Simulation of biodiesel industrial production via solid base catalystin a fixed-bed reactor
Li Haoyang Pan Xiaomei Xiao Yang Xiao Guomin Huang Jinjin
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
Simulation of biodiesel industrial production via solid base catalystin a fixed-bed reactor
Li Haoyang Pan Xiaomei Xiao Yang Xiao Guomin Huang Jinjin
(School of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, China)
Biodiesel industrial production based on a solid base catalyst in a fixed-bed was simulated. The lab and bench scale experiments were carried out effectively, in which the kinetic model is established and it can describe the transesterification reaction well. The Antoine equation of biodiesel is regressed with the vapor-liquid data cited of literature. The non-random two liquid (NRTL) model is applied to describe the system of fatty acid methyl ester (FAME), methanol and glycerol and parameters are obtained. The Ternary phase map is obtained from Aspen Plus via the liquid-liquid equilibrium (LLE) data. In order to describe the production in a fixed-bed performs in industrial scale after being magnified 1000 times, the Aspen Plus simulation is employed, where two flowsheets are simulated to predict material and energy consumption. The simulation results prove that at least 350.42 kW energy consumption can be reduced per hour to produce per ton biodiesel compared with data reported in previous references.
solid base catalyst; fixed-bed reactor; Aspen Plus simulation; biodiesel industrial production
With the rapid decrease in fossil fuels and the increasing consumption of resources, searching for alternative energy sources has become even more urgent. Much attentions has been paid to research in renewable, economical and environment friendly fuels by both developed and developing nations. Biodiesel (fatty acid methyl ester, FAME), a kind of “green fuel”, is environment-friendly, safe and renewable, making it fundamentally useful in improving the utilization of this green power[1]. As a result, it is being widely favored and investigated by companies and institutes. The exploitation of industrialization of FAME production is required urgently[2].
One method of biodiesel production is the transesterification of triglycerides with low alcohol, such as methanol or ethanol, and glycerol obtained as a by-product. Traditionally, homogeneous catalysts including H2SO4, NaOH, KOH and NaOCH3solutions[3-6]were used to power biodiesel production, in which mass transfer is insignificant due to a low diffusion resistance. Therefore, reactions powered by homogeneous catalysts are fast and have high conversions. But some shortcomings[7]of homogeneous catalyst are obvious as well. Homogeneous acids and bases erode equipment and they are with difficulty separated from products, not only increasing the production cost but also generating plenty of waste water, which is strictly contrary to the notion of sustainable development. Non-catalytic supercritical reaction is of short reaction time with a conversion of 95% or more. It is not necessary to wash products at all. But the molar ratio of alcohol to oil is quite high and the reaction temperature and pressure should exceed the critical values of methanol. Heterogeneous catalysts, however, are able to overcome the former’s disadvantages. Transesterification catalyzed by a solid base has remarkably advantages. These kinds of processes are easily operated and conveniently disposed of and output no waste water. Heterogeneous transesterification is considered to be a green process[8], requiring neither catalyst recovery nor aqueous treatment steps. A high biodiesel conversion can also be achieved. Heterogeneous catalysts in biodiesel production have been extensively investigated in the last few years. Several metal oxides have been studied for the transesterification process including alkali earth metal oxides, transition metal oxides, mixed metal oxides and supported metal oxides[9].
In addition, the process of transesterification is another factor which should be paid attention to, because it impacts remarkably on material and energy consumption. Presently, batch reactors are employed in FAME production and have become a set of mature processes. However, there are several disadvantages concerning costs in that process: a long reaction time, large energy consumption, poor mass transfer, low conversion, difficulty in control and a lack of continuous production[10]. In order to promote operation efficiency, continuous technique reforms and innovations are required. The main directions may be as follows: 1) Developing new heterogeneous catalysts, extending the life of catalysts, finding new catalyst reactivation methods, decreasing costs and preventing environment pollution simultaneously; 2) Applying new techniques such as catalysis coupled with separation, decreasing the alcohol to oil ratio to reduce energy consumption in alcohol’s recycling; 3) Investigating transesterification reactions in super critical conditions or other new FAME producing processes with independent intellectual property rights[11]. Various new high-efficiency reactors are applied in the FAME production. The lab scale and bench scale[12-14]experiments were conducted well in our previous work. Kinetic and mass transfer models have been established and they can describe the transesterification reaction well. In order to predict how the catalyst, fixed-bed and the models perform on an industrial scale after being magnified 1 000 times, the industrial scale production of biodiesel was simulated. In this system, solid base KF/Ca-Mg-Al hydrotalcite[13]is prepared and used as catalyst. Soybean oil and methanol are employed as feedstock of transesterification to calculate energy and material consumption in industrial scale of biodiesel producing. Based on lab and bench scale, this simulation provides model data for industrial design, such as separating procedure selections and the further references for equipment sizes calculation.
1 Methodology
1.1 Kinetic model
1.1.1 Intrinsic-kinetic model
An intrinsic-kinetic model was developed by Gao et al[13]. Based on the proposed assumptions, the reaction can be written as
(1)
This equation describes the overall reaction of triglyceride (A) and methanol (B) to form three FAME (C) and glycerol (D). Since the series of assumptions, our work conducted with soybean oil agrees with the kinetic based on palm oil as a reactant. The kinetic equation, therefore, can be written as
rA=k1CACB-k2CCCD=
(2)
whererAis the reaction rate of A considering no diffusion effects, kmol/(kg·s)-1;k1is the rate constant of positive reaction, m6/(kmol·kg·s)-1;k2is the rate constant of inverse reaction, m6/(kmol·kg·s)-1;Ci0is the initial concentration in the bulk fluid of componenti, kmol/m3;Ciis the concentration in the bulk fluid of componenti, kmol/m3;mis the molar ratio of alcohol to oil andxis the conversion of transesterification.
Pre-exponential factors of the reversible reaction and activation energy were calculated and regressed by the Arrhenius equation. Rate constants at different temperatures of both positive and inverse reactions were computed by this data consequently.
1.1.2 Macro-kinetic model
While internal and external diffusion are included[12], the kinetic model should be modified as
(3)
wherereiis the reaction rate including both external and internal diffusion effects, kmol/(kg·s)-1;kis the rate constant of transesterification reaction, m6/(kmol·kg·s)-1;Csiis the concentration at the external surface of catalyst of componenti, kmol/m3;Kis the reaction equilibrium constant andKiis the adsorption equilibrium constant of componenti, m3/kmol.
In previous work, this model agreed well with transesterification via a solid base catalyst in a fixed-bed, with which 95% conversion can be achieved at the temperature 65 ℃, the recommended condition in the literature. Our work was carried out on the basis of these kinetic models for lab and bench scales.
1.2 Reactor model of fixed-bed
Besides the kinetic models, the reactor model also contributes to the veracity of this simulation. Xiao et al.[12]put a one-dimensional heterogeneous model of a fixed-bed as
(4)
wherexis the conversion of transesterification;Lis the height of catalyst packed in the fixed bed reactor, m;reis the reaction rate including external diffusion effects, kmol/(kg·s)-1;u1is the liquid mixture velocity, m/s;ηis the internal diffusion effectiveness factor;ρbis the packed density of fixed bed reactor, kg/m.
Impacts such as liquid hourly space velocity(LHSV), h-1, temperature, and the molar ratio of methanol-to-oil on transesterification conversion are illustrated. The computed result of this model suggests that the conversion reaches 95% under the conditions LHSV of 0.76 to 0.25 h-1, the molar ratio of methanol-to-oil of 9.16 to 13.7, and the temperature of 338 to 347 K, which agrees with experimental data.
1.3 Pure components parameters
Soybean oil can be defined as C57H104O6. It performs appropriately in presenting most properties of soybean oil. The molecule format of FAME is presented as C19H36O2, a product via transesterification of soybean oil and methanol. Parameters of pure components come from the chemical simulation software Aspen Plus and can represent most properties of reactants and products, except for FAME[15].
(5)
wherePis the pressure, Pa; andTis the absolute temperature,K.
The Antoine equation (5) is used to present boiling points of FAME under different pressures. Parameters of the Antoine equation are regressed from the equation and the fraction data of 14 compositions in soybean oil. With this equation, conditions and results of separation in the following processes are determined and obtained exactly.
1.4 Parameters of Ternary mixture
Aspen Plus has almost all the property parameters of common chemicals, but still lacks some data from certain chemicals. The products in this process are FAME and glycerol, mixed with excess methanol. Thus regressing the liquid-liquid equilibrium (LLE) is needed. The LLE data of FAME, methanol and glycerol are from Ref.[16] and regressed by the NRTL (non-random-two-liquid) model with Aspen Plus. It is essential to obtain information of the phase equilibrium of the reactive mixture to explore possible operation conditions for the reactor and the downstream separation processes.
1.5 Flowsheet simulation
According to section 1.1 and 1.2, the preheaters were set at 65 ℃, and the following reaction conducted at such a temperature that can guarantee the conversion of 95%. Glycerol, the by-product, dissolves partially in methanol while biodiesel exists[17]. Biodiesel would be miscible with glycerol while with methanol, vaporized to some degree. Therefore, this work investigates two procedures on the measures of dealing with methanol and products. Two flowsheets, depicted in Fig.1, are drawn to compare energy and material consumption with the other processes. In the first several steps, the two flowsheets are the same: soybean oil and methanol are pumped into two pre-heaters, respectively. The reactants were heated by the preheaters and then pumped into the fixed-bed, in which the solid base KF/Ca-Mg-Al hydrotalcite catalyst was packed to power the transesterification. After the reaction, FAME, the by-product glycerol and unreacted methanol, were dealt with differently in the two flow sheets.
Flowsheet 1 (FS1) separated FAME and glycerol in a 25 ℃ temperature decanter V-101, in which, two liquid phases were formed: a glycerol-rich phase on the lower layer and a FAME-rich phase on the upper layer. The two phases were delivered to column T-101 and T-103 to recover methanol, respectively. Methanol mass fraction of each distillated vapor was near 100% while the stages and reflux ratio were 4 and 1. Out of column T-101, the FAME-rich phase was sent to T-102 for further purification, of which 20 stages and reflux ratio of 4 were set to obtain 99.8% mass fraction of FAME. The raw glycerol obtained in column T-103 was sent to T-104 for further purification.

(a)

(b)
On the contrary, flowsheet 2 (FS2) recovered methanol from the crude FAME mixed with glycerol in the column T-101 first and then separated the glycerol and FAME in the decanter V-102. Little stages (five theoretical stages) and low reflux ratio (reflux ratio=1) were enough to achieve the separation purpose since the boiling point of methanol is much lower than those of the other components. Similar to FS1, the phase separation was conducted at 25 ℃[18]to satisfy the requirement of the maximum triglyceride content in biodiesel (≤0.2 %, mass fraction) specified in EN 14214. The by-product glycerol was purified in column T-102.
Traditionally, increasing stage numbers can elevate the purification of FAME and glycerol. 20 stages were set in column T-102 of FS1 to purify FAME. Pressure was as low as 2 kPa to avoid methyl ester thermal decomposition. Stage number and reflux ratio were crucial factors affecting energy and mass consumption. T-104 of FS1 and T-102 of FS2 were employed to purify glycerol with 18 and 14 stages respectively and the reflux ratio of 4 for both. Under such conditions, the mass fraction of FAME reached 0.998 in biodiesel purification and glycerol reached 0.9996 in both glycerol purification columns.
2 Results and Discussion
2.1 Parameters of pure components
Some FAME regressed parameters are listed in Tab.1, and compared with the data regressed by parameters cited in Ref.[15]. The absolute errors of the temperature between the two classes were below 5 ℃ except in the situation of the pressure of 13.332 kPa. Therefore, the Antoine equation with the regressed parameters was appropriate to present the real boiling points, and thus data obtained in the processes of separating methanol, FAME and glycerol is credible.
Tab.1 Comparison of boiling points at different pressures

P/kPaTemperature/℃Ref.[15]ThisworkAbsoluteerrorsRelativeerror101.325355.919355.4480.4710.001393.326351.563351.4160.1470.000486.660347.703347.839-0.1360.000479.993343.602344.035-0.4340.001373.327339.221339.968-0.7470.002266.661334.513335.593-1.0790.003259.995329.417330.850-1.4330.004353.329323.849325.661-1.8120.005646.663317.698319.919-2.2220.007039.997310.800313.469-2.6690.008633.331302.911306.075-3.1650.010426.664293.634297.356-3.7230.012719.998282.250286.619-4.3690.015513.332267.223272.370-5.1460.0193
2.2 Phase equilibrium data
The NRTL model is suitable for characterizing the LLE data as it shows an ideal performance in predicting regressed parameters at all global temperatures[17]. This model not only can compute the VLE system but also suits LLE binary activity coefficient calculation. Based on these considerations, this model is applied to the partial miscible system.[19].
As illustrated in Tab.2,cij’s are 0.3, between 0.2 and 0.47, suitable for describing the non-associated liquid such as the FAME-methanol-glycerol system[20]. The Ternary phase map at 25 ℃ regressed by the NRTL model with Aspen Plus is shown in Fig.2.
Tab.2 The regressed binary parameters of NRTL model
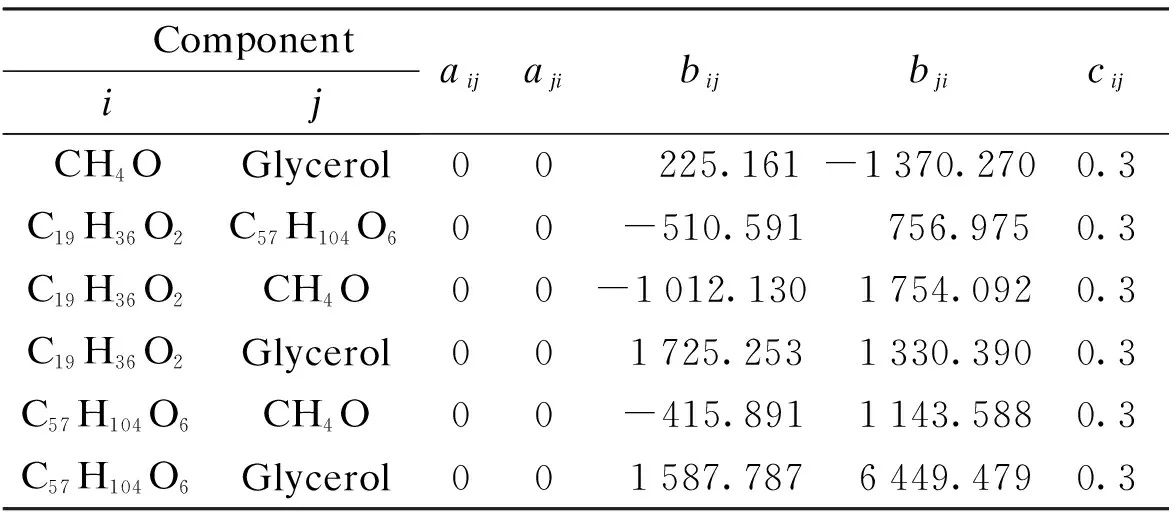
ComponentijaijajibijbjicijCH4OGlycerol00225.161-1370.2700.3C19H36O2C57H104O600-510.591756.9750.3C19H36O2CH4O00-1012.1301754.0920.3C19H36O2Glycerol001725.2531330.3900.3C57H104O6CH4O00-415.8911143.5880.3C57H104O6Glycerol001587.7876449.4790.3
Notes:aij,aji,bij,bjiandcijare the parameters of NRTL equation.
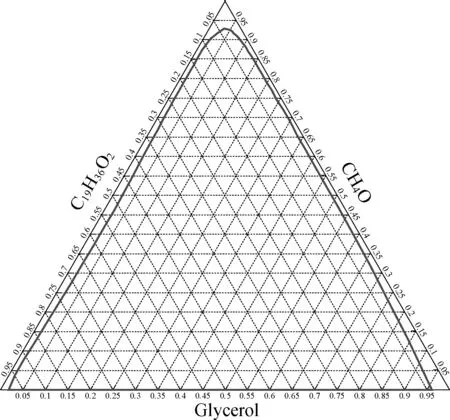
Fig.2 Ternary phase map of FAME-methanol-glycerol
It is obvious that FAME, methanol, and glycerol are not completely miscible and the fraction of FAME dissolved in glycerol decreases with the increasing mole fraction of methanol while the solution of glycerol in FAME almost holds invariantly. It makes the separation processes after reaction simplified and thus energy and mass are saved. By tie lines, the phase separation can be determined at any component fraction, which is the basis of LLE in this simulation.
2.3 Energy and mass consumption
Two series of data of material and energy consumed in producing 1 t FAME by the two flowsheets are listed in Tab.3. FS1 consumed more material of the 11.33 kg, including 9.31 kg soybean oil and 2.02 kg methanol, and energy of 165.05 kW but produced less glycerol of 11.66 kg than FS2. In FS1, methanol was recovered in column T-101 while the biodiesel was purified in T-102 and glycerol purified in T-103 and T-104. For FS2, such processes were conducted in T-101, V-101 and T-102, respectively. Compared with FS2, two more columns were employed in FS1. The biggest gap of energy consumed was formed at columns T-102 and T-103, the processes of biodiesel and glycerol recovery.
Tab.3 Comparison of material and energy consumption and output of products in two flowsheets
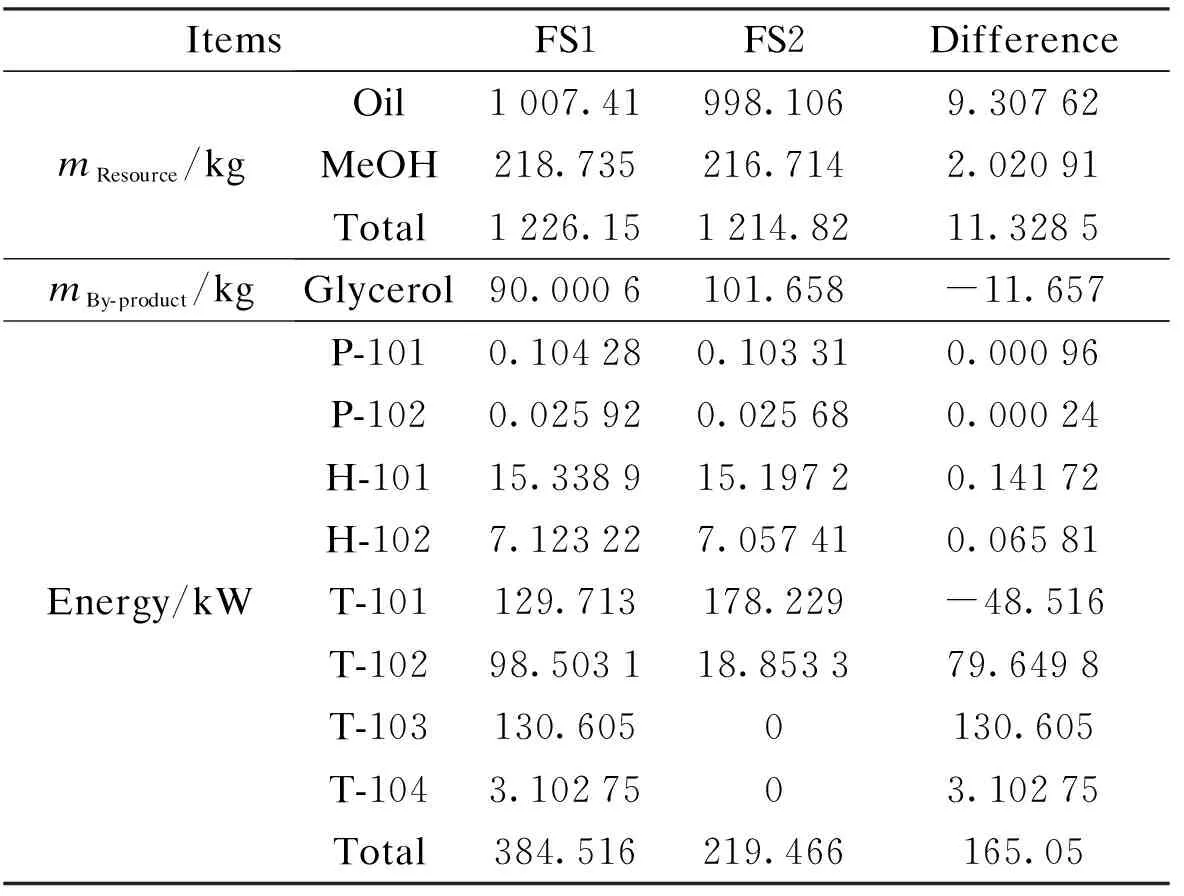
ItemsFS1FS2DifferencemResource/kgOil1007.41998.1069.30762MeOH218.735216.7142.02091Total1226.151214.8211.3285mBy-product/kgGlycerol90.0006101.658-11.657Energy/kWP-1010.104280.103310.00096P-1020.025920.025680.00024H-10115.338915.19720.14172H-1027.123227.057410.06581T-101129.713178.229-48.516T-10298.503118.853379.6498T-103130.6050130.605T-1043.1027503.10275Total384.516219.466165.05
Stream conditions of the two flow sheets are listed in Tab.4 and Tab.5 to compare the purities of products and the consumption of feedstock. In order to compare the impact of different processes on material and energy consumption, similar mass fractions were specified. The mass fractions of FAME, methanol and glycerol are 99.83%, 100%, 99.97% in streams PL114, PL111 and PL118, respectively in Tab.4; and they are 99.86%, 100% and 100% in streams PL211, PL208 and PL214, respectively, as shown in Tab.5.
Note that material and energy consumptions are less even though the products’ purity and material recovery are slightly higher in FS2. The reason for these phenomena may be explained by that the methanol in FS1 was distributed in the two phases of FAME and glycerol, and thus more columns were used to separate methanol to purify product and recover methanol. As a result, more material was wasted in these devices, while after being decanted in FS2, the purity of FAME is 99.86 % and this process consumes a little energy that can be ignored.
Ref.[18] reported energy consumption based on three production processes in simulation of producing 40000 t FAME per year. The first process, named Alkali-FVO, was an alkali-catalyzed process using fresh canola oil as the feedstock. The second process, named Alkali-WVO, was an alkali-catalyzed process with an acid-catalyzed pre-treatment step of waste canola oil. The last process, named SC-WVO, was a supercritical process using waste canola oil. All of those included pressuring and heating of raw material, reaction, methanol recovery and biodiesel purification. The Alkali-WVO procedure needs pre-treatment for WVO consisting of many free fat acids (FFAs) which cannot be catalyzed by alkali. However, in super-critical and FVO processes, this treatment is not needed. Catalyst was also needed to treat alkali catalyzed processes after the reaction. As revealed in Tab.6, Alkali-WVO consumes more energy than Alkali-FVO due to the high energy consumption of the methanol distillation columns. In comparison with the alkali-catalyzed processes, the super-critical processes have a large requirement for pumping and heating raw material to achieve the harsh reaction conditions. Alkali-FVO is the most energy-consuming among the three processes, but it consumes more energy, about 350.42 kW, compared with FS2.
Tab.4 Stream data of FS1

StreamnamePL101PL104PL107PL111PL114PL116PL118Totalflow/(kg·h-1)1000217.1251217.1257.42994.60399.60289.602Temperature/℃25256520.6338194.314280.21286.941Pressure/MPa0.10.10.20.10.020.10.02Massfraction/(kg·kg-1)C19H36O2000.8245200.998330.038120.00294C57H104O6100.0008200.00100CH3OH010.08928100.001930Glycerol000.0853700.000970.959890.99966
Tab.5 Stream data of FS2

StreamnamePL201PL204PL207PL208PL211PL212PL214Totalflow/(kg·h-1)1000217.1251217.12108.51003.27105.351101.851Temperature/℃25256520.63382525181.582Pressure/MPa0.10.10.20.10.10.10.02Massfraction/(kg·kg-1)C19H36O2000.8245200.998630.015680C57H104O6100.0008200.0009900CH3OH010.08928100.0010Glycerol000.0853700.000320.983241
Tab.6 Comparison of energy consumption per hour of five processes
kW
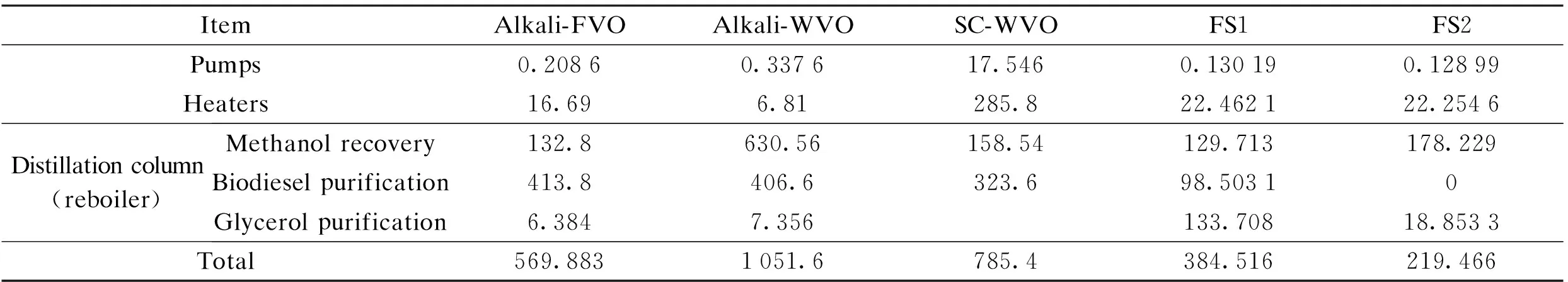
ItemAlkali-FVOAlkali-WVOSC-WVOFS1FS2Pumps0.20860.337617.5460.130190.12899Heaters16.696.81285.822.462122.2546Distillationcolumn(reboiler)Methanolrecovery132.8630.56158.54129.713178.229Biodieselpurification413.8406.6323.698.50310Glycerolpurification6.3847.356133.70818.8533Total569.8831051.6785.4384.516219.466
3 Conclusion
Intrinsic and macro kinetic models of transesterification catalyzed by the hydrotalcite catalyst were reviewed. The Antoine Equation and NRTL parameters along with LLE data of FAME, methanol and glycerol are accurately regressed by Aspen Plus to simulate our processes in this work. Two flowsheets were established to simulate the procedure of biodiesel. Energy and material consumption are 384.516 kW and 1226.15 kg in FS1 and 219.466 kW and 1214.82 kg in FS2 per hour. The result suggests that decanting product following vaporize methanol can save much more energy and materials. FS2 saves the energy of 350.42 kW in producing 1 t biodiesel per hour compared with process Alkali-FVO. Consequently, the two flowsheets are energy saving compared with the other three processes and FS2 are more appropriate. So much energy and material are reduced due to our catalyst KF/Ca-Mg-Al hydrotalcite’s high catalytic conversion, heterogeneous property and recovering methanol followed by separating FAME and the glycerol process strategy. Therefore, the process catalyzed by solid base in a fixed-bed is proved to be feasible while being magnified 1 000 times to industrial scale successfully.
[1]Nie X, Chang X, Zhang T, et al. Energy-saving and economic assessment for continuous pipeline production of biodiesel [J].ChemindForestProd, 2011, 31(4): 8-11. (in Chinese)
[2]Kiss A A. Separative reactors for integrated production of bioethanol and biodiesel [J].ComputChemEng, 2010, 34(5): 812-820.
[3]Demirbas A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey [J].EnergConversManage, 2003, 44(13): 2093-2109.
[4]Zhang Y, Dube M A, McLean D D, et al. Biodiesel production from waste cooking oil: 1. process design and technological assessment [J].BioresourTechnol, 2003, 89(1): 1-16.
[5]Ma F, Hanna M A. Biodiesel production: a review [J].BioresourTechnol, 1999, 70(1): 1-15.
[6]Vicente G, Martinez M, Aracil J. Integrated biodiesel production: a comparison of different homogeneous catalysts systems [J].BioresourTechnol, 2004, 92(3): 297-305.
[7]Li W M, Zheng X L, Xu C M, et al. Preparation and its properties of biodiesel by using solid base catalyst [J].JournalofChemicalandIndustryEngineering(China), 2005, 56(4): 711-716. (in Chinese)
[8]Chouhan A P S, Sarma A K. Modern heterogeneous catalysts for biodiesel production: a comprehensive review [J].RenewSustEnergRev, 2011, 15(9): 4378-4399.
[9]Refaat A A. Biodiesel production using solid metal oxide catalysts [J].IntJEnvironSciTechnol, 2011, 8(1): 203-221.
[10]Han X L, Huang X M. Present status of biodiesel production technology [J].ModChemInd, 2007, 27(S1): 129-133. (in Chinese)
[11]Guo W J, Min E Z. An investigation on developing China’s biodiesel industry [J].ACTAPetrolSin, 2003, 19(2): 1-6. (in Chinese)
[12]Xiao Y, Gao L J, Xiao G M, et al. Experimental and modeling study of continuous catalytic transesterification to biodiesel in a bench-scale fixed-bed reactor [J].ChemEngResDes, 2012, 51(37): 11860-11865.
[13]Gao L J, Xiao G M, Xiao Y. The intrinsic kinetics of transesterification of palm oil for biodiesel production in fixed-bed [C]//Proceedingsofthe6thChineseNationalChemicalandBiochemicalEngineeringAnnualMeeting. Changsha, China, 2010. (in Chinese)
[14]Xiao Y, Gao L J, Xiao G M, et al. Kinetics of the transesterification reaction catalyzed by solid base in a fixed-bed reactor [J].EnergFuels, 2010, 24(11): 5829-5833.
[15]Yuan W, Hansen A C, Zhang Q. Vapor pressure and normal boiling point predictions for pure methyl esters and biodiesel fuels [J].Fuel, 2005, 84(7/8): 943-950.
[16]Andreatta A E, Casa’s L M, Hegel P, et al. Phase equilibria in ternary mixtures of methyl oleate, glycerol, and methanol [J].IndEngChemRes, 2008, 47(15): 5157-5164.
[17]Chen Y C. Research on vapor-vapor phase equilibrium in biodiesel system [D]. Hangzhou: Department of Chemical and Biological Engineering, Zhejiang University, 2010. (in Chinese)
[18]Lee S, Posarac D, Ellis N. Process simulation and economic analysis of biodiesel production processes using fresh and waste vegetable oil and supercritical methanol [J].ChemEngResDes, 2011, 89(12): 2626-2642.
[19]Tang Y T, Huang H P, Chien I L. Design of a complete ethyl acetate reactive distillation system [J].JChemEngJpn, 2003, 36(11): 1352-1363.
[20]Jiang X W, Wang Y, Guan C X. Calculation for vapor-liquid phase equilibrium of nonideal system with NRTL and SRK equation [J].ChemEngDes, 2007, 17(5): 11-15. (in Chinese)
固体碱催化固定床法生产生物柴油的工艺研究
李浩扬 潘晓梅 肖 洋 肖国民 黄金金
(东南大学化学化工学院, 南京 211189)
对固体碱催化剂催化固定床法工业化生产生物柴油进行了模拟.顺利进行了小试实验,并建立了生物柴油合成的酯交换反应的动力学模型,该模型能较为准确地描述酯交换反应.通过对文献中的气液平衡数据的回归得到了生物柴油的安托因方程.选择NRTL模型对脂肪酸甲酯(FAME)-甲醇-甘油体系进行描述,得到了体系NRTL的二组分参数,利用Aspen Plus对该体系液液平衡数据(LLE)的回归得到了三组分相图.为了预测固定床工艺生产在工业规模放大1000倍后的效果,Aspen Plus对2个流程进行了仿真模拟,预测物料和能量消耗.模拟结果表明,与先前文献报道的数据相比,每小时生产每吨生物柴油至少可以减少350.42 kW的能量消耗.
固体碱催化剂;固定床反应器;Aspen Plus模拟;生物柴油生产
TQ028.4
s:The National Basic Research Program of China (973 Program)(No.2010CB732206), the National Natural Science Foundation of China (No.21076044, 21276050).
:Li Haoyang, Pan Xiaomei, Xiao Yang, et al.Simulation of biodiesel industrial production via solid base catalyst in a fixed-bed reactor[J].Journal of Southeast University (English Edition),2014,30(3):380-386.
10.3969/j.issn.1003-7985.2014.03.023
10.3969/j.issn.1003-7985.2014.03.023
Received 2013-12-24.
Biographies:Li Haoyang (1988—), male, graduate; Pan Xiaomei(corresponding author), female, doctor, associate professor, pan xiaomei@seu.edu.cn.
 Journal of Southeast University(English Edition)2014年3期
Journal of Southeast University(English Edition)2014年3期
- Journal of Southeast University(English Edition)的其它文章
- P-FFT and FG-FFT with real coefficients algorithm for the EFIE
- Compressed sensing estimation of sparse underwateracoustic channels with a large time delay spread
- Improved metrics for evaluating fault detection efficiency of test suite
- Early-stage Internet traffic identification based on packet payload size
- An adaptive generation method for free curve trajectory based on NURBS
- Stability analysis of time-varying systems via parameter-dependent homogeneous Lyapunov functions
