly promoted the growth of the Lycoris radiata suspension cells and alkaloids accumulation in the cells
Lycoris radiata, suspension cell line, precursors, alkaloids
July 9, 2013; Accepted: September 5, 2013
Zhaorong Dong. Tel: +86-551-65786955; E-mail: d3030@163.com
张玉琼, 李勇, 周建辉, 等. 前体物对石蒜悬浮细胞生长和生物碱积累的影响. 生物工程学报, 2014, 30(2): 247–254.
Zhang YQ, Li Y, Zhou JH, et al. Effect of precursor on growth and accumulation of alkaloids of Lycoris radiata suspension cells. Chin J Biotech, 2014, 30(2): 247–254.
Supported by: Key Technologies Research and Development Program of China (No. 2013BAJ10B12), Natural Science Foundation of Anhui Province (No. 1208085mc48).
国家科技支撑计划项目(No. 2013BAJ10B12),安徽省自然科学基金 (No. 1208085mc48) 资助。
时间:2013-10-17 网络出版地址:http://www.cnki.net/kcms/detail/11.1998.Q.20131017.1242.004.html

