Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol
DING Xiao-Chun CHEN Xiu ZHOU Jian-Hua WANG Tao SUN Dun HE Jian-Ping
(College of Material Science and Technology,Nanjing University of Aeronautics and Astronautics,Nanjing 210016,P.R.China)
Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol
DING Xiao-Chun CHEN Xiu ZHOU Jian-Hua WANG Tao SUN Dun HE Jian-Ping*
(College of Material Science and Technology,Nanjing University of Aeronautics and Astronautics,Nanjing 210016,P.R.China)
Abstract: Pt-Ni alloy catalysts with different atomic ratios were deposited on CMK-5(carbon replicated from SBA-15 silica)by NaBH4reduction.X-ray diffraction(XRD)suggests alloy formation between Pt and Ni.X-ray photoelectron spectroscopy(XPS)shows that Pt-Ni/CMK-5(5:1)has more detectable oxidized Ni.More metallic Pt is present on Pt-Ni/CMK-5(5:1)(atomic ratio)than on Pt/CMK-5.Oxidized Ni species,such as NiO,Ni(OH)2,and NiOOH,favor the adsorption of methanol and the dissociation of methanol from the surface of Pt.Cyclic voltammetry shows that Pt-Ni/CMK-5(5:1)has the highest specific activity among the as-made catalysts and its electrochemical active area is 63.9 m2·g-1.It has more resistance to CO poisoning than Pt/CMK-5.
Key Words:CMK-5;Pt/CMK-5 catalyst;Pt-Ni/CMK-5 catalyst;Methanol;Electrooxidation
1 Introduction
Fuel cells are appealing alternative power sources as they offer high energy density with zero or low emission of pollutants.Among the diverse types of fuel cells,the proton exchange membrane fuel cells(PEMFC)and direct methanol fuel cells(DMFC)are the most suitable candidates for transportation applications,portable electronics,and residential power sources due to their relatively low operating temperature(<100°C)and fast starting-up function.However,the commercial viability of PEMFC and DMFC is still hindered by several drawbacks,including the low catalytic activity of electrodes,the high cost of the Pt-based catalysts,and the poor durability and reliability.One of the main obstacles for the application of PEMFC in vehicles is the long-term durability of the cathodecatalysts,especially when the fuel cells are operated under the cycle duty.Up to now,the carbon-supported Pt is still a conventional electro-catalyst for PEMFC and DMFC.The degradation of Pt/C cathode catalysts results from both the reduction of electrochemical active surface area(EAS)of Pt and the corrosion of carbon support.1The overpotential caused by the highly irreversible oxygen reduction reaction(ORR)and the methanol crossed over from anode poisoning cathode is the major performance limitation for cathode catalyst.2So there are two solutions to the above problems,one is to quest for alternative catalyst supports,such as carbon nanotubes,carbon spheres,graphitic carbon nanofibers,3-7which are beneficial to improve the dispersion of Pt and consequently enhance its electro-catalytic activity.The other approach is to prepare Pt-based alloy,such as Pt-Ru,Pt-Ni,Pt-Co.8-10Based upon bifunctional mechanism,CO-poisoned Pt nanoparticles can be regenerated via the reaction of surface CO with O-type species associated with a second metal yielding CO2.11Over the last two decades,various Pt-based alloy catalysts had been widely investigated,among which Pt-Ni bimetallic catalyst had attracted more interest.12-21Ni can decrease the oxidation activation potential of H2O,which can dissociate into active oxygen species at a lower potential.The formed active Ni-(OH)adscan react with CO into CO2.Besides,various oxidized Ni accelerate the reaction of Pt-CO with oxygen-containing species produced by oxidized Ni,and thus decreasing the CO-poisoning of Pt.Therefore,Pt-Ni alloy catalyst shows improved electrocatalytic activity.17
In order to enhance the catalytic activity of the Pt-Ni alloy catalyst,the choice of the support plays a very important role in obtaining high-performance catalysts.CMK-5,a carbon replicated from SBA-15 silica,is among promising support candidates due to its large pore volume,high structural stability and large surface area.22,23Based on our previous research work,the electrochemical active surface of Pt/CMK-5 approximately equals to that of Pt/C(E-ETK).24
In the present work,CMK-5 was applied to support catalyst nanoparticles via the NaBH4-reduction method.With the fixed total Pt-Ni loading,more Pt loading can absorb more methanol,however displaying a lower electrocatalytic activity.Because less Ni loading forms less Ni-(OH)ads,unfavorable for the oxidation activation of methanol.However a lower Pt loading provides less active sites for absorbing methanol.The present work is undertaken to determine the optimum nominal Pt-Ni atomic ratio among 1:1,3:1,5:1,and 7:1.The physical and morphological characteristics of these bimetallic catalysts were systematically investigated.And the electro-catalytic properties of the catalysts for hydrogen and methanol oxidation were evaluated by cyclic voltammetry.Furthermore,the relationship between the structure and the electrochemical performance and the mechanism interpretation for catalysts were investigated in detail.
2 Experimental
2.1 Synthesis of catalyst
Nano-casting carbon of ordered large pore structure was synthesized via a nanocasting process using SBA-15 as a template,furfuryl alcohol(FA)as a carbon precursor.It was denoted as CMK-5,22,23employed as the catalyst support.The catalyst was obtained via the chemical reduction method by NaBH4.40 mg of CMK-5 was impregnated with 0.038 mol·L-1H2PtCl6in the mixture of water and isopropanol.Then the suspension was constantly stirred to obtain a homogenously dispersed solution,adjusting pH to 9 with NaOH,and subsequently the temperature was increased to 60°C.Afterwards,excessive 0.1 mol·L-1NaBH4solution(31.8 mg NaBH4added into 80 mL of 2 g·L-1NaOH solution)were added dropwise into the suspension under vigorous stirring,followed by 3 h of continuous stirring for the complete reduction of Pt(and Ni).Finally,the resulting material was washed with distilled water several times and dried in a vacuum oven at 80°C,labeled as Pt/CMK-5.The mixture of 0.038 mol·L-1H2PtCl6and 0.01 mol·L-1Ni(NO3)2solution with Pt-Ni atomic ratios of respective 1:1,3:1,5:1,7:1 was used as the Pt-Ni alloy catalyst precursor solution,the following experimental steps were the same as above.And the final samples were signified as Pt-Ni/CMK-5(1:1),Pt-Ni/CMK-5(3:1),Pt-Ni/CMK-5(5:1),and Pt-Ni/CMK-5(7:1),respectively.The metal loading(mass fraction)of all catalysts was 20%.
2.2 Characterization
The porous structure of the carbon support was measured by N2adsorption isotherm using Micromeritics ASAP 2010 at 77 K.X-ray diffraction(XRD)patterns of the catalysts were recorded by a Bruker D8 ADVANCE diffractometer using Cu Kαradiation(λ=0.154056 nm).Transmission electron microscopy(TEM,FEI Tecnai G2)operating at 200 kV was applied to characterize the morphology and the particle size distribution of all catalysts.The samples for TEM measurement were prepared by ultrasonically suspending the powder in ethanol and placing a drop of the suspension on a carbon film supported by Cu grids.X-ray photoelectron spectroscopy(XPS)analysis was carried out on an ESCALAB 250(Thermo Electron Co.,America)spectrometer with monochromatic Al Kαradiation(150 W,15 kV).The compositions of the samples were analyzed by inductively coupled plasma atomic emission spectroscopy(ICP-AES,Jarrell-Ash 1100).
An electrochemical interface(Solartron 1287)and a conventional three-electrode system were employed to conduct the cyclic voltammetry of catalysts in 0.5 mol·L-1H2SO4and 1 mol·L-1H2SO4+2 mol·L-1CH3OH solutions.The working electrode was prepared as follows:5 mg of the catalyst was mixed with 1 mL of ethanol and 50 μL of 5%(mass fraction)Nafion solution(Du Pont).The mixture was sonicated for 30 min to obtain inky slurry.Approximately 25 μL of the slurry was applied onto the surface of the glassy carbon electrode to form a thin layer of ca 0.1256 cm2in geometrical area.A saturated calomel electrode(SCE)and a platinum foil were used as the referenceelectrode and the counter electrode,respectively.The cyclic voltammograms were collected between-0.22 and 0.98 V in H2SO4system(or between 0 and 1 V in methanol system)versusSCE with a scan rate of 20 mV·s−1at room temperature.From the cyclic voltammetry curve,we can calculate the electrochemical active surface area(EASA)of Pt,which are based on Eq.(1).25,26

where,QHis the total charge of hydrogen atoms electro-absorpted on the Pt surface,mPtis the mass of Pt andQHrefis assumed to be 0.21 mC·cm-2corresponding to a Pt surface density of 1.3×1015cm-2.
3 Results and discussion
3.1 Structural analysis
Wide-angle XRD,presented in Fig.1,is utilized to characterize the crystalline structure of the catalysts.The wide peak observed at about 24°is associated with C(002)-plane diffraction.27Four diffraction peaks observed at 2θof 39°,46°,67°,and 81°are indexed to(111),(200),(220),and(311)reflections,suggesting the face-centered-cubic(fcc)structure for Pt.Furthermore,compared with pure Pt supported catalyst,there emerges a slight shift of Pt(111)-plane peak toward the higher diffraction angle in Pt-Ni alloy catalysts,indicative of the alloy formation between Pt and Ni.28As can be noted from the diffractograms,no characteristic lines of Ni fcc structure are observed.The absence of lines corresponding to metallic Ni fcc structure(along with Pt lattice)may be due to the metallic grains that are intermixed with amorphous Ni oxides such as NiO,Ni(OH)2,and NiOOH.17
According to the wide-angle XRD patterns,Table 1 lists the corresponding parameters,including the displacement angle of Pt(111)-plane peak(DA),the mean particle size(D),and the lattice constant values(afcc),wherein,Dis evaluated by the parameters of the Pt(220)peak according to Scherrer′s equation,andafccis calculated on the assumption that the alloy particles are completely homogeneously-dispersed.29,30In Table 1,as the content of Ni in binary catalysts increases,the crystalline structure of Pt changes,showing that the adding of a foreign metal influences the crystalline structure.31It was noted that with the proportion of Ni in the Pt-Ni alloys decreasing,all diffraction peaks were shifted synchronously to lower 2θvalues.The shift is an indication of the reduction in lattice constant.According to Vegard′s law,lattice constant can be used to measure the extent of alloying.afccfor Pt-Ni/CMK-5 presents a decrease monotonically with the Ni content.The reduction ofafccin Pt-Ni/CMK-5 arose primarily from the substitution of platinum at-oms by Ni atoms,which led to the contraction of the fcc lattice,an indication of the formation of Pt-Ni alloys.17

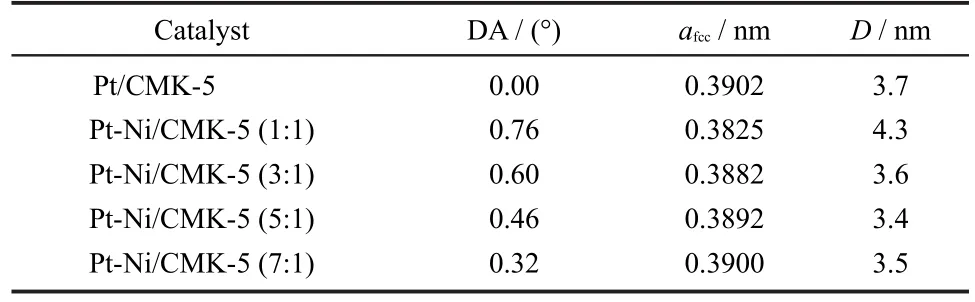
Table 1 Lattice parameters,particle sizes of catalysts calculated based upon XRD patterns
X-ray photoelectron spectroscopy(XPS)analysis is performed to investigate the oxidation states of Pt and Ni.As shown in Fig.2(a),there emerges a doublet at 71.2 eV/74.6 eV indicative of metallic Pt.In Fig.2(b),Pt 4fregion of the spectrum can be deconvoluted into three pairs of doublets,which are signature of Pt(0),Pt(II)and Pt(IV),respectively.The Ni 2p3/2spectrum shows a corresponding complex structure and different nickel species,including Ni,NiO,Ni(OH)2,and NiOOH with the binding energies located at 852.6,853.78,855.5 and 857.3 eV,respectively.28Furthermore,the relative quantitative analysis can be measured by the integrated intensities of the deconvoluted XPS signals.As shown in Table 2,the Pt-Ni alloy presents a much enhanced enrichment of metallic Pt on the surface as compared with pure Pt catalyst,probably because of the electron transfer from a lower electronegativity of Ni(1.19)to a higher electronegativity of Pt(2.28),which is consistent with the abundant amorphous Ni oxides detectable in Fig.2(c).32
The micrometric morphology of supported catalysts is generally characterized by the TEM images.In Fig.3(b),the Pt-Ni catalyst with the atomic ratio of 5:1 is small-sized and uniformly anchored onto CMK-5.Comparatively,in Fig.3(c),pure Pt catalyst presents a slight agglomeration,with some relatively large-sized nanoparticles in several regions of carbon support.Besides,as for Pt-Ni(1:1)catalyst,there appears large-area agglomeration phenomenon for alloy nanoparticles,showing the most severe agglomeration among such three alloy nanoparticles.It is known that,given a similar size,the metal having a lower sublimation tends to surface segregate in binary alloys.The heats of vaporization of Pt and Ni are 509.6 and 370.3 kJ·mol-1,respectively.28Therefore,Ni is enriched on the surface,resulting in the most severe alloy catalyst segregation among such three catalysts.Conclusively,appropriate Ni in Pt-Ni alloy catalyst facilitates dispersing nanoparticles on the support.

Table 2 Valance states,binding energy(EB),and atomic ratios(AR)of integrated intensity of pure Pt in Pt/CMK-5,as well as Pt-Ni and Ni in Pt-Ni/CMK-5(5:1)

3.2 Electro-catalytic performances
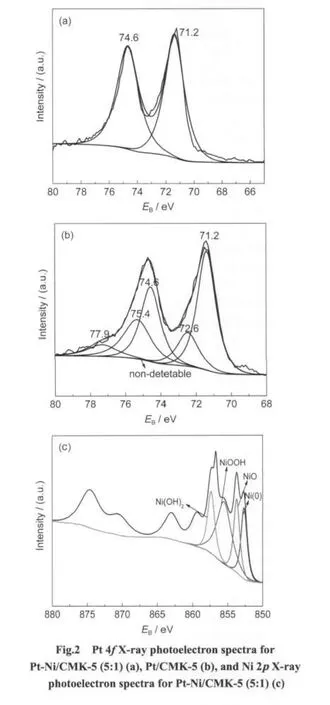
To evaluate the electro-catalytic properties of supported Pt,CV curves are generally referred to as a means of electrocatalytic characterization.25,26The CV curves for different catalysts in 0.5 mol·L-1H2SO4solution are shown in Fig.4.The reversible hydrogen adsorption/desorption and preoxidation/reduction doublet peaks of Pt are clearly seen for all catalysts except for Pt-Ni/CMK-5(1:1),suggesting that excessive alloy metal is unfavorable to the formation of uniformly-dispersed catalyst particles,and thus resulting in the relative poor electro-catalytic property.
The electrochemical active surface area(EASA)of metal nanoparticles is one most important parameter in the evaluation of hydrogen electro-oxidation properties.25,26As listed in Table 3,among the as-prepared catalysts,the EASA of Pt-Ni/CMK-5(5:1)reaches a peak value of 63.9 m2·g−1,higher than that reported in literatures(56 m2·g-1).27Compared with Pt/CMK-5,the adding of appropriate Ni can significantly increase the EASA.

Table 3 Electrochemical active surface area of different catalysts
Methanol electro-oxidation of all catalysts is showed in Fig.5.The Pt-Ni/CMK-5(5:1)catalyst exhibits better performance than Pt/CMK-5.As the generally accepted interpretation of bifunctional mechanism explained,33metallic Pt facilitates the adsorption/dissociation process of methanol anchored on the surface of Pt.More oxidative Ni can remove the intermediary products derived from the oxidation of methanol,and release more active sites provided by metallic Pt,28as is confirmed by the above XPS analysis.Moreover,the enhanced activity of Pt-Ni/CMK-5(5:1)catalyst can be attributed to optimized electronic properties in Pt 4fwhen it is alloyed with Ni.Electron transfer from Ni to Pt can be explained by the electronegativities of Ni(1.91)and Pt(2.28).The shift indelectron density from Ni to Pt would be expected to lower the density of states(DOS)at the Fermi level and to reduce the bond energy of Pt and CO as a byproduct of methanol electrooxidation.It has already been pointed out that Ni(hydro)oxides on the Pt/Ni nanoparticles could promote methanol oxidationviaa surface redox process.These two contributions to enhancing methanol electrooxidation would exist in the Pt/Ni based electrodes.34
The ratio of the forward anodic peak current(If)to the backward anodic peak current(Ib)is commonly used to determine the tolerance of catalysts to carbonaceous species accumulation.35Ordinarily,a higherIf/Ibvalue implies more tolerant toward CO-poisoning.In our experiments,the ratio(listed in Table 3)was estimated to be higher for bimetallic catalyst(except Pt-Ni/CMK-5(1:1))than the pure Pt catalyst.A highIf/Ibindicates that most of the intermediate carbonaceous species were oxidized to CO2in the forward scan,further suggesting that the presence of Ni oxides(detectable in XPS)in the catalyst provides an oxygen source for CO oxidation at lower potential.9,18Therefore,Pt-Ni alloy catalyst exhibits an improved resistance to CO poisoning.TheIf/Ibvalue of Pt-Ni/CMK-5(1:1)catalyst is lowest,probably due to the poorly-dispersed Pt nanoparticles.

4 Conclusions
In this paper,pure Pt and Pt-Ni alloy catalysts are supported on CMK-5 by chemical reduction method.Based on XRD and XPS results,it is hypothesized that Ni is present in an oxide/hydroxid amorphous form,as confirmed by the XPS.The physical characterization shows that Pt-Ni with the atomic ratio of 5:1 possesses the best dispersity,and provides far more metallic Pt.Due to the favorable structural property,Pt-Ni/CMK-5(5:1)offers the best electro-chemical performance amongst all the as-prepared catalysts.Conclusively,the research work of doping Ni into the lattice of Pt,undoubtedly,is meaningful in solving the problems encountered by fuel cells.
(1) Liu,X.;Chen,J.;Liu,G.;Zhang,L.;Zhang,H.M.;Yi,B.L.J.Power Sources2010,195,4098.
(2)Li,W.Z.;Zhou,W.J.;Li,H.Q.;Zhou,Z.H.;Zhou,B.;Sun,G.Q.;Xin,Q.Electrochim.Acta2004,49,1045.
(3)Yang,C.W.;Wang,D.L.;Hu,X.G.;Dai,C.S.;Liang,Z.J.Alloy.Compd.2008,448,109.
(4) Wang,X.M.;Li,N.;Pfefferle,L.D.;Haller,G.L.J.Phys.Chem.,C2010,114,16996.
(5)Tang,H.;Chen,J.H.;Nie,L.H.;Liu,D.Y.;Deng,W.;Kuang,Y.F.;Yao,S.Z.J.Colloid Interface Sci.2004,269,26.
(6) Steigerwalt,E.S.;Deluga,G.A.;Lukehart,C.M.J.Nanosci.Nanotechnol.2003,3,247.
(7)Yen,C.H.;Shimizu,K.;Lin,Y.Y.;Bailey,F.;Cheng,I.F.;Wai,C.M.Energy Fuels2007,21,2268.
(8)Shimazaki,Y.;Hayasaka,S.;Koyama,T.;Nagao,D.;Kobayashi,Y.;Konno,M.J.Colloid Interface Sci.2010,350,580.
(9) Zhao,Y.;E,Y.F.;Fan,L.Z.;Qiu,Y.F.;Yang,S.H.Electrochim.Acta2007,52,5873.
(10) Do,J.S.;Chen,Y.T.;Lee,M.H.J.Power Sources2007,172,623.
(11) Choi,J.H.;Park,K.W.;Kwon,B.K.;Sung,Y.E.J.Electrochem.Soc.2003,150,773.
(12) Liu,F.;Lee,J.Y.;Zhou,W.J.J.Phys.Chem.B2004,108,17959.
(13) Jeon,T.Y.;Yoo,S.J.;Cho,Y.H.;Lee,K.S.;Kang,S.H.;Sung,Y.E.J.Phys.Chem.C2009,113,19732.
(14)Jiang,S.J.;Ma,Y.W.;Tao,H.S.;Jian,G.Q.;Wang,X.Z.;Fan,Y.N.;Zhu,J.M.;Hu,Z.J.Nanosci.Nanotechnol.2010,10,3895.
(15)Yano,H.;Kataoka,M.;Yamashita,H.;Uchida,H.;Watanabe,M.Langmuir2007,23,6438.
(16) He,C.Z.;Kunz,H.R.;Fenton,J.M.J.Electrochem.Soc.2003,150,A1071.
(17)Mathiyarasu,J.;Remona,A.M.;Mani,A.;Phani,K.L.N.;Yegnaraman,V.J.Solid State Electrochem.2004,8,968.
(18)Liu,Z.L.;Ling,X.Y.;Su,X.D.;Lee,J.Y.J.Phys.Chem.B 2004,108,8234.
(19) Wang,Z.B.;Yin,G.P.;Shi,P.F.J.Electrochem.Soc.2005,153,A2406.
(20) Park,K.W.;Choi,J.H.;Ahn,K.S.;Sung,Y.E.J.Phys.Chem.B 2004,108,5989.
(21) Sun,D.;He,J.P.;Zhou,J.H.;Wang,T.;Di,Z.Y.;Ding,X.C.Acta Phys.-Chim.Sin.2010,26,1219.[孙 盾,何建平,周建华,王 涛,狄志勇,丁晓春.物理化学学报,2010,26,1219.]
(22)Lu,A.H.;Li,W.C.;Schmidt,W.G.;Schuth,F.Microporous Mesoporous Mat.2005,80,117.
(23) Antolini,E.;Salgado,J.R.C.;Gonzalez,E.R.J.Electroanal.Chem.2005,580,145.
(24)Zhou,J.H.;He,J.P.;Dang,W.J.;Zhao,G.W.;Zhang,C.X.;Mei,T.Q.Electrochem.Solid-State Lett.2007,10,B191.
(25) Pozio,A.;Francesco,D.M.;Cemmi,A.J.Power Sources 2002,105,13.
(26)Yang,R.Z.;Liu,X.P.;Zhang,H.R.Carbon 2005,43,11.
(27)Zhou,J.H.;He,J.P.;Dang,W.J.;Zhao,G.W.;Zhang,C.X.Electrochem.Solid-State Lett.2007,10,B191.
(28)Park,K.W.;Choi,J.H.;Kwon,B.K.;Lee,S.A.;Sung,Y.E.J.Phys.Chem.B 2002,106,1869.
(29) Gojkovic,S.L.;Vidakovic,T.R.;Durovic,D.R.Electrochim.Acta 2003,48,3607.
(30) Radmilovic,V.;Gasteiger,H.A.;Ross,P.N.J.Catal.1995,154,98.
(31)Geng,D.S.;Lu,G.X.J.Phys.Chem.C 2007,111,11897.
(32) Liu,F.;Lee,J.Y.;Zhou,W.J.Small 2006,2,121.
(33)Watanabe,M.;Uchida,M.;Motoo,S.J.Electroanal.Chem.1987,229,395.
(34) Park,K.W.;Choi,J.H.;Sung,Y.E.J.Phys.Chem.B 2003,107,5851.
(35)Lin,Y.;Cui,X.;Yen,C.;Wai,C.M.J.Phys.Chem.B 2005,109,14410.
CMK-5负载Pt-Ni合金催化剂及其甲醇电化学氧化性能
丁晓春 陈 秀 周建华 王 涛 孙 盾 何建平*
(南京航空航天大学材料科学与技术学院,南京210016)
采用NaBH4还原法将不同原子比的铂镍负载于CMK-5(由SBA-15模板所得的碳载体)表面.X射线衍射(XRD)和X射线光电子能谱(XPS)测试结果表明,所得催化剂是以铂镍合金的形式存在,相对于Pt/CMK-5而言,这种合金化的催化剂中Pt表现出更多的金属态.电化学测试结果显示,在催化剂中主要以化合态存在的镍(包括NiO、Ni(OH)2和NiOOH)可能更有利于甲醇的吸附和氧化产物从催化剂表面的脱附.另外,从循环伏安测试结果可知,Pt-Ni/CMK-5(5:1)(原子比)具有较大的比表面活性,其电化学活性面积高达63.9 m2·g-1,且与Pt/CMK-5相比抗CO中毒能力有明显改善.
CMK-5;Pt/CMK-5催化剂;Pt-Ni/CMK-5催化剂; 甲醇; 电化学氧化
O646
Received:October 27,2010;Revised:January 10,2011;Published on Web:February 16,2011.
∗Corresponding author.Email:jianph@nuaa.edu.cn;Tel:+86-25-52112900;Fax:+86-25-52112626.The project was supported by the National Natural Science Foundation of China(50871053).
国家自然科学基金(50871053)资助项目
- 物理化学学报的其它文章
- Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Electronic Structures and Optical Properties of Ilmenite-Type Hexagonal ZnTiO3
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
- Controlled Synthesis of Mesoporous MnO2Nanospindles

