Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first-episode schizophrenia over 12 months of treatment with risperidone or quetiapine
Jianjun LIU, Jushui SUN*, Xinghua SHEN, Weigang GUO, Shengli ZHI, Guangming SONG, Qiuxia XU,Juanfen SONG
•Original article•
Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first-episode schizophrenia over 12 months of treatment with risperidone or quetiapine
Jianjun LIU1, Jushui SUN1*, Xinghua SHEN1, Weigang GUO1, Shengli ZHI1, Guangming SONG1, Qiuxia XU1,Juanfen SONG2
schizophrenia, weight gain, first-episode, risperidone, quetiapine, long-term follow-up, China
1. Introduction
Most patients with schizophrenia need long-term treatment with antipsychotic medication. However,weight gain and hyperprolactinemia are common side effects of many modern antipsychotics and are common complaints among patients with schizophrenia treated with antipsychotics.[1]Short-term studies have shown that weight gain and hyperprolactinemia are more prominent among female patients of reproductive age.[2,3]There have been few long-term studies on this topic.Both risperidone and quetiapine are two commonly used antipsychotics to treat schizophrenia that have different pharmacological mechanisms of action.The current study compares the long-term effects of risperidone and quetiapine on serum prolactin and on weight among female patients with schizophrenia. We hypothesize that there are differences in the effects of risperidone and quetiapine on serum prolactin and on weight among reproductive-aged female patients with first-episode schizophrenia.
2. Methods
2.1 Sample
All female patients with first-episode schizophrenia who were admitted to the Third People’s Hospital of Huzhou from 1 January to 31 December, 2011 were potential subjects for the study (Figure 1). Enrolled patients met the following inclusion criteria: (a) diagnostic criteria for schizophrenia as specified in the Chinese Classification of Mental Disorders-3rdedition (CCMD-3)[4]; (b) female; (c)18 to 44 years of age; (d) a score of greater than 60 on the Positive and Negative Syndrome Scale (PANSS)[5]; (e)regular menstrual periods; (f) a body mass index (BMI)greater than 19; and (g) the patient or the patient’s legal guardian signed informed consent for the study. Patients with any of the following conditions were excluded: (a)a serious physical illness (e.g., cardiovascular diseases,hepatic diseases, renal diseases, or endocrine system or nervous system diseases); (b) substance dependence;(c) a history of allergies; (d) abnormal results in routineadmission laboratory tests; or (e) currently pregnant or planning to get pregnant. Participants were dropped from the study during follow-up if any of the following occurred: (a) pregnancy; (b) serious physical illness; (c)severe adverse reactions to the medications; (d) use of adjunctive antipsychotics or conversion to other antipsychotic medication; or (e) treating clinician decided to remove the patient from the study because of clinical changes in the patient.
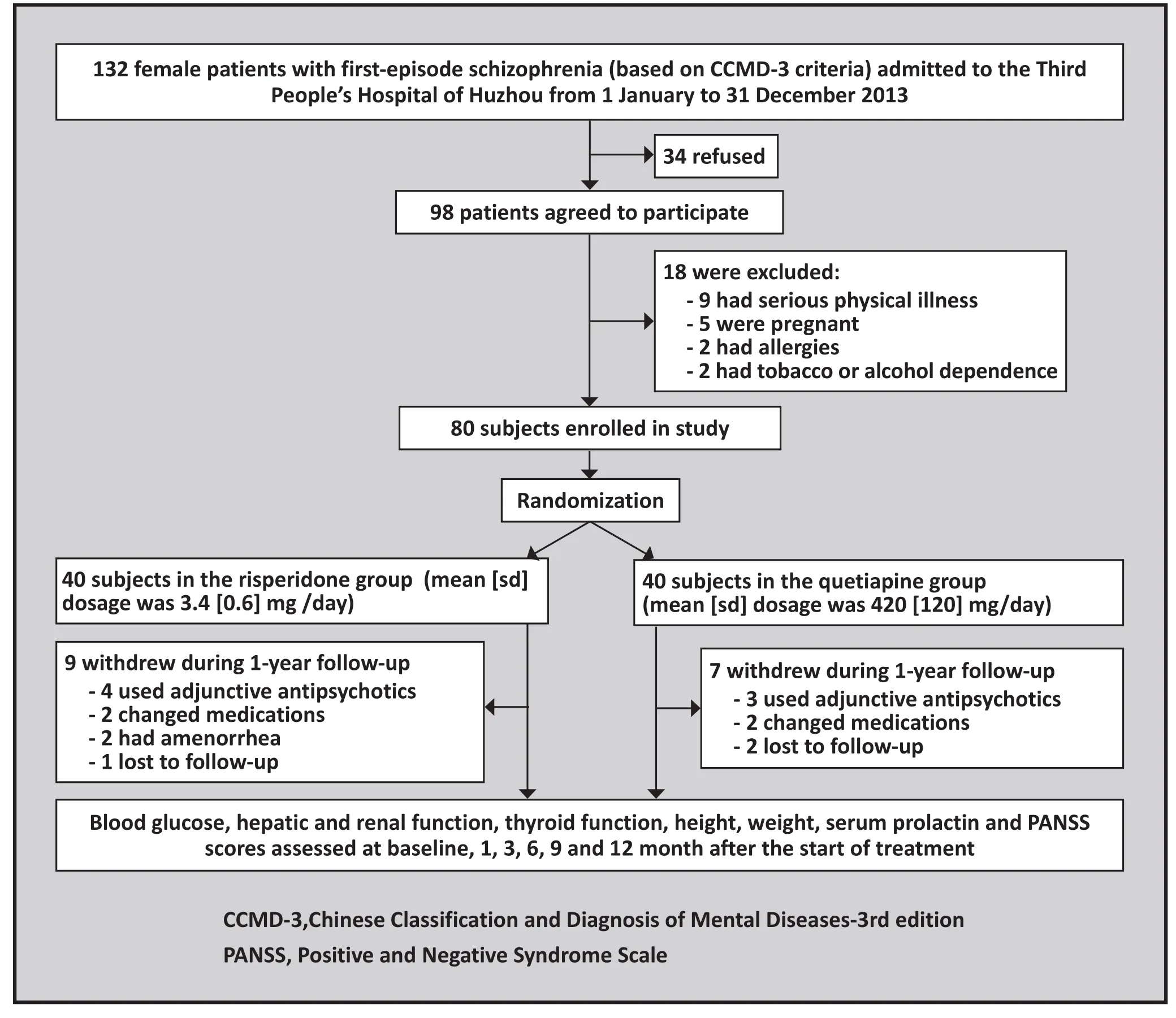
Figure 1. Flow chart of the study
A total of 80 patients were enrolled; 40 were randomized (using a random number table) to the risperidone group and 40 to the quetiapine group.Individuals in the risperidone group had a mean (sd)age of 29 (9) years (range=18 to 42 years), their mean duration of illness was 4.5(2) months (range=1 to 9 months), 10 of them had a family history of mental disorders, and at baseline their mean PANSS score was 80.4 (10.2), their mean serum prolactin concentration was 22.6 (4.2) ng/ml, and their mean weight was 59.0(5.9) kg. The 40 patients in the quetiapine group had a mean age of 30 (8) years (range=19 to 44 years), a mean duration of illness of 5.5 (3) months (range=1 to 11 months), 8 of them had a family history of mental disorders, and at baseline their mean PANSS score was 82.6 (11.7), their mean serum prolactin concentration was 23.6 (3.7) ng/ml and their mean weight was 60.2 (6.6) kg. There were no statistically significant differences in baseline demographic characteristics,prolactin level, weights or PANSS scores between the two groups.
The study was approved by the ethics committee of the Third People’s Hospital of Huzhou.
2.2 Procedure
This is an open-label randomized controlled trial.Risperidone (produced by Xian-Janssen Pharmaceutical Ltd; trade name: Risperdal; 1 mg/ tablet) was given at a starting dosage of 1 mg/day and increased to full dosage within two weeks (exact dosages varied). After symptoms were controlled, maintenance dosage, which was half to two-thirds of the full dosage, was used until the end of the 12-month follow-up. The mean (sd)dosage was 3.4 (0.6) mg/day during the 12 months.Quetiapine (produced by Astra Zeneca Limited; trade name: Seroquel; 25 mg/ tablet or 200 mg/ tablet) was given at a starting dosage of 100 mg/day and increased to full dosage within 2 weeks. After symptoms were controlled, maintenance dosage, which was half to two thirds of the full dosage, was used until the end of the 12-month follow-up. The mean (sd) dosage was 420(120) mg/day during the 12 months. The only other medications allowed during the course of the study were benzodiazepines, propranolol hydrochloride and benzhexol hydrochloride tablets.
There were two phases of the treatment: the first phase was hospital-based inpatient treatment which lasted for an average of three months; the second phase was home-based outpatient treatment during which patients came to the outpatient clinic for follow-up visits every three months. As shown in Figure 1, 16 of the 80 enrolled patients (20%) withdrew during the one-year follow-up, 9 from the risperidone group (4 required additional antipsychotic medications, 2 changed medications, 2 had amenorrhea, and 1 was lost to follow-up) and 7 from the quetiapine group (3 required additional antipsychotic medications, 2 changed medications, and 2 were lost to follow-up). The number of individuals who completed assessments at baseline,1 month, 3 months, 6 months, 9 months and 12 months in the risperidone group were 40, 40, 35, 33, 31, and 31,respectively; the corresponding number of individuals who completed assessments in the quetiapine group were 40, 40, 35, 34, 33, and 33, respectively. There were no statistically significant differences in the baseline demographic characteristics, prolactin concentration,weights or PANSS scores between individuals who did and did not complete the 12-month follow-up.
2.3 Assessments
Three psychiatrists previously trained in the use of the PANSS (who were not blind to the treatment group of the subjects) assessed the severity of patients’symptoms with the PANSS at baseline and at 1 month,3 months, 6 months, 9 months and 12 months after enrollment. Previous studies have shown that the Chinese version of the PANSS has good inter-rater reliability (ICC=0.83-0.87).[5]
Fasting blood samples were collected from enrolled patients at 07:00 at baseline and at each of the five follow-up time points to assess serum prolactin level,blood glucose, hepatic and renal function and thyroid function. Serum prolactin was assessed using the ACCESS automated chemoluminescenceimmunoassay system (manufactured by the Beckman Coulter Co.,Ltd, United States) and matched kits with inter-assay coefficient of variation of 5% and intra-assay coefficient of variation of 8%. The assessments were conducted strictly in accordance with the manufacturer’s instructions. A serum prolactin concentration of >25ng/ml was considered hyperprolactinemia.[6]
Height was measured at enrollment and weight was assessed at baseline and at each follow-up assessment.
2.4 Statistical analysis
Analysis was conducted using SPSS13.0 software.Missing data were replaced using the last observation carried forward (LOCF) method. Differences between groups, across time and the interaction between the two were analyzed using repeated measures analysis of variance. When an overall statistically significant difference was found, pairwise comparisons were conducted using Bonferroni method to correct for typeierror inflation. The incidence of hyperprolactinemia after treatment between the two groups was compared using Fisher’s exact tests. The correlation between changes in prolactin concentration and changes in weight (combining 170 pairs of changes in the risperidone group [baseline to 1 month, 1 month to 3 months, 3 months to 6 months, 6 months to 9 months and 9 months to 12 months] and 175 pairs of change in the quetiapine group) was assessed using Spearman rank correlation coefficients. All statistical tests were two-tailed and 0.05 was set as the level of statistical significance.
3. Results
3.1 Prolactin concentration
Repeated measures analysis of variance showed a statistically significant difference in the serum prolactin concentrations between the risperidone group and the quetiapine group (F=79.42, p<0.001); serum prolactin concentrations were significantly different across time(F=14.09, p<0.001) and the time by treatment group interaction term was also significantly different (F=12.81,p<0.001). As shown in Table 1, in the risperidone group serum prolactin at each time point was significantly higher compared to baseline by a factor of 3.5-fold to 5.2-fold; the level peaked at the end of the 3rdmonth of treatment, decreased by the 6thmonth of treatment(though still 3-fold higher than baseline) and then remained stable for the last 6 months of treatment.In the quetiapine group, no statistically significant differences were found in serum prolactin levels over time. Throughout the 12 months of treatment serum prolactin levels were significantly higher in the risperidone group than in the quetiapine group.
3.2 Comparison of PANSS scores
Repeated measures analysis of variance showed that the total PANSS scores were significantly different between the two groups (F=24.93, p<0.001); there was a statistically significant difference across time (F=176.63,p<0.001) and a significant time by treatment group interaction (F=18.34, p<0.001). Post-hoc comparisons revealed that in both groups the PANSS score at each follow-up point was significantly lower than the baseline score (p<0.001); moreover, at the end of the third and sixth month, the PANSS scores of the risperidone group were significantly lower than those of the quetiapine group (third month: F=34.82, p<0.001; six month:F=2.99, p<0.046).
3.3 Comparison of BMI
Repeated measures analysis of variance showed no statistically significant difference in BMI between the two groups (F=0.93, p=0.339) after controlling for time. Compared to baseline, BMI measurements were significantly different across time (F=93.05,p<0.001) after controlling for treatment assignment.There was no interaction between the treatment group and time effects (F=0.173, p=0.984). Post-hoc comparisons using Bonferroni corrections showed that compared to baseline BMI, the BMI was higher in both the risperidone and quetiapine groups at all of the follow-up time points (all p<0.001). There were no statistically significant differences in BMI between the two treatment groups at any of the follow-up time points. In both groups 62% of the 1-year increase in BMI had already occurred by the end of the 3rdmonth of treatment.

Table 1. Comparison of mean (sd) serum prolactin, scores on the Positive and Negative Syndrome Scale(PANSS) and body mass index (BMI) between females with first-episode schizophrenia treated with one year of risperidone (n=40) or quetiapine (n=40) using the last observation carried forward (LOCF)method to deal with dropouts during the follow-up

Table 2. Comparison of the prevalence of hyperprolactinemia among females with first-episode schizophrenia treated with risperidone or quetiapine for one year
3.4 Comparison of the prevalence of hyperprolactinemia
As shown in Table 2, the prevalence of hyperprolactinemia, defined as a serum prolactin concentration of >25ng/ml, was greater in the risperidone group than in the quetiapine group at all follow-up time points. By the end of 12 months of treatment 62.5%(25/40) of the patients in the risperidone group met criteria of hyperprolactinemia while only 2.5% (1/40)of the patients in the quetiapine group met criteria of hyperprolactinemia.
3.5 Correlation between weight and the prolactin concentration
The mean weight of patients in the risperidone group increased from 59.0 (5.9) kg to 63.9 (7.8) kg over the year of treatment (a 8.3% increase in weight) while that of patients in the quetiapine group increased from 60.2 (6.6) kg to 64.4 (7.5) kg (a 7.0% increase in weight).We assessed the correlation of changes in weight with changes in prolactin level combining the results for the five time periods considered in the study (baseline-1 month, 1-3 months, 3-6 months, 6-9 months and 9-12 months) using Spearman rank correlation coefficients and found a weak, non-significant positive relationship:in the risperidone group there were 170 pairs of change values (rs=0.17, p=0.104), in the quetiapine group there were 175 pairs of change values (rs=0.07, p=0.862).
4. Discussion
4.1 Main findings
We found that one year of treatment with standard doses of risperidone and quetiapine in unselected first episode female patients with schizophrenia had similar treatment effects -- though the maximum improvement was achieved more quickly with risperidone than with quetiapine. The effect of both medications on weight gain was similar but the prevalence of hyperprolactinemia was much higher in patients treated with risperidone than in those treated with quetiapine.
The different effects of risperidone and quetiapine on serum prolactin concentration may be attributed to their different pharmacological mechanisms: quetiapine has a higher binding affinity to 5-HT receptors while risperidone has a higher binding affinity to D2.[7,8]The difference in the prevalence of hyperprolactinemia has potential long-term consequences. Chronichyperprolactinemia may cause menstrual irregularity (two patients in the risperidone group dropped out due to amenorrhea) and it can affect calcium metabolism which can potentially lead to osteoporosis.[9,10]
Antipsychotics-induced weight gain is mainly due to the inhibition of the 5-HT2A, 5-HT2C, H1 and M3 receptors.[10]The effects of different antipsychotics on weight are different: most studies[11,12]found the largest weight gain among patients treated with clozapine or olanzapine and the smallest weight gain among patients taking ziprasidone or aripiprazole; weight gain among patients treated with risperidone and quetiapine is intermediate. Previous studies reported that the effects of risperidone and quetiapine on weight were different,[13,14]but our study found no difference in the changes in BMI over one year of treatment with these two medications. We also found that most of the weight gain occurs in the initial three to six months of treatment; a finding that is in line with the findings of the study by Neovius and colleagues.[15]
Oberweis and colleagues[16]postulated that the inhibition of dopamine receptors by antipsychotic medications leads both to weight gain and to increased serum prolactin concentration. In vitro experiments showing that hyperprolactinemia reduces insulin sensibility of adipose cells supports this hypothesis about the relationship of insulin resistance and hyperprolactinemia to weight gain. However, our study did not find any clear correlation between changes in weight and changes in prolactin levels.
4.2 Limitations
The 20% dropout rate (16/80) over the one-year treatment study is lower than that reported in similar studies[17]but this could, nevertheless, potentially affect our results. There were, however, similar numbers of subjects who dropped out from the two treatment groups and there were no statistically significant differences in the baseline characteristics of those who did and did not complete the study. Moreover, we used the LOCF methodology in our analysis to ensure that all enrolled patients were represented in the data at all follow-up time points. We conclude that the risk of substantial bias in our results is small.
Serum prolactin concentrations are assumed to be normally distributed in the general population but the distribution of values in the sample was not normally distributed. This may have skewed the results of the repeated measures analysis of variance used to compare prolactin levels between the two groups over time. However, when we dichotomized results (which eliminates the problem of a non-normal distribution of results) into those who do and do not met standard criteria for hyperprolactinemia the results were similar to the findings for the repeated measures analysis of variance.
The study used first-episode female patients so these results may not be relevant for male patients, for older patients or for patients with a longer treatment history.
4.3 Implications
This randomized controlled trial used young, firstepisode female patients so the results are not confounded by prior treatment history or by the effects of menopause on prolactin. There was no difference in the pattern of weight gain between risperidone and quetiapine over the first year of treatment; in both groups the majority of the weight gain occurred early in treatment so this is clearly the time when the greatest effort is needed to help patients change their diet and physical activity to combat these weight increases.In this study risperidone achieved its maximum effectiveness more rapidly than quetiapine but the level of improvement achieved by the two medications was similar by nine months of treatment; further study with unselected first-episode patients is needed to confirm this difference but it may be important in patients for who more rapid improvement will help sustain their willingness to continue taking antipsychotic medication.The much higher prevalence of hyperprolactinemia in the risperidone group compared to the quetiapine group (62.5% v. 2.5% at the end of 1 year of treatment)is concerning; long-term studies are needed to determine whether or not the prolactin levels in women treated with risperidone remain high and to evaluate potential negative consequences on menstruation and bone metabolism.
Conflict of interest
The authors report no conflict of interest related to this article.
Funding
This study was funded by the Huzhou Ministry of Technology.
1. Freudenreich O, McEvoy JP. Optimizing outcome with antipsychotic treatment in first-episode schizophrenia:balancing efficacy and side effects. Clin Schizophr Relat Psychoses. 2012;6(3): 115-121. doi: http://dx.doi.org/10.3371/CSRP.6.3.3
2. Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. 2009;35(5): 937-948.doi: http://dx.doi.org/10.1093/schbul/sbn023
3. Citrome L, Stauffer VL, Chen L, Kinon BJ, Kurtz DL, Jacobson JG, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia:an analysis of correlations with efficacy, weight gain,and prolactin concentration. J Clin Psychopharmacol.2009;29(3): 278-283. doi: http://dx.doi.org/10.1097/JCP.0b013e3181a289cb
4. Society of Psychiatry, Chinese Medical Association. [Chinese Classification of Mental Disorders, 3rdedition (CCMD-3)].Shandong Province: Shangdong Science and Technology Publishing House; 2001. Chinese
5. Si TM, Yang JZ, Shu L, Wang XL, Kong QM, Zhou Mo, et al. [The reliability, validity of PANSS and its implication]. Zhongguo Xin Li Wei Sheng Za Zhi. 2004;18(1): 45-47. Chinese. doi:http://dx.doi.org/10.3321/j.issn:1000-6729.2004.01.016
6. Karasek M, Pawlikowski M, Lewiński A. Hyperprolactinemia:causes, diagnosis, and treatment. 2006;57(6): 656-662
7. Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol.2008;22(2 Suppl):12-19. doi: http://dx.doi.org/10.1177/0269216307087148
8. Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychoticinduced weight gain: insights into mechanisms of action.CNS Drugs.2011;25(12):1035-1059. doi: http://dx.doi.org/10.2165/11596300-000000000-00000
9. Renn JH, Yang NP, Chueh CM. Bone mass in schizophrenia and normal populations across different decades of life.BMC Musculoskelet Disord. 2009;10: 1
10. Misra M, Papakostas GI, Klibanski A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J Clin Psychiatry. 2004;65(12): 1607-1618.doi: http://dx.doi.org/10.4088/JCP.v65n1205
11. Heal DJ, Gosden J, Jackson HC, Cheetham SC,Smith SL.Metabolic consequences of antipsychotic therapy: preclinical and clinical perspectives on diabetes, diabetic ketoacidosis,and obesity. Handb Exp Pharmacol. 2012;212: 135-164
12. Wang LJ, Ree SC, Huang YS, Hsiao CC,Chen CK. Adjunctive effects of aripiprazole on metabolic profiles: comparison of patients treated with olanzapine to patients treated with other atypical antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40: 260-266.doi: http://dx.doi.org/10.1016/j.pnpbp.2012.10.010
13. Choong E, Bondolfi G, Etter M, Jermann F, Aubry JM,Bartolomei J,et al. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46(4): 540-548. doi: http://dx.doi.org/10.1016/j.jpsychires.2012.01.014
14. Lee SY, Park MH, Patkar AA, Pae CU. A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2): 490-496. doi: http://dx.doi.org/10.1016/j.pnpbp.2010.12.003
15. Neovius M, Eberhard J, Lindström E, Levander S. Weight development in patients treated with risperidone: a 5-year naturalistic study. Acta Psychiatr Scand. 2007;115(4): 277-278
16. Oberweis B, Gragnoli C. Potential role of prolactin in antipsychotic-mediated association of schizophrenia and type 2 diabetes. J Cell Physiol. 2012;227(8): 3001-3006. doi:http://dx.doi.org/10.1002/jcp.24023
17. Fang MS, Zhao JP, Guo XF, Zhai JG, Lu Z, Sun XL, et al.[Maintenance treatment effectiveness of seven antipsychotic drugs in schizophrenic patients: a 1-year follow-up study].Zhong Hua Jing Shen Ke Za Zhi. 2009;42: 1-6. doi: http://dx.doi.org/10.3760/cma.j.issn.1006-7884.2009.01.001
2013-07-09; accepted: 2013-11-18)

Jianjun Liu graduated from Anhui Medical University with a major in Clinical Medicine in 1994 and worked as a psychiatrist at Suzhou Psychiatric Hospital, Anhui Province. He obtained his master’s degree in Psychiatry from Luzhou Medical College (Sichuan Province) in July 2009 and has since worked at the Department of Psychiatry of the Third Hospital of Huzhou. He is an associate chief psychiatrist in the Department of Psychiatry and his main research interests are pharmacology and the clinical study of schizophrenia.
利培酮或喹硫平治疗女性首发精神分裂症患者12个月中血清催乳素及体重变化的随机对照研究
刘建君,孙菊水,沈鑫华,郭纬刚,支胜利,宋光明,许秋霞,宋娟芬
精神分裂症,体重增加,首发,利培酮,喹硫平,长期随访,中国
Background:Increased serum prolactin and weight gain are common side effects of atypical antipsychotics but few studies have assessed the long-term pattern of these adverse effects.Aim:Compare the effects of risperidone and quetiapine on serum prolactin and weight over 12 months of treatment among female patients with first-episode schizophrenia.Methods:Eighty female inpatients with first-episode schizophrenia were randomly assigned to receive risperidone (n=40) or quetiapine (n=40) for 12 months. Prolactin concentration, weight and height were measured one day before starting treatment and 1, 3, 6, 9 and 12 months after initiating treatment. Severity of symptoms was assessed at the same time periods using the Positive and Negative Syndrome Scale(PANSS).Results:Thirty-one patients in the risperidone group and 33 patients in the quetiapine group completed the 12 months of treatment. PANSS scores decreased at each follow-up assessment for both groups; the improvement was significantly greater in the risperidone group after 3 months and 6 months of treatment but by the 9th month of treatment the level of improvement in the two groups was similar. In the quetiapine group serum prolactin remained stable throughout the 12 months but in the risperidone group the serum prolactin level increased 3.5- to 5.2- fold over the one-year follow-up. Weight gain was seen in both groups,particularly during the first 3 months of treatment: 62% of the increase in BMI in both groups had occurred by the end of the 3rd month of treatment. No between-group differences in weight changes were observed.The correlation between changes in weight and changes in prolactin levels were weakly positive: rs=0.17(p=0.104) in the risperidone group and r=0.07 (p=0.862) in the quetiapine group.Conclusions:Risperidone and quetiapine had similar efficacy in the first year of treatment of first-episode schizophrenia though risperidone was more rapidly effective. Use of risperidone was associated with chronic hyperprolactinemia but this did not occur with quetiapine. Long-term use of both drugs was associated with sustained weight gain; the timing and magnitude of the weight gain is similar for the two drugs. Weight gain was not strongly related to changes in prolactin levels.
http://dx.doi.org/10.3969/j.issn.1002-0829.2014.02.005
1Psychiatry Department, Third People’s Hospital of Huzhou, Huzhou City, Zhejiang Province, China2Laboratory Department, Third People’s Hospital of Huzhou, Huzhou City, Zhejiang Province, China*correspondence: さp@163.com
A full-text Chinese translation will be available at www.saponline.org on May 15, 2014.
背景:血清催乳素升高和体重增加是非典型抗精神病药物常见的副作用,但很少有研究评估这些副作用的长期情况。目标:比较利培酮或喹硫平治疗女性首发精神分裂症患者12个月后对血清催乳素及体重变化的影响。方法:80例女性首发精神分裂症住院患者被随机分配接受利培酮(N=40)或喹硫平(N=40)为期12个月的治疗。在开始治疗前一天和开始治疗后第1,3,6,9和12个月分别进行催乳素浓度,体重和身高的测定。在每一个时间段同时采用阳性和阴性症状量表(PANSS)评估症状的严重程度。结果:利培酮组31例患者和喹硫平组33例患者完成12个月的治疗。两组PANSS评分在每次后续的评估中都有所下降;利培酮组在治疗3个月和6个月时的改善显著,但在治疗第9个月两组的水平改善相似。喹硫平组血清催乳素在12个月内保持稳定,但利培酮组的血清催乳素水平在一年的随访中升高3.5至5.2倍。两组均出现体重增加,特别是在治疗的头3个月:两组的体重指数在治疗3个月时增加了62%。体重变化组间没有显著差异。体重变化和催乳激素水平变化之间的相关性呈弱阳性:利培酮组rs=0.17(p=0.104),喹硫平组r=0.07(p=0.862)。结论:虽然利培酮更迅速有效,但利培酮和喹硫平在治疗首发精神分裂症的第一年有相似的疗效。慢性高泌乳素血症与利培酮使用有关,但喹硫平没有。长期服用这两种药物与体重持续增加有关;两种药物在体重增加的时间性和幅度上相似。体重增加和催乳素水平变化不是密切相关的。
- 上海精神医学的其它文章
- The dopamine system and alcohol dependence
- Suicide in India: a systematic review
- Effectiveness of self-management training in community residents with chronic schizophrenia: a single-blind randomized controlled trial in Shanghai, China
- Retrospective comparison of cognitive behavioral therapy and symptom-specific medication to treat anxiety and depression in throat cancer patients after laryngectomy
- Providing free treatment for severe mental disorders in China
- Case report on lithium intoxication with normal lithium blood levels

