Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
ZHANG Qi-Bo HUA Yi-Xin
(Key Laboratory of Ionic Liquids Metallurgy,Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,P.R.China)
Effect of the Ionic Liquid Additive-[BMIM]HSO4on the Kinetics of Oxygen Evolution during Zinc Electrowinning
ZHANG Qi-Bo*HUA Yi-Xin
(Key Laboratory of Ionic Liquids Metallurgy,Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,P.R.China)
Abstract:The effect of the ionic liquid additive 1-butyl-3-methylimidazolium hydrogen sulfate([BMIM]HSO4)on the kinetics of oxygen evolution during zinc electrowinning from an acidic sulfate solution was investigated.We used potentiodynamic polarization,electrochemical impedance spectroscopy,scanning electron microscopy,and X-ray diffraction for this study.Potentiodynamic polarization curves and the corresponding kinetic parameter analysis show that[BMIM]HSO4has a catalytic effect on oxygen evolution by stimulating the reaction rate constant.Impedance data reveal that[BMIM]HSO4can markedly reduce the oxygen evolution charge transfer resistance.The addition of 5 mg·L-1[BMIM]HSO4obviously decreased the resistance value by at least 50%over the studied potential range from 1.85 to 2.10 V.In addition,the results of the impedance measurements also suggest an inhibition effect of[BMIM]HSO4on the secondary reactions and this is due to the adsorption of the additive on the anode surface,which decreased the amount of active sites for anion adsorption.All electrochemical results were corroborated with a morphological and orientation analysis of the anodic surface after 120 h of anodic polarization.The addition of[BMIM]HSO4inhibited the generation of the intermediate productβ-PbO2and it promoted the generation of larger,loose,and porousα-PbO2,which benefited the oxygen evolution reaction.
Key Words:Electrochemical impedance spectroscopy; Zinc electrowinning; Oxygen evolution;Ionic liquid additive; Surface morphology
Lead and its alloy have been wildly used as permanent anodes for electrowinning and plating of zinc due to their high corrosion-resistance property in the sulfuric acid used in the electrolytic solutions[1].Pure lead is a weak material and it tends to creep and warp during use.In practice,lead alloys are usually used instead of pure lead,since alloying with some amounts of certain metals can significantly improve its corrosion resistance,mechanical characteristics and also castability[1-2].Pb-Ag alloy,typically containing 0.25%to 1.0%(w,mass fraction)silver is the main anode material in the production of anodes for hydrometallurgical electrowinning of zinc[3-6].Silver addition is found to improve the lead corrosion resistance,longer anode life,lower anode potential,and consequently,reduce oxygen overpotential[1,3,7].However,due to the high cost of silver,many attempts have been made to find material which by forming of binary[8-9],ternary[9-13],or quaternary[2,14]lead alloys,would be able to substitute or decrease its quantity in the anodes.
During the deposition process,oxygen will release at the anode surface as a gas and then oxidize materials in the electrolyte,corrode the anode,or form peroxides at the anode surface.The oxygen corrodes the anode directly by the formation of PbO,PbSO4and then PbO2with different crystalline phases(α and β)on the surface[15-19].The morphology of the PbO2layer plays an important role in inhibiting the spalling and corrosion of anodes which leads to cathode contamination.Alloy additions and anode fabrication techniques can promote a more continuous,adherent PbO2layer,which serves to evolve oxygen as a gas instead of corroding the underlying lead surface.For hydrometallurgical zinc electrowinning,the energy consumption saving improvement is bound up with a reduction in the anodic voltage(oxygen evolution voltage),which represents 50%-60%of cell voltage.An effective way to decrease the oxygen evolution overvoltage and reduce solution ohmic resistance is the use of additives(anodic depolarizers).As is well known,additives are widely used in zinc electrodeposition due to their special functions in deposition process.These additives are found to affect both the deposition and crystal-building processes through their adsorbates at the electrode surface[20].Appropriate addition is necessary for the formation of finegrained,smooth,compact deposits[21].However,most of the literature focuses on the effects of additives on the current efficiency and polarization behavior as well as their effects on deposit morphology and crystallographic orientations of the cathode during the zinc electrodeposition process;scant information is available on the effects of additives on the oxygen evolution on the anode during the deposition process.Afifi et al.[22]studied the effect of some organic agents on the zinc electrodeposition from zinc sulfate acid solution.The results showed that organic substances,such as ethanol,ethylene glycol,or acetic acid,were effective in decreasing the overall cell voltage.Similarly,Chapman and Yen[23]reported that the addition of 0.1 mol·L-1ethylene glycol can reduce the cell voltage by over 5%.In our previous report[24-25],[BMIM]HSO4was found to be an excellent levelling agent in both zinc and copper electrodeposition.The addition of[BMIM]HSO4is observed to increase current efficiency and reduce specific electric energy consumption for zinc electrodeposition and slightly change the reduction mechanism for copper electrodeposition.It is demonstrated that this additive has a pronounced inhibiting effect on both Zn2+and Cu2+electroreduction and leads to more leveled and fine-grained cathodic deposits.
The present study aims at elucidating the effect of[BMIM]HSO4on the kinetics of the oxygen evolution on the anode during zinc electrodeposition from acidic sulfate solution.To this purpose,various experimental methods,such as potentiodynamic polarization,electrochemical impedance spectroscopy(EIS),scanning electron microscopy(SEM),and X-ray diffraction(XRD)have been used.
1 Experimental
1.1 Reagents
The solution of zinc electrolyte consisted of 55 g·L-1zinc and 150 g·L-1sulfuric acid was prepared by ZnSO4·7H2O and H2SO4and the specific experimental procedures were similar to as described previously[24].The ionic liquid additive[BMIM]HSO4was synthesized by our laboratory and the specific synthetic methods were mentioned elsewhere[26].All the reagents used wereAR grade.
1.2 Electrolysis
Small-scale galvanostatic electrolysis was performed in a rectangular flow cell with dimension of 20 cm×10 cm×8 cm made of plexiglass by chronopotentiometric measurements.The flow rate of the electrolyte was maintained at 1.2 dm3·h-1during the deposition process.A pure vertical planar aluminum(>99.95%)sheet and two parallel lead-silver alloy(Ag,0.2%)plates of 12 cm2as effective area were used as the cathode and anode,respectively.The interelectrode distance was 3.0 cm.Zinc was deposited on both sides of the cathode onto a total area of 10 cm2.All electrolysis experiments were run in a constant temperature bath at(40±1)°C.In all cases,the current density was held constant at 400 A·m-2during the deposition time of 120 h with the cathode aluminum sheet changing at 24 h interval.For each experiment,a freshly prepared solution was used and the solution temperature was thermostatically controlled at a desired value.
1.3 Electrochemical measurements
Electrochemical studies were based on the analysis of poten-tiodynamic polarization and electrochemical impedance spectroscopy.All the electrochemical measurements were measured by using an electrochemical work station(GAMRY USA,PCl4/300)and carried out at 40°C under atmospheric condition.A conventional three-electrode electrochemical cell was used for these experiments.A lead-silver alloy(Ag,0.2%)disk electrode(Ф4 mm,99.995%)inserted in a Teflon tube with exposed surface of 0.1256 cm2was used as working electrode.A graphite rod and a saturated calomel electrode(SCE)mounted inside a Luggin capillary were used as the counter and reference electrodes,respectively.Prior to each experiment,the electrolyte was deoxygenated by bubbling ultrapure argon for at least 10 min.The working electrode was grinded with 1200 grit silicon carbide paper and polished using 0.5 μm high-purity alumina,and then degreased with anhydrous alcohol in an ultrasonic bath for 1 min,washed with doubly distilled water and finally dried.
Potentiodynamic polarization measurements were scanned at a constant scan rate of 5 mV·s-1from the initial potential of 1.80 V to the final potential of 2.15 V.In the case of EIS measurements,the working electrode potential was held at a series of constant potentials chosen from the activation controlled region over a frequency range of 100 kHz-6 mHz with a signal amplitude perturbation of 5 mV.
1.4 Deposit examination
After electrolysis,the anode was removed from the cell and washed thoroughly with distilled water and dried.A microscope(Tescan Czech,VEGA II XMH)and an X-ray diffractometer(Rigaku Japan,D/max 2200)were used to examine the surface morphology and the orientation of the anodic layers,respectively.
2 Results and discussion
2.1 Polarization studies
The anodic potentiodynamic polarization curves obtained on a clean Pb-Ag anode in the absence and presence of ionic liquid additive[BMIM]HSO4are presented in Fig.1.A significant decrease in anodic polarization is observed with the addition of[BMIM]HSO4in the electrolyte and such an effect is more pronounced with increasing[BMIM]HSO4concentrations.Since the main reaction occurred on the Pb-Ag anode is that of oxygen evolution taking place on a PbO2layer in the case without the presence of Mn2+in the electrolyte,therefore,this depolarizing effect could be attributed to the increase in the rate of oxygen evolution[27-28].However,further work needs to be done on aged industrial anodes having a significant layer of MnO2to establish whether the depolarizing effect is still observed.
The kinetic parameters Tafel slope,a(mV·decade-1),transfer coefficient,αa,and anodic exchange current density,i0,a(mA·cm-2),for the anodic reaction were calculated from their respective polarization curves.The results are given in Table 1 for unstirred electrolyte which is mixed by the gas evolution.The marked decrease in Tafel slopes with the addition of[BMIM]HSO4indicates that the oxygen evolution reaction is affected strongly by the presence of[BMIM]HSO4in the solution,and this effect becomes more pronounced at higher[BMIM]HSO4concentrations.This is confirmed by the significant increase of the transfer coefficient and exchange current density when[BMIM]HSO4is present in the solution.This change is probably ascribed to the catalytic effect of[BMIM]HSO4on the oxygen evolution by stimulating the reaction rate constant of oxygen evolution on the PbO2layer.

2.2 Impedance measurement
Electrochemical impedance measurement was used to investigate the electrochemical reaction occurred on the anode in the absence and presence of[BMIM]HSO4during the zinc electrodeposition process.The electrochemical impedance has been analyzed at different potential domains(the low and high polarization domains,respectively),where different electrode reactions occurred.
In Fig.2,the impedance plots obtained at the low potential domain(E=1.85 V)on the polarization curves of Fig.1 at points A(without additive)and C(with addition of 5 mg·L-1[BMIM]HSO4)give a high-frequency capacitive loop,corresponding to the charge transfer resistance of the formation of the PbSO4layer on the anode surface.The current for the lead oxidation(generating PbSO4)remains very low(blow 0.5 mA·cm-2)in comparison with the total current.Therefore,it can hardly be detected in the polarization curves(Fig.1).The charge transfer resistance is estimated from the diameter of the high-frequency capacitive loop and the corresponding values obtained at various potential are presented in Table 2.As can be observed from Fig.2,the resistance value is found to decrease in the presence of[BMIM]HSO4,indicating that the ad-ditive can promote the electrode reaction.The similar profile of the complex-plane impedance spectra observed in the absence and presence of additive at points A and C reveals that the electrode reaction mechanism does not change with the addition of[BMIM]HSO4.This promotion effect of additive may be due to these high conductive additive molecules adsorbed on electrode surface,which improves the conductivity of the PbO sublayer and thus promotes the growth of the PbSO4.

Table 1 Effects of[BMIM]HSO4on the kinetic parameters of oxygen evolution reaction
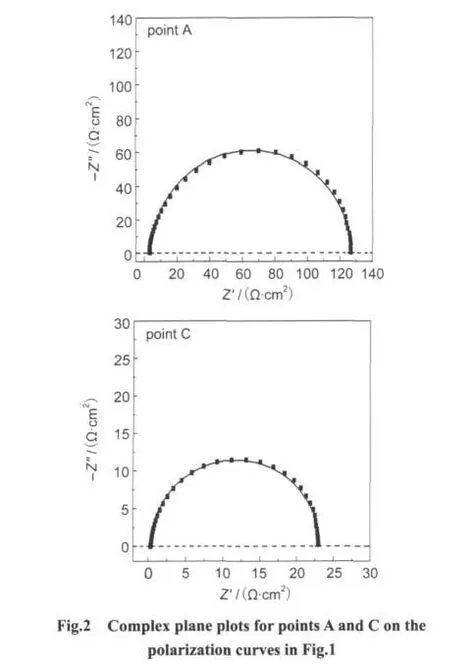

Table 2 Variation of the resistance with the anodic polarization of Pb-Ag electrode

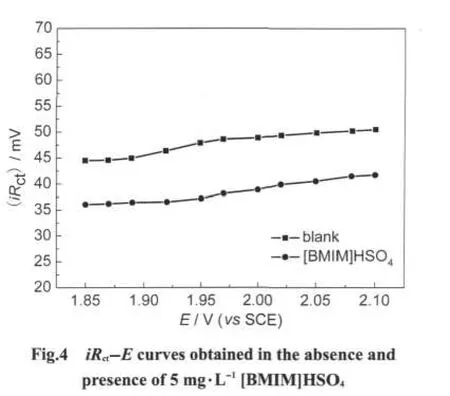
Fig.3 shows the impedance plots obtained at high potential domain(E=2.08 V)for the additive-free electrolyte at point A'and for the electrolytes containing 1,5,10 mg·L-1[BMIM]HSO4at points B',C',D',respectively.As can be seen from Fig.3,for the additive-free electrolyte,the impedance plot exhibits two capacitive features at high frequencies followed by an inductive loop at low frequency values(Fig.3(point A')).The high frequency capacitive loop is usually related to thefrom 4.2 to 0.66 Ω·cm2in comparison to additive-free solution(Table 2),suggesting that the electrode reaction is more ready to occur.These results confirm the catalytic effect of[BMIM]HSO4on the oxygen evolution at electrode surface obtained by anodic polarization studies.In addition,it appears that the iRctproduct can be regarded as potential independent,as shown in Fig.4.As compared to the pure electrolyte,the iRctvalues decrease with the addition of[BMIM]HSO4,which also proves the catalytic effect of the additive on the charge transfer process.charge transfer across the double layer for oxygen evolution which is the main reaction.The second capacitive feature observed at medium frequencies is attributed to the relaxation of the electrode coverage by an absorbed silver salt from a secondary reaction[29]and reflects an inhibition of the main reaction by some adsorbed species resulting from secondary reactions taking place on the alloy electrode surface[30-31].The low frequency inductive loop could be interpreted in terms of relaxations of a slow electrode activation with increasing polarization,which is probably connected to an increase in the PbOnlayer conductivity[32-33].In contrast,for the[BMIM]HSO4containing electrolytes,the medium frequency capacitive loop becomes weak,suggesting an inhibition effect of additive on secondary reactions.In addition,this effect becomes more pronounced at higher additive concentrations.In the case of 10 mg·L-1[BMIM]HSO4containing electrolyte,the medium frequency capacitive loop nearly disappears.This inhibition effect may be explained as follows:there is a general agreement in the literature[28,30-31]that electrolyte anions,such as SO42-ions,can adsorb on the β phase and induce an inhibition of oxygen evolution.Additionally,the absorbed silver salt on the electrode surface is found to facilitate the anions adsorption.With the addition of[BMIM]HSO4,the imidazolium compound molecules can be adsorbed on the active sites of anode surface and compete with silver salt that will reduce the active sites for anion adsorption and thus block secondary reactions from taking place.
Similar to the case of low polarization domains,the charge transfer resistance,Rct,decreases in the presence of[BMIM]HSO4.With the addition of 5 mg·L-1[BMIM]HSO4at the high potential of 2.08 V,the charge transfer resistance decreases
2.3 Morphology and orientation studies
The morphologies of anodic layers after 120 h anodic polarization obtained by small-scale electrolysis from the electrolyte in the absence and presence of 5 mg·L-1[BMIM]HSO4were characterized using scanning electron microscope.Typical SEM photomicrographs are shown in Fig.5.
As it can be seen from Fig.5(A-C),the investigated additiveshows a marked effect on the surface quality of the anodic layers as compared with those obtained from electrolyte without additive(Fig.5(a-c)),which is porous with relatively large and coarse grains.In the presence of the investigated additive,the oxide layers obtained is more levelling and compact.In both cases,the anodic layers consist of α-PbO2and β-PbO2two crystallites:both oxide layers are tetragonal texture with bigger crystallites α-PbO2surrounding by smaller crystallites β-PbO2.However,with the addition of 5 mg·L-1[BMIM]HSO4the relative content of β-PbO2is found to decrease,which is more evidence by comparing with their backscattering electron images(Fig.5(c,C)).The result indicates an inhibition of the generation of β-PbO2.It is demonstrated that the presence of[BMIM]HSO4in the electrolyte generates two opposite effects:a catalytic effect on polarization curves due to a stimulated rate constant of oxygen evolution on the anodic oxide layer(PbO2),and a transient inhibition of the generation of β-PbO2.These two opposite effects become more pronounced with increasing additive concentration.Since the generation of β-PbO2is inhibited,a relative enhancement of the generation of α-PbO2is induced.In addition,the decrease in the relative content of β phase in the anodic oxide layers leads to a reduction in the active sites for anion adsorption that induce an inhibition of oxygen evolution.This results in a promotion effect on the oxygen evolution at electrode surface.
The influence of[BMIM]HSO4on the crystallographic structure of the anodic layers was examined by XRD and the diffraction patterns for selected deposits are shown in Fig.6.These patterns indicate that the oxide layers mainly contain α-PbO2and β-PbO2crystallites without preferred crystallographic texture.The primary change in the diffraction patterns with the addition of[BMIM]HSO4is the weakness of the β-PbO2corresponding diffraction peak intensity,which indicates a decrease in the relative content of β-PbO2in the anodic oxide layers.This result is in agreement with the anodic layer morphology observed as discussion above.

3 Conclusions
The effect of ionic liquid additive-[BMIM]HSO4on the kinetics of oxygen evolution during zinc electrowinning from acidic sulfate solution has been studied.In the cases of[BMIM]HSO4containing electrolytes,a stimulation of oxygen evolution is observed.This catalytic effect essentially results from the stimulation of the reaction rate constant for oxygen evolution.The impedance data reveal a transient inhibition of[BMIM]HSO4on the secondary reactions apparently under the form of a medium-frequency capacitive feature which becomes more obvious with increasing additive concentrations.It could be concluded that this inhibition is ascribed to the adsorption of the additive on the active sites of anode surface in competition with the silver-salt adsorbate.The morphology of anodic layers and their orientation analysis exhibit an inhibition effect of[BMIM]HSO4on the generation of β-PbO2which provides active sites for the adsorption of these anions.These phenomena consequently appear that the determining effect of the additive lies in the reduction in the active sites for anion adsorption and creates more active sites able to stimulate the reaction rate constant of oxygen evolution.
1 Petrova,M.;Noncheva,Z.;Dobrev,T.;Rashkov,S.;Kounchev,N.;Petrov,D.;Vlaev,S.;Mihnev,V.;Zarev,S.;Georgieva,L.;Buttinelli,D.Hydrometallurgy,1996,40:293
2 Ivanov,I.;Stefanov,Y.;Noncheva,Z.;Petrova,M.;Dobrev,T.;Mirkova,L.;Vermeersch,R.;Demaerel,J.P.Hydrometallurgy,2000,57:109
3 Felder,A.;Prengaman,R.D.Journal of the Minerals,Metals and Material Society,2006,58:28
4 Jiang,L.X.;Zhong,S.P.;Lai,Y.Q.;Lü,X.J.;Hong,B.;Peng,H.J.;Zhou,X.Y.;Li,J.;Liu,Y.X.Acta Phys.-Chim.Sin.,2010,26:2369 [蒋良兴,衷水平,赖延清,吕晓军,洪 波,彭红建,周向阳,李 劼,刘业翔.物理化学学报,2010,26:2369]
5 Lai,Y.Q.;Jiang,L.X.;Li,J.;Zhong,S.P.;Lü,X.J.;Peng,H.J.;Liu,Y.X.Hydrometallurgy,2010,102:73
6 Lai,Y.Q.;Jiang,L.X.;Li,J.;Zhong,S.P.;Lü,X.J.;Peng,H.J.;Liu,Y.X.Hydrometallurgy,2010,102:81
7 Pavlov,D.;Rogachev,T.Electrochim.Acta,1986,31:241
8 Rashkov,S.;Dobrev,T.;Noncheva,Z.;Stefanov,Y.;Rashkova,B.;Petrova,M.Hydrometallurgy,1999,52:223
9 Lupi,C.;Pilone,D.Hydrometallurgy,1997,44:347
10 Siegmund,A.;Prengaman,R.D.;Dutrizac,J.E.Lead-Zinc 2000//Dutrizac,J.E.Warrendale.PA:TMS,2000:589-597
11 Newnham,R.H.J.Appl.Electrochem.,1992,22:116
12 Zhong,S.P.;Lai,Y.Q.;Jiang,L.X.;Tian,Z.L.;Li,J.;Liu,Y.X.Chin.J.Process Eng.,2008,8:289 [衷水平,赖延清,蒋良兴,田忠良,李 劼,刘业翔.过程工程学报,2008,8:289]
13 Lai,Y.Q.;Zhong,S.P.;Jiang,L.X.;Lü,X.J.;Chen,P.R.;Li,J.;Liu,Y.X.J.Cent.South Univ.Tech.,2009,16:236
14 Petrova,M.;Stefanov,Y.;Noncheva,Z.;Dobrev,T.;Rashkov,S.British Corrosion Journal,1999,34:198
15 Pavlov,D.Electrochim.Acta,1978,23:845
16 Pavlov,D.;Dinev,Z.J.Electrochem.Soc.,1980,127:855
17 Yamamoto,Y.;Fumino,K.;Ueda,T.;Nambu,M.Electrochim.Acta,1992,37:199
18 Ruetchi,P.;Cahan,B.D.J.Electrochem.Soc.,1957,104:406
19 Burbank,J.J.Electrochem.Soc.,1959,106:369
20 Paunovic,M.;Schlesinger,M.Fundamental of electrochemical deposition.2nd ed.New York:John Willey&Sons Inc.Publication,2006:177-198
21 Saba,A.E.;Elsherief,A.E.Hydrometallurgy,2000,54:91
22 Afifi,S.E.;Ebraid,A.R.Journal of the Minerals,1992,1:32
23 Chapman,T.W.;Yen,S.C.Anode depolarisation in electrowinning.AIME Meeting,Las Vegas,Nov.,1980
24 Zhang,Q.B.;Hua,Y.X.J.Appl.Electrochem.,2009,39:261
25 Zhang,Q.B.;Hua,Y.X.;Wang,Y.T.;Lu,H.J.;Zhang,X.Y.Hydrometallurgy,2009,98:291
26 Whitehead,J.A.;Lawrance,G.A.;McCluskey,A.Aust.J.Chem.,2004,57:151
27 Rerolle,C.;Wiart,R.Electrochim.Acta,1995,40:939
28 Cachet,C.;Rerolle,C.;Wiart,R.Electrochim.Acta,1996,41:83
29 Cachet,C.;Pape-Rerolle,C.L.E.;Wiart,R.J.Appl.Electrochem.,1999,29:813
30 Berube,L.P.;Piron,D.J.Electrochem.Soc.,1987,134:562
31 Katz,E.R.;Stucki,S.J.Electroanal.Chem.,1987,228:407
32 Ruetschi,P.;Cahan,B.D.J.Electrochem.Soc.,1958,105:369
33 Lappe,F.J.Phys.Chem.Solids,1962,23:1563
离子液体添加剂[BMIM]HSO4对锌电积过程析氧反应动力学的影响
张启波*华一新
(离子液体冶金重点实验室,昆明理工大学冶金与能源工程学院,昆明650093)
研究了1-丁基-3-甲基咪唑硫酸氢盐([BMIM]HSO4)离子液体对锌电积过程析氧反应的影响.研究工作借助于动电位极化,电化学阻抗谱,扫描电镜和X射线衍射等测试技术.动电位极化曲线及对应的动力学参数分析表明,[BMIM]HSO4对阳极析氧反应具有催化作用,可提高析氧反应速率常数.电化学阻抗谱数据表明,[BMIM]HSO4能显著降低阳极析氧电荷传递电阻,在所研究的1.85-2.10 V电位范围内添加5 mg·L-1[BMIM]HSO4,电阻值至少降低50%.此外,添加剂对阳极表面二次反应具有抑制作用,其在阳极表面的吸附,阻碍了阴离子的阳极活化位点吸附过程.电化学测试结果与长时间(120 h)阳极极化后所得阳极表面形貌和结晶取向分析结果相一致.[BMIM]HSO4的添加能有效抑制中间产物β-PbO2的形成,促进铅银电极表面大块且疏松多孔α-PbO2的生成,加速阳极析氧的进行.
电化学阻抗谱;锌电积;析氧;离子液体添加剂;表面形貌
O646
Received:September 30,2010;Revised:November 16,2010;Published on Web:December 8,2010.
∗Corresponding author.Email:qibozhang@yahoo.com.cn;Tel:+86-871-5162008.
The project was supported by the National Natural Science Foundation of China(50864009,50904031)and Research Fund for the Doctoral Program of Higher Education of China(20070674001).
国家自然科学基金(50864009,50904031)及高等学校博士学科点专项科研基金(20070674001)资助项目
- 物理化学学报的其它文章
- Molecular Dynamics Simulations ofα-Tocopherol in Model Biomembranes
- Effects of Substrate-Target Distance and Si Co-Doping on the Properties of Al-Doped ZnO Films Deposited by Magnetron Sputtering
- Cathodic and Thermal Stabilities of the P(VdF-HFP)-Based Ionic Liquid Composite Polymer Electrolyte
- 表面活性剂对驱油聚合物界面剪切流变性质的影响
- Catalytic Decomposition of Cellulose in Cooperative Ionic Liquids
- Pt-Ni Catalyst Supported on CMK-5 for the Electrochemical Oxidation of Methanol

