Hepatoprotective mechanism of lycorine against carbon tetrachloride induced toxicity in swiss albino mice - A proteomic approach
Soundarrajan Ilavenil, Dhanaraj Karthik, Mariadhas Valan Arasu, Mayakrishnan Vijayakumar, Srisesharam Srigopalram, Selvaraj Arokiyaraj, Sivanesan Ravikumar*, Ki Choon Choi*
1Grassland and Forage division, National Institute of Animal Science, South Korea
2Department of Biotechnology, PRIST University, Vallam, Thanjavur, Tamilnadu, India
3Department of Botany and Microbiology, King Saud University, Saudi Arabia
4Department of Horticulture, Sunchon National University, South Korea
5Institute of Green Bio-Science and Technology, Seoul National University, South Korea
Hepatoprotective mechanism of lycorine against carbon tetrachloride induced toxicity in swiss albino mice - A proteomic approach
Soundarrajan Ilavenil1,2, Dhanaraj Karthik2, Mariadhas Valan Arasu3, Mayakrishnan Vijayakumar1, Srisesharam Srigopalram4, Selvaraj Arokiyaraj5, Sivanesan Ravikumar2*, Ki Choon Choi1*
1Grassland and Forage division, National Institute of Animal Science, South Korea
2Department of Biotechnology, PRIST University, Vallam, Thanjavur, Tamilnadu, India
3Department of Botany and Microbiology, King Saud University, Saudi Arabia
4Department of Horticulture, Sunchon National University, South Korea
5Institute of Green Bio-Science and Technology, Seoul National University, South Korea
ARTICLE INFO
Article history:
Received 12 September 2014
Received in revised form 11 February 2015
Accepted 21 March 2015
Available online 20 June 2015
Lycorine
CCl4
Oxidative stress
2D gel
MALDI-TOF-MS ATP synthase
Regucalcin
HSP 60
Objective:To investigate the differential of protein expression in CCl4induced mice treated with lycorine.Methods:The present study was carried out to identify the differentially expressed protein in carbon tetrachloride (CCl4) induced oxidative stress mice treated with lycorine (5 mg/kg. bw) using 2D gel and MALDI-TOF.Results:We observed many kinds of differentially expressed protein in experimental liver. Among these, three are much differently expressed protein which is identified as ATP synthase, regucalcin and HSP60; these proteins are involved in the ATP synthesis, calcium regulation and rescue the integrity cellular proteins respectively.
Conclusion:This investigation provided a molecular mechanism of the lycorine during CCl4induced oxidative stress in mice liver.
1. Introduction
The liver is the main metabolic organ which metabolizes the toxic substance via xenobiotic metabolism and it’s very sensitive to free radical related diseases, hepatitis, cirrhosis, liver cancer and other alcohol related disorders. A major cause of liver disorder is due to exposure of different environmental pollutants and xenobiotics such as Paracetamol, CCl4thioacetamide and alcohol, which damage the liver by producing reactive oxygen species (ROS). The ROS is highly toxic which produce the tissue injury through covalent DNA base oxidation, lipid peroxidation and protein oxidation[1]. Antioxidant is an important molecule capable for stabilizing and deactivating the free radical before they attack[2]. At low level of free radical provides beneficial effects on cellular and immune functions, however, excess of free radical generate the oxidative stress which damages the cellular and immune functions[3]. At present CCl4is an important substance used to elucidate the mechanism of hepatotoxicity effects[4]. CCl4damage the hepatocytes directly by changing the permeability of the plasma, lysosomal and mitochondrial membranes. It generates the highly reactive free radicals through the activation of oxidase reaction by CYP2E1 and leads to the severe centrilobular necrosis[5]. Generally, free radicals can extract hydrogen atoms from the membrane lipids of the liver cells and produce the lipid hydroperoxides. Antioxidants (Super oxide dismutase (SOD), Glutathione perxidase (GPx), and catalase (CAT) are the first line defense mechanism for scavenging the reactive oxygen species[6]. Imbalance between the reactive oxygen species production and antioxidant defense result in oxidativestress which deregulated the cellular function as a result in various pathological conditions like cancer etc.. Many studies have been suggested that the natural antioxidants and their mechanism against oxidative stress[7]. The differential protein expression analysis using 2 dimensional electrophoresis (2-DGE) Matrix assisted laser desorption/ ionization time of flight mass spectroscopy (MALDITOF-MS) has been used to detect the differential protein expressions for last few decades to present state[8].
Lycorine is a major alkaloid found in the leaf and root bulb of the amaryllidaceae family plants. This alkaloid has been shown to behave as potent therapeutic agent in numerous experimental models. It possesses many pharmacological activities such as hepotoprotective activity[9, 10], antioxidant[11], anti-inflammatory[12], and antibacterial and analgesics agents[13]. The present study has been designed to identify the proteomic mechanism of lycorine during CCl4induced oxidative stress in the swiss albino mice.
2. Materials and methods
2.1. Chemicals
Required chemicals for 2DE and MS were obtained from Sigma Chemicals [USA] and stored at 2-4 ℃ and protected from sunlight. All other chemicals were analytical grades and were obtained from standard commercial suppliers.
2.2. Experimental design
The Swiss albino mice were randomly divided into four groups each consist of six mice. Group I served as normal and was given olive oil daily for a period of 8 weeks. Group II served as lycorine alone [5 mg/kg]. For inducing hepatotoxicity, animals of groups III and IV were administered orally [1 mL/kg bw] with carbon tetrachloride twice a week for a period of 8 weeks. Group III served as CCl4induced group. Group IV administered orally with lycorine[9] [5 mg/kg i.p.] daily for a period of 8 weeks. At the end of the experiment, animals were sacrificed by cervical dislocation. Liver samples were dissected out and washed immediately with ice cold saline to remove the blood as possible, and immediately stored at -70 ℃ until analysis. All the animal experimentations were premeditated and executed in compliance with the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and Institutional Animal Ethics Committee Guidelines [743/03/abc/ CPCSEA dt 3.3.03].
2.3. Sample preparation
Approximately 10 mg of tissue samples were cut into small fragments and suspended in phosphate buffer saline (PBS). The suspensions were homogenized using a rotating blade homogenizer for 50 s followed by sonication for 10 times and then centrifuged at 10 000 rpm, 4 ℃ for 10min and collect the supernatant, added an equal volume of ice cold acetone with 10% TCA and incubated for overnight at -20 ℃. after that centrifuged at 10 000 rpm for 15 min at 4 ℃ and removed the supernatant and then pellet was rinsed with a fourfold volume of ice cold acetone for five times and then centrifuged. The precipitate was collected and suspended in 200 μL of lysis buffer containing 85 mM Tris [pH 6.8] and 2% SDS stored at -80 ℃ for further use The extracted protein concentrations was determined as previously reported[14].
2.4. High Resolution Two-dimensional gel electrophoresis
350 μg of extracted protein from each tissue was loaded into (160 × 3) mm diameter rod gel with a pH range between 3-10. Isoelectric focusing was conducted using a vertical gel system according to the following procedure: 15 min at 200 V, 30 min at 300 V, 16 h at 400 V. The rod gel was equilibrated with 6 M urea, 6 M urea, 75 mM Tris-HCl pH 8.8, 29.3% glycerol, 2% SDS and 0.002% bromophenol blue for 25 min with gentle shaking. For second dimensional separation, the rod gel was placed on the top of the SDS-PAGE (12%) gel and sealed with 0.5% agarose in the SDS electrophoresis buffer. Electrophoresis was performed at 10 ℃in the vertical PAGE system, according to the following program; 100 V for 30 min, then 200 V gel for 3 h until the bromophenol blue front had migrated to the end of the gel. Gels were stained with colloidal Coomassie blue for overnight. To account for experimental variation, we run and analyzed triplicate gels for each experimental group[15].
2.5. MALDI-TOF-Mass Spectroscopy
The selected protein spots were cut and washed twice with 25 mM NH4HCO3, pH 8.0 and 50% acetonitrile in 25 mM NH4HCO3, and pH 8.0. For digestion, added 5 μL trypsin solutions (10 ng/μL; sequencing grade, Promega) and incubated at 37 ℃ for 5 h. The extraction was performed in two steps by the addition of 50% acetonitrile and 1% trifluoroacetic acid and dried. Then it was dissolved in 1:1 ratio of MALDI matrix [5 mg/mL recrystallized 2-cyano-3-[4-hydroxyphenyl] acrylic acid]. In the final step before the MALDI-TOF [time-of-flight] analysis, 1 μL dissolved sample was spotted on the target slides[15].
2.6. Protein identification
Peptide mass fingerprinting [PMF] was performed by using ultraflex MALDI-TOF mass spectrometer equipped with Linear mode 25KvA, laser shots 150 [337 nm, 50 hz, N2laser [Bruker-Daltonics, Germany]. Protein identification was performed by searching for Mus musculus proteins in the latest version of the NCBI database using the mascot search engine[15]. The followingparameters were applied: monoisotopic; missed cleavages, 1; allowed variable modifications, oxidation [Met] and fixed modification, Carbamidomethyl [C] and peptide tolerance, 0.5 Da[16].
3. Results
Figure 1 show images from the IEF and SDS-PAGE which exhibited many protein spots in the experimental liver tissues. The alteration in the liver protein expressions are reproducible and excised the three reliable protein spots from experimental tissues, which are subjected into MALDI-TOF analysis after tryptic digestion. Two protein spots were excised from the lycorine treated mice and another one from the CCl4induced group of mouse liver. MALDI spectra were characterized by the presence of several mass signals originated by contaminant keratins and fragment of auto-catalyzed trypsin. The results were shown in Figure 2, 3, 4 and Table 1-4.

Table 1 Identified proteins expression in mice liver of control and experimental groups.
The peptide pattern obtained from each spot were subjected to database analysis using a mascot search engine with allowing one missed cleavage of trypsin per peptide and a mass tolerance of one Dalton. Spot 1 was identified as an ATP synthase, which was 54.62 kDa and has a pI of 5.10. Spot 2 was identified as a Regucalcin, which was 33.40 kDa and has a PI 5.15.Spot 3 was identified as HSP 60 which was 56.67 kDa and has a pI of 5.25.
The cellular locations of these proteins were determined using UniPort and these proteins were associated with many subcellular locations in the cytoplasm and nucleolus of liver cells. The identified protein such as HSP 60 was expressed in CCl4induced liver. It indicated that CCl4could be induced the oxidative stress in the liver, which stimulates the HSP 60 expression. ATP synthase and regucalcin were expressed in CCl4induced rats treated with the lycorine. This result displayed the protective nature of lycorine against CCl4induced oxidative stress in swiss albino mice.
4. Discussion
Proteomics database was used to determine the alteration of protein expression patterns in the experimental animals, which might be revealed the metabolic variations in the living system. For example, investigation of reactive oxygen species [ROS] role in the mitochondrial diseases under the normal physiological conditions, approximately 2%-4% of oxygen was consumed by the mitochondria and changed into superoxide anion instead of water due to the unproductive electron transfer within the complex I and III[17]. When the respiratory chain reaction is slow down, more electrons are diverted from their normal pathway which leads to stimulates more ROS production. This kind of situation can be found in the case of ATP synthase deficiency. The limited rate of ATP synthesis results in a high membrane potential which slow down the electron transfer along with respiratory chain[18]. The previous research revealed that the excess of free radical productions causes the mitochondrial respiratory chain reaction damage which leads to depletion of ATP synthase activity. The present study, ATP synthase expression was observed in the lycorine treatment during the CCl4induction in the mice. This condition provides some information about the CCl4and lycorine treatment, CCl4enhances the ROS production which causes the mitochondrial function and reduced ATP production. But, lycorine treatment to the CCl4treated mice may stimulate the ATP synthase which produce more ATP for energy production for neutralizing the generated free radicals or may be reduced free radical activity by the scavenging activity through the antioxidant pathway. Similarly, lycorine have potent antioxidant and hepatocytes protective nature against CCl4induced toxicity in Swiss albino mice and it also provides evidence that protective effects arepossible due to increases of free radicals scavenging activities and restored the antioxidant status in the hepatic tissues[9].

Table 2 Peptide fragment observed in the MALDI mass spectrum of ATP synthase tryptic digest and a comparison with the output of the NCBI database.

Table 3 Peptide fragment observed in the MALDI mass spectrum of Regucalcin tryptic digest and a comparison with the output of the NCBI database.
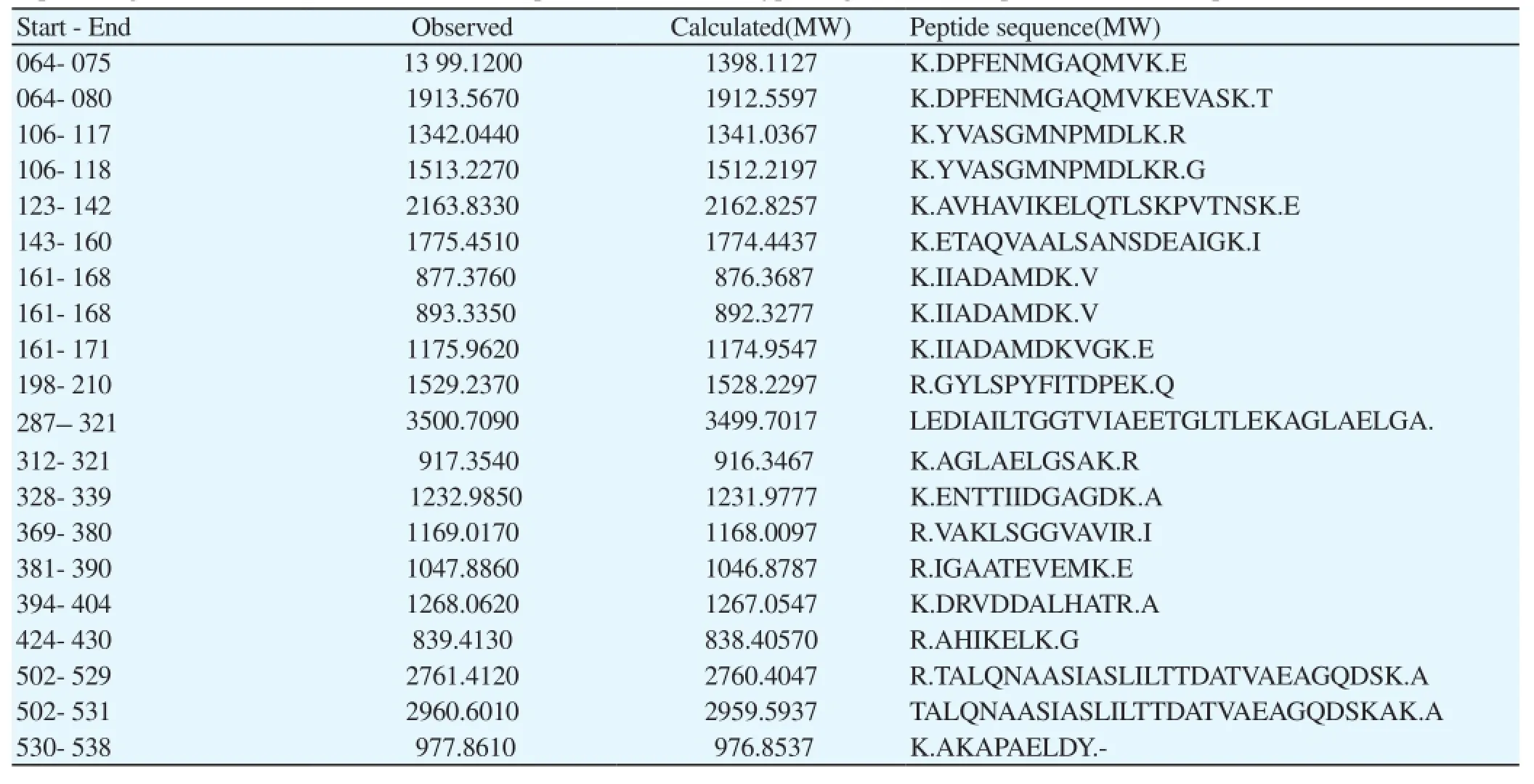
Table 4 Peptide fragment observed in the MALDI mass spectrum of HSP 60 tryptic digest and a comparison with the output of the NCBI database.
Calcium ion [Ca2+] plays a very important role in the regulation of numerous cellular functions through its Ca2+binding proteins[19]. The Ca2+effect has been modulated by the Ca2+binding proteins, regucalcin is a calcium binding protein which plays an important role in the regulation of intracellular Ca2+homeostasis and the activity of several proteins which are involved in the signaling pathways such as kinases, phosphates etc[20]. Oral administration of carbon tetrachloride has been shown to be down regulated the regucalcin mRNA expression in hepatic cells, which is known as chemical inducer of liver injury[21]. The protein and their mRNA levels of regucalcin in the liver were significantly down regulated at 6, 8 and 30 days after CCl4treatment[22]. The present study identified the regucalcin expression in the CCl4induced mice treated with lycorine. This investigation, demonstrated that the lycorine may stimulate the regucalcin expression by the scavenging CCl4produced free radicals. Many reports claimed that the regucalcin mRNA expression was down regulated by the carbon tetrachloride, Phenobarbital, galactosamine, ethanol[20] and streptozotocin-diabetic state, which cause a disorder of liver metabolism. The decrease in regucalcin leads to an acceleration of signal transduction from the cytoplasm to nucleus, and such a decrease weakens the suppressive effect of regucalcin on the over expression of liver cell proliferation and protein synthesis.
Heat shock proteins are most evolutionarily conserved proteins[23]. The amino acid sequence of the HSP60 was almost similar to its homolog in plants, bacteria and humans[24]. These proteins are primarily responsible for maintaining the integrity of cellular proteins especially, in response to environmental changes, temperature, imbalance of concentration, pH changes and other toxins can induce the heat shock proteins for maintaining the conformation of the cellular proteins. In this study, we identified the HSP-60 protein expression in CCl4treated mouse liver. This result evidenced that CCl4administration stimulates the HSP-60 expression liver of mice, because CCl4administration can be making more stress to the liver cells, therefore, HSP-60 synthesized for preventing CCl4mediated oxidative stress. The HSP-60 expression did not show the visible expression in the lycorine treated and control mouse. The up regulation of HSP60 expression allows for the maintenance of other cellular processes occurring in the cell, especially during stressful times. HSP60 constitutes approximately 15%-30% of all cellular proteins[25]. The HSP60 expression protects the cells from hydrogen peroxide or indomethacin induced cellular injury under the both necrotic and apoptotic conditions[26]. Mice treated with DOPA stimulate the HSP-60 up regulation in the mitochondria and HSP-70 in the cytoplasm. It concluded that the heat shock signal pathway serving as the basic defense mechanism for neurotoxicity induced by the oxygen and nitrogen species free radical [27].
The present study, we identified the differentially expressed proteins in the liver of experimental animals. Mass spectra analysis and database searching shows three differentially expressed proteins like ATP synthase, regucalcin and HSP60 which are associated with liver functions such as energy productions, calcium regulation and maintaining integrity of cellular proteins. These results showed that lycorine could restore the ATP synthase, regucalcin and HSP60 protein expression during the CCl4administration by its antioxidant mechanism.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Laskin JD, Black AT, Jan JH, Sinko PJ, Heindel ND, Sunil V, et al. Oxidants and antioxidants in sulfur mustard-induced injury. Ann NY Acad Sci 2010 ; 1203: 92-100.
[2] Khalid Rahman. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2007; 2: 219-236.
[3] Valko M, Leibfritz D, Moncola J, Cronin MD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Review Int J Biochem Cell Biol 2007; 39: 44-84
[4] Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 2003; 33(2): 105-36.
[5] Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2007; 25: 185-209.
[6] Arikan DC, Bakan V, Kurutas EB, Sayar H, Coskun A. Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J Pediatr Surg 2010 ; 45: 2203-2209.
[7] Brewer MS. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci F 2011; 10: 221-247.
[8] Tannu NS, Wu J, Rao VK, Gadgil HS, Pabst MJ, Gerling IC, et al. Paraffinwax-coated plates as matrix-assisted laser desorption/ionization sample support for high-throughput identification of proteins by peptide mass fingerprinting. Anal Biochem 2004; 327: 222-232
[9] Ilavenil S, Kaleeswaran B, Raviumar. S. Protective effect of Lycorine against CCl4induced hepatotoxicity in Swiss Albino mice. Fund Clin Pharmacol 2012; 26: 393-401.
[10] Ilavenil S, Kaleeswaran B, Raviumar S. Protection of human erythrocytes by using Crinum asiaticum and Lycorine against APPH induced oxidative damage induced by 2-amidinopropane. Saud J Biol Sci 2010; 18: 181- 187. [11] Ilavenil S, Kaleeswaran B, Raviumar S. Antioxidant and Hepatoprotective activity of lycorine against Carbon tetrachloride-induced oxidative stress in Swiss albino mice. Der Pharm Chem 2010; 2: 267-272.
[12] Wiart C. Medicinal plants of Southeast Asia. Kuala Lumpur: Pelanduk Publications; 2000, p. 100.
[13] Lewis JR. Amaryllidaceae alkaloids. Nat Prod Rep 1990; 7: 549-556.
[14] Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
[15] Karthik D, Ilavenil S, Kaleeshwaran B, Sunil S, Ravikumar S. Proteomic analysis of plasma proteins in diabetic rats by 2D electrophoresis and MALDI-TOF-MS. App Biochem Biotechnol 2012; 166: 1507-1519.
[16] Kathik D, Ravikumar S. Characterization of the brain proteome of rats with diabetes mellitus through two-dimensional electrophoresis and mass spectrometry. Brain Research 2011; 1371: 171-179.
[17] Beyer RE. An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem Cell Biol 1992 ; 70: 390-403.
[18] Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997; 416: 15-18.
[19] Rossi MR, Somji SH, Garrett S, Sens MA, Nath J, Sens DA. Expression of hsp 27, hsp 60, hsc 70, and hsp 70 stress response genes in cultured human urothelial cells exposed to lethal and sub-lethal concentrations of sodium arsenite. Environ Health Pers 2002; 110: 1225-1232.
[20] Marques R, Maia CJ, Vaz C, Correia S, Socorro S. The diverse roles of calcium-binding protein regucalcin in cell biology: from tissue expression and signaling to disease. Cell Mol Life Sci 2014; 71: 93-111.
[21] Yamaguchi M. The role of regucalcin in nuclear regulation of regenerating liver. Biochem Biophys Res Commun 2000; 276: 1-6.
[22] Isogai M, Oishi K, Yamaguchi M. Serum release of hepatic calcium binding protein regucalcin by liver injury with galactosamine administration in rats. Mol Cell Biochem 1994; 136: 85-90.
[23] Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci 2007; 64: 742-751.
[24] Johnson RB, Fearon K, Mason T, Jindal S. Cloning and characterization of the yeast chaperonin HSP60 gene. Gene 2003; 84: 295-302.
[25] Trivedi V, Gadhvi P, Chorawala M, Shah G. Role of heat shock proteins in immune response and immunotherapy for human cancer. Int J Pharm Sci Rev Res 2010; 2: 57-62.
[26] Takada M, Otaka M, Takahashi T, Izumi Y, Tamaki K, Shibuya T, et al. Over expression of a 60-kDa heat shock protein enhances cytoprotective function of small intestinal epithelial cells. Life Sciences 2010; 86: 499-504.
[27] Calabrese V, Mancuso C, Ravagna A. In vivo induction of heat shock proteins in the substantia nigra following L-DOPAadministration is associated with increased activity of mitochondrial complex I and nitrosative stress in rats: regulation by glutathione redox state. J Neurochem 2007; 101: 709-717.
ment heading
doi:10.1016/S2305-0500(15)30008-7
*Corresponding author: Ki Choon Choi, Grassland and Forage Division, National Institute of Animal Science, RDA, Korea.
E-mail: choiwh@korea.kr
Dr. S. Ravikumar Department of Biotechnology, PRIST University, India.
E-mail: drravinikesh@yahoo.co.in
 Asian Pacific Journal of Reproduction2015年2期
Asian Pacific Journal of Reproduction2015年2期
- Asian Pacific Journal of Reproduction的其它文章
- Potential of pre-gestational intake of Laportea interrupta L. (stinging nettle) leaf decoction as an aid for fetal-maternal health
- Antifertility potential of hydroalcoholic extract of Cordia dichotoma G Forst. leaves: A folklore medicine used by Meena community in Rajasthan state in India
- Effect of genistein on proinflammatory cytokines and estrogen receptorβ in mice model of endometriosis
- Effect of Piper betel leaf stalk extract on protein metabolism in reproductive tissues of male albino rats
- Association of polymorphism in cell death pathway gene FASLG with human male infertility
- Antitumor activity of aqueous extract of Ziziphus jujube fruit in breast cancer: An in vitro and in vivo study
