Effect of sulfation during carbonation on CO2 capture in calcium looping cycle
Wang Chunbo Liu Hongcai Chen Liang Lufei Jia Yewen Tan
(1School of Energy and Power Engineering, North China Electric Power University, Baoding 071003, China)(2CanmetENERGY, Natural Resources Canada, 1 Haanel Drive, Ottawa, Ontario, Canada K1A 1M1)
Effect of sulfation during carbonation on CO2capture in calcium looping cycle
Wang Chunbo1Liu Hongcai1Chen Liang1Lufei Jia2Yewen Tan2
(1School of Energy and Power Engineering, North China Electric Power University, Baoding 071003, China)(2CanmetENERGY, Natural Resources Canada, 1 Haanel Drive, Ottawa, Ontario, Canada K1A 1M1)
Two Canadian limestones with different properties were tested to determine the effect of SO2during the carbonation of sorbent on the CO2capture performance in Ca-looping. When the reaction gas is mixed with SO2, the carbonation ratio of the sorbent is always lower than that without SO2for each cycle under the same conditions, and the sulfation ratio increases almost linearly with the increase in the cycle times. At 650 ℃, there is little difference in the carbonation ratio of the sorbent during the first four cycles for the two carbonation time, 5 and 10 min at 0.18%SO2. The indirect sulfation reaction that occurs simultaneously with the carbonation of CaO is responsible for the degradation of the sorbent for CO2capture, and the carbonation duration is not the main factor that affects the ability of the sorbent. 680 ℃ is the best carbonation temperature among the three tested temperatures and the highest carbonation ratio can be obtained. Also, the sulfation ratio is the highest. The probable cause is the different effects of temperature on the carbonation rate and sulfation rate. A higher SO2concentration will decrease the carbonation ratio clearly, but the decrease in the carbonation capability of the sorbent is not proportional to the increase of the SO2concentration in flue gases.
Ca-based sorbent; carbonation; sulfation; looping; CO2capture
Calcium looping is an emerging technology for high-temperature post-combustion CO2capture[1-5]. It has experienced the fastest developing pace due to the strong similarities and synergies with existing combustion technology in circulating fluidized beds, including recent oxy-fired CFB development[6]. Both carbonation and calcination reactions are carried out at very high temperatures, around 650 ℃ for carbonation,
CaO+CO2→CaCO3
(1)
and over 900 ℃ for calcination in a rich atmosphere of CO2,
CaCO3→CaO+CaO
(2)
However, there is some SO2in coal fired flue gases. Limestone has great potential for capturing sulphur dioxide, despite SO2concentrations being two orders of magnitude lower than that of CO2. The desulphurization of the combustion flue gases is achieved via the following reaction under the atmospheric conditions:
CaO+SO2+1/2O2→CaSO4
(3)
which is usually called the indirect sulfation of limestone.
In combustion systems where the partial pressure of CO2is high enough that CaCO3is not calcined to CaO, the removal of SO2can be realized via direct sulfation, i.e., direct reaction of the gaseous SO2with calcium carbonate in the presence of O2:
CaCO3+SO2+1/2O2→CaSO4+CO2
(4)
Sulfation is irreversible under typical FBC conditions, although it proceeds at a much slower rate than carbonation. As sulfation proceeds, the CaSO4formed leads to pore blockage due to the high volume per unit mass occupied by the primary sulfation product compared to the calcine[7]. Pore closure mainly occurs on the surface of the particles, obstructing direct contact with the interior calcine[7-10]. Since the pore of CaO will be blocked and the CaSO4product layer will be resistant to CO2diffusion on the surface of the sorbents, the SO2will bring a negative effect on the CO2capture for this process. Ryu et al.[11-13]suggested that the presence of SO2leads to the fast deterioration of limestone CO2capture, mainly due to the competition between carbonation and both sulfation reactions. Basinas et al.[14]found that the sorbents sulphated via the un-reacted core mode converted more available calcium, but this adversely affected the reversibility of cyclic CO2capture. The reversibility strongly deteriorated when a higher total pressure was combined with increasing SO2partial pressure. The effect of the presence of SO2was also studied by Coppola et al[15]. Results showed that the presence of SO2in the flue gas significantly decreased the sorbent CO2capacity, most likely because of the formation of an impervious CaSO4layer at the periphery of the particle[16].
As tested, the CO2capture capacity for all sorbents calcined in CO2in the presence of SO2was effectively eliminated after 2 to 3 cycles. These results suggest that the presence of SO2must be avoided if the object is CO2capture from flue gas[17]. In this study, the sulfation following the carbonation of CaO is tested. The effect factors, such as the carbonation temperature, SO2concentration etc, are tested. Especially the influence of duration is tested, which is hoped to obtain the best balance between CO2capture and SO2negative effects. Also, a high SO2concentration of 0.18% was tested, aiming to find how much negative effect of SO2exists only in the carbonation stage on the Ca-sorbents utilization.
1 Experimental Procedure
Two limestones, Massieci and Kelly Rock, were used for the test. Prior to the sorption test, both limestones were milled and sieved to ensure the particle size ranging from 250 to 425 μm for all the tests performed. The main components of the sorbents are presented in Tab.1.

Tab.1 The component of the two limestones %
TherMax 700 TGA was used for testing. The temperature and the weight of the sample were recorded continuously, and the flow rate was kept at 50 mL/min for both calcination and carbonation. The initial calcination of the limestone occurred under the non-isothermal conditions as the temperature was raised from room temperature to 850 ℃. The samples were then maintained at this temperature for a sufficient time (5 min) to ensure complete calcination, and then it was cooled down to the carbonation temperature. Pure nitrogen was used for the calcination process in all cases. When the furnace temperature reached the set carbonation temperature, the gas was switched to the mixed gases containing SO2for specified duration. After carbonation duration, the inlet gas was then switched back to pure nitrogen, and it was heated to 850 ℃ in N2. The cyclic process was repeated 8 times. The testing conditions are shown in Tab.2.
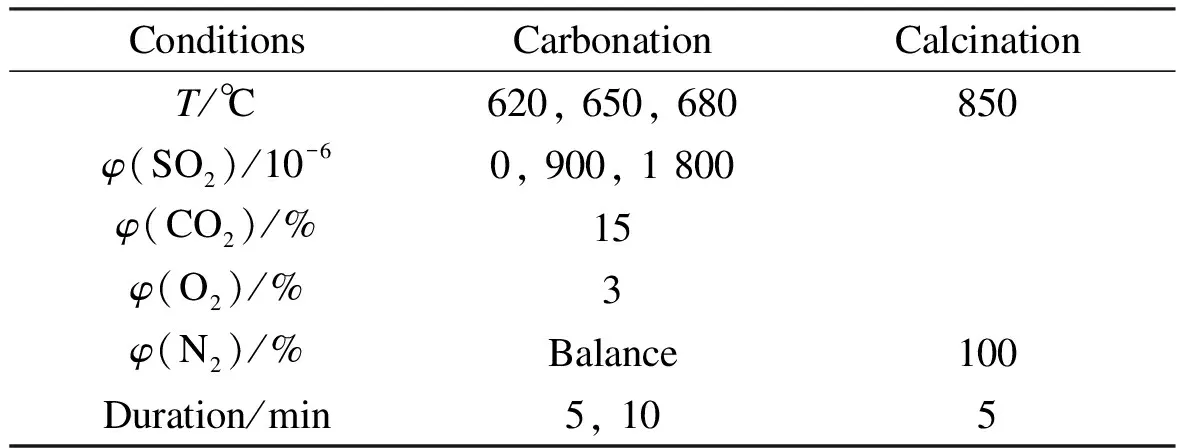
Tab.2 Testing conditions
2 Results and Discussion
2.1 Effect of SO2on carbonation kinetics and conversion
Massieci limestone is chosen for testing the effect of SO2on the carbonation first, and the carbonation temperature is 650 ℃. For comparison, the carbonation in 15%CO2and 85%N2(without SO2) was also tested. The carbonation duration for both tests is 5 min, as shown in Fig.1.
The most common characteristic shown in Fig.1 is that when the reaction gas is without SO2, the limestone calcined to CaO completely, and this can be found from the
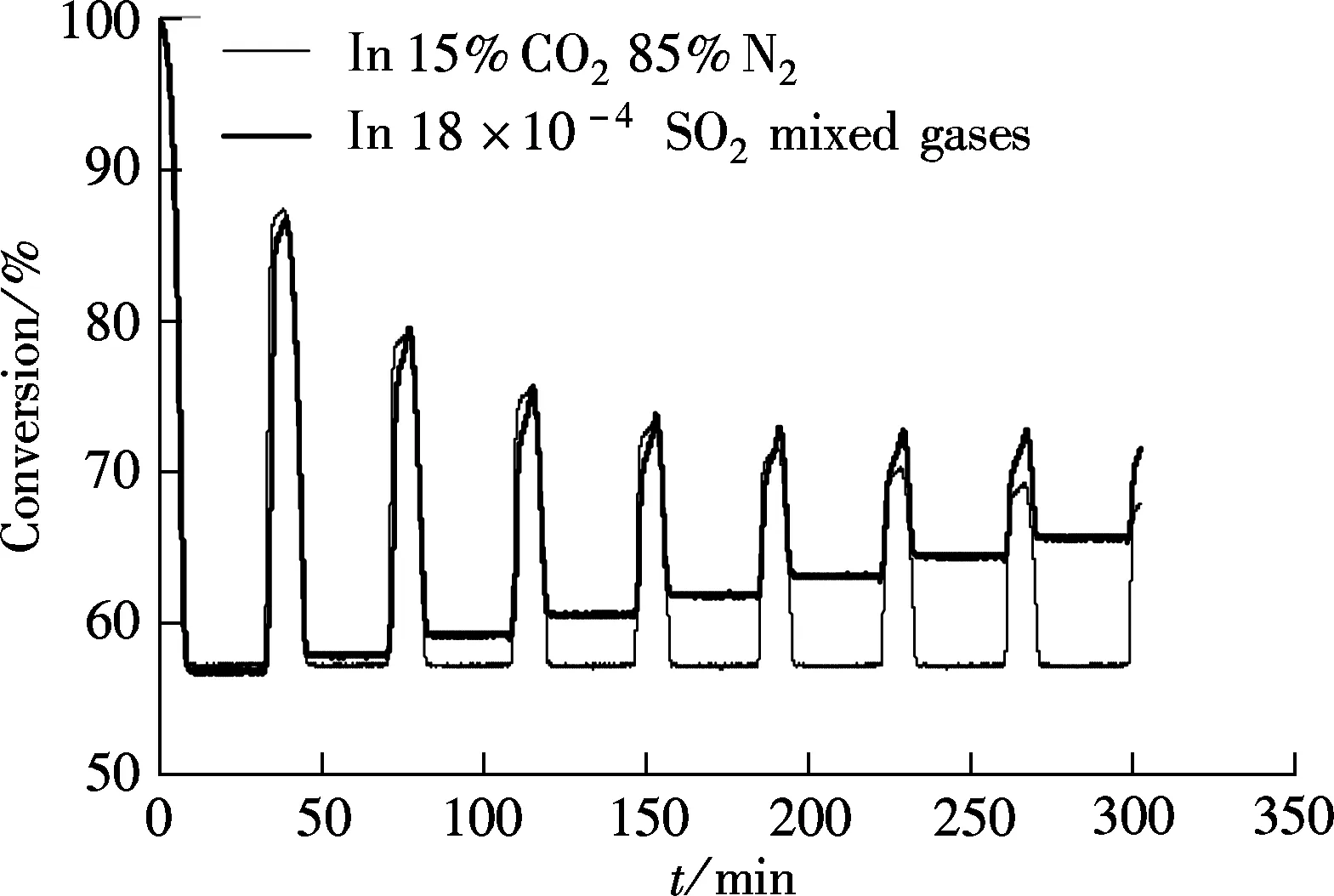
Fig.1 Effect of SO2 on the conversion of Massieci limestone in Ca-looping at 650 ℃
same degree of decomposition for every cycle although the ability of capture CO2always decreases with the increase in the cycle times. However, when the reaction gas contains 0.018%SO2, the final conversion degree increases with the increase in the cycles’ times. For example, for the first cycle, the conversion degree is 56.8% and it is 65.8% for the 8th cycle. The only reason for this is that CaSO4is formed during CaO carbonation, and this will degrade the capability of the sorbent for capturing CO2.
2.2 Effect of duration on conversion of carbonation and sulfation
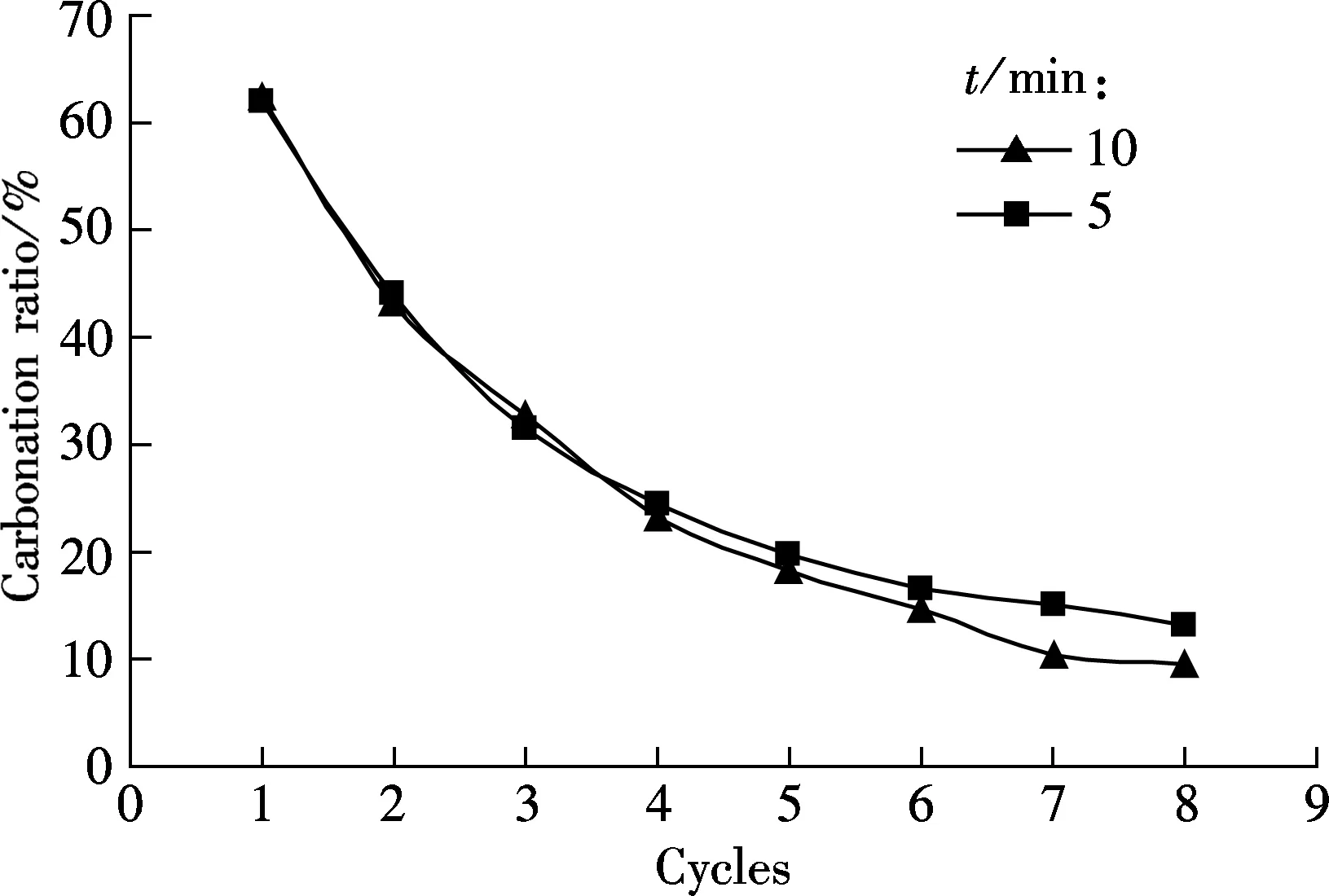
To obtain a good carbonation utilization of sorbent, a suitable carbonation duration should be evaluated. The carbonation duration of 5 and 10 min are tested, and the testing conditions are the same as those in Fig.1 (see Fig.2).
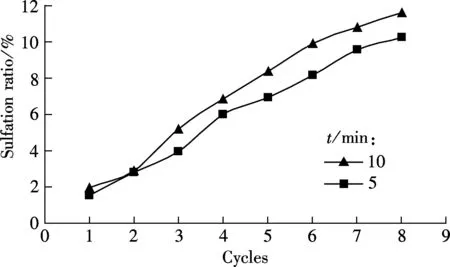
As shown in Fig.2(a), the difference in the carbonation ratio between the two carbonation durations, 5 and 10 min are not obvious for all the eight cycles. Also, a similar tendency occurs for the sulfation ratio of sorbents for the two durations, as shown in Fig.2(b). For example, 11.65% for 10 min and 10.29% for 5 min at the 8th cycle. It seems that a longer carbonation duration (5 and 10 min) will not greatly decrease the carbonation ratio.
(a)
(b)
To verify this further, the carbonation ratio under the conditions without SO2in reaction gases are tested, as shown in Fig.3.
Fig.3 shows that there is little difference in the carbonation ratios between the two durations. It can be speculated that the difference in carbonation ratios between the two durations (see Fig.2) is mainly caused by SO2rather than carbonation duration. Since the sulfation reaction occuring after 5 min is direct sulfation which is very slow at 650 ℃, it will not produce seriously adverse effects on the carbonation of CaO even after a longer carbonation duration. So, the carbonation duration is not an influential factor that affects the ability of the sorbent at 650 ℃ in the calcium looping cycles.

Fig.3 The carbonation ratio of Massieci limestone without SO2 at 650 ℃
2.3 Effect of temperature on conversion of carbonation and sulfation
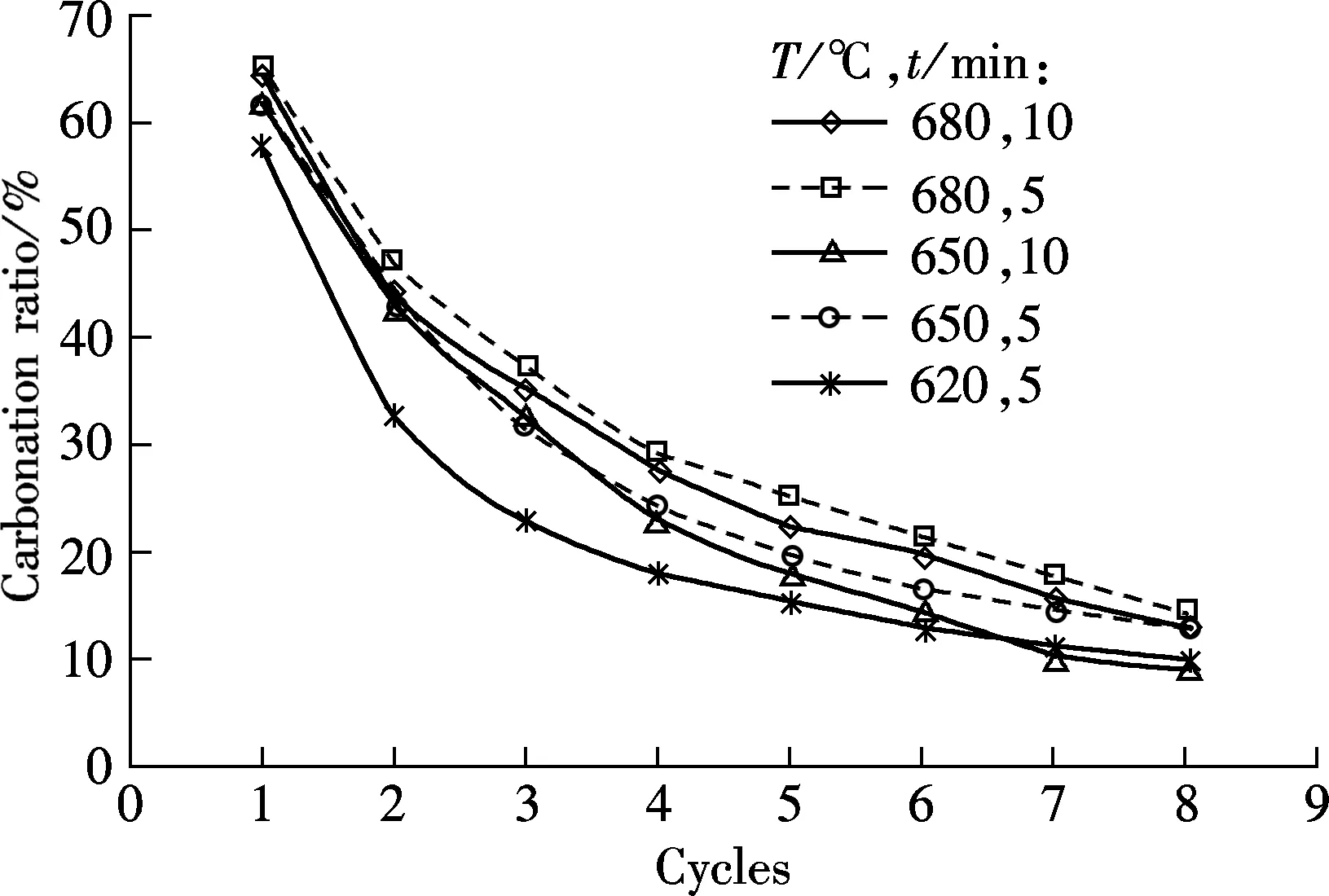
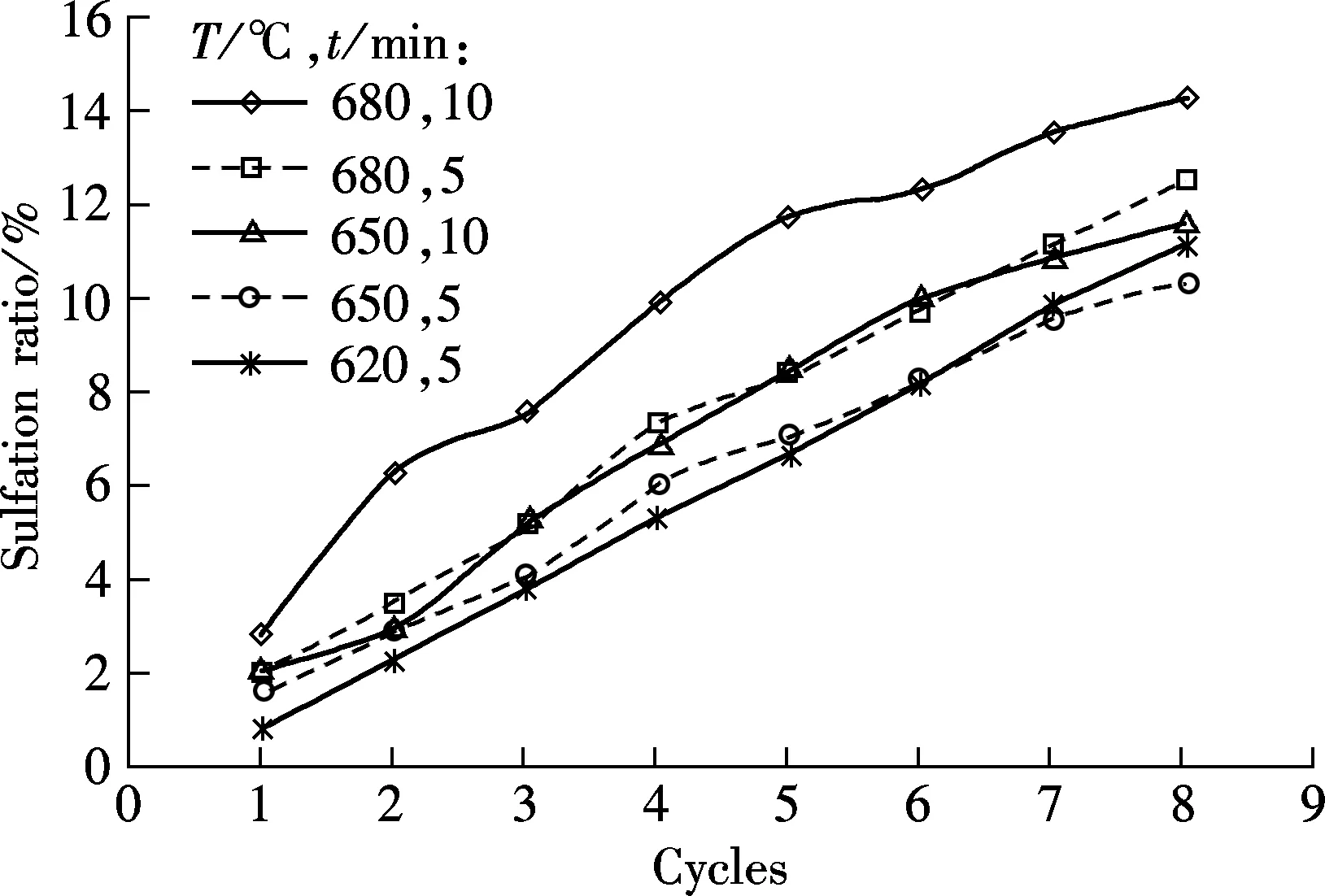
Fig.4 shows the carbonation and sulfation characteristics of sorbent at temperatures of 620, 650 and 680 ℃, respectively, and the SO2concentration is 0.18%.
(a)
(b)
As shown in Fig.4(a), for the 5 min carbonation duration, the best carbonation is obtained at 680 ℃, then at 650 ℃, and the worst is at 620 ℃. For the tests of 10 min duration, the carbonation ratio is higher at 680 ℃ than that at 650 ℃. It shows that temperature is an important factor that determines the carbonation of the sorbent, and 680 ℃ is the best carbonation temperature among the three testing temperatures. However, as an negative influence on carbonation, the sulfation ratio is the highest at 680 ℃, as shown in Fig.4(b). Since the sulfation reaction of sorbent can retard the carbonation of the sorbent, the highest sulfation ratio of sorbent should result in the lowest carbonation ratio. A probable reason for this phenomenon is that the increase in the carbonation ratio caused by the increasing temperature is greater than the negative effect caused by sulfation reaction. Although sulfation will be enhanced by high temperatures, the carbonation improvement will be more obvious.
2.4 Effect of SO2concentration on conversion of carbonation and sulfation
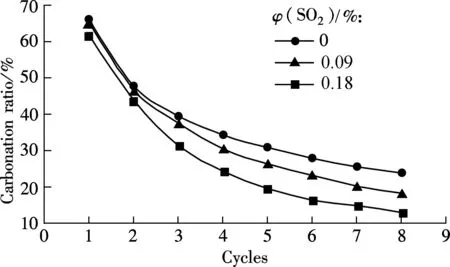
As known from Fig.1, 0.18%SO2will bring a negative effect on the capability of sorbent for CO2capture. For the low sulphur content coal, the SO2concentration in flue gas is less than 0.18%, and the effect of a relatively low concentration SO2is investigated. The mixed gas with 0.09%SO2is used for the next test at the carbonation temperature of 650 ℃ (see Fig.5).
(a)

(b)
It can be seen from Fig.5(a) that a higher concentration of SO2will greatly decrease the carbonation ratio, especially from the second cycle. For example, the carbonation ratio is 18.33% at 0.09%SO2and only 13.06% at 0.18%SO2for the 8th cycle, and the difference is 5.27%. However, the relative sulfation ratio difference is greater than that of the carbonation ratio, as shown in Fig.5(b). For example, the sulfaion ratio is 10.29% for 0.18%SO2and only 2.47% for 0.09% SO2at the 8th cycle. It shows that the decrease in the carbonation capability of the sorbent caused by SO2is not proportional to the increase of the SO2concentration in flue gases, and even a little CaSO4product formed in the carbonation will lead to a decrease in the capability of the sorbent for CO2capture.
2.5 Effect of different limestones
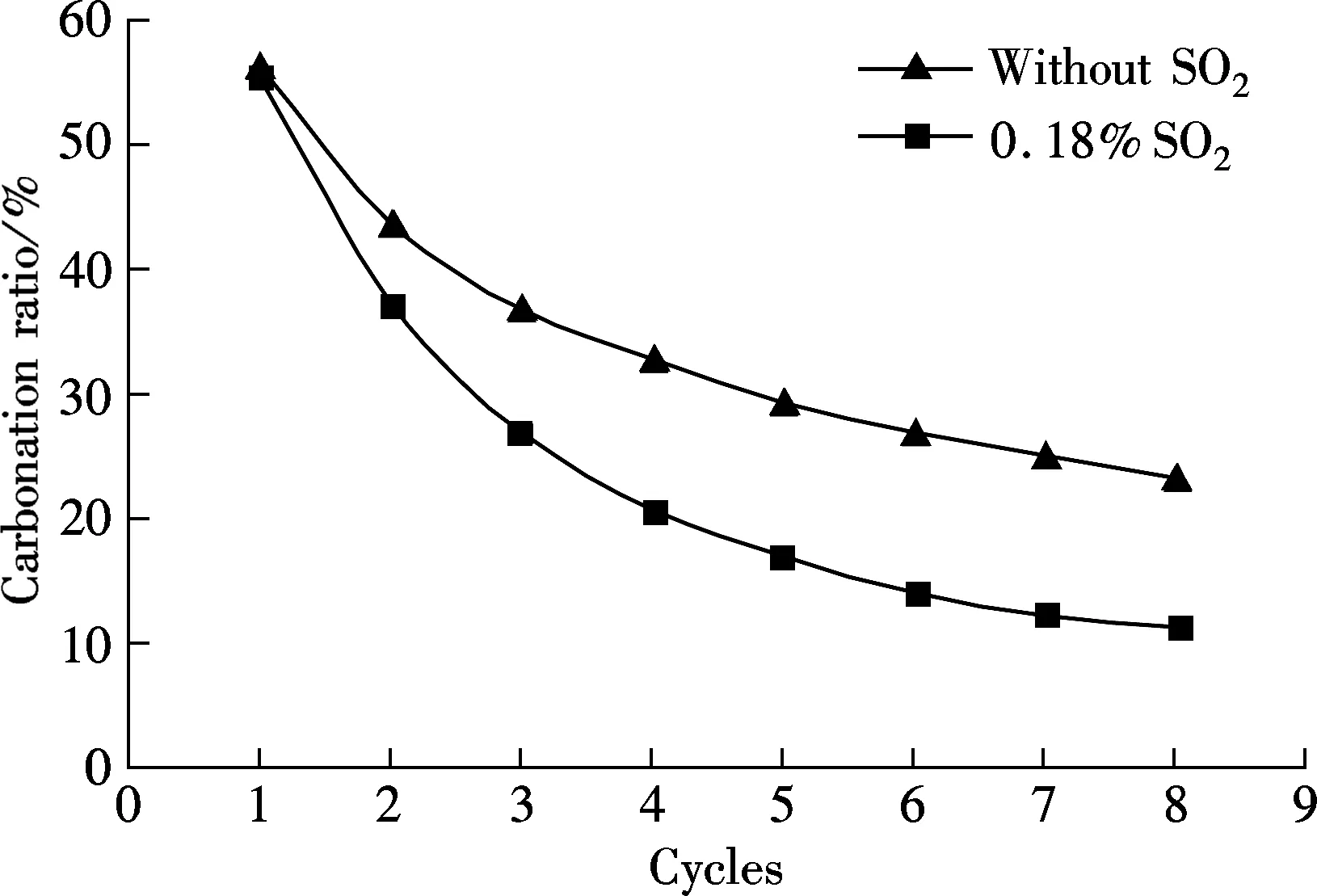
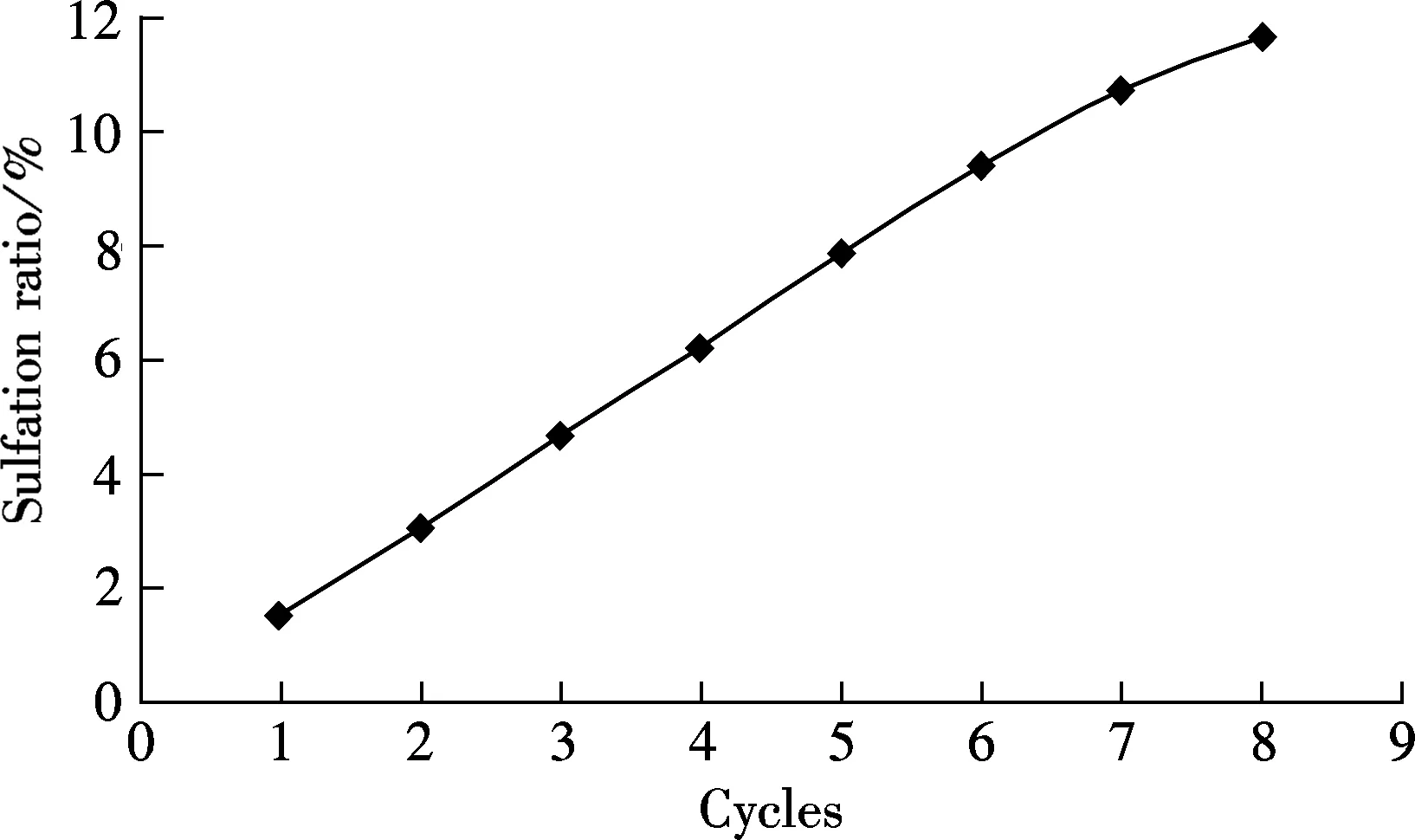
Another limestone, Kelly Rock limestone, was tested to check if the phenomena occurring above are only applicable to one specific limestone. For comparison, the carbonation in 15%CO2and 85%N2at the carbonation temperature of 650 ℃ is also tested (see Fig.6). As shown in Fig.6, when the reaction gases are mixed with 0.18%SO2, the carbonation ratio of the sorbent is always lower than that without SO2for each cycle. For example, the carbonation ratio is 23% without SO2and only 11.17% in 0.18% SO2mixed gases. The sulfation occurred along with the carbonation of sorbent brings more negative effect with more looping cycles, just like that of Massieci limestone. Also, the sulfation ratio of sorbent increases with the cycles almost linearly, from the first cycle of 1.51% up to 11.71% of the 8th cycle.
(a)
(b)
3 Conclusion
When reaction gases are mixed with 0.18%SO2, the carbonation ratio of the sorbent is always lower than that without SO2for each cycle. The carbonation ratio of the sorbent decreased with cycles whether with SO2or not, but a fast decrease occurred when the reaction gases contained SO2. Sulfation of the sorbent occurred during carbonation will bring more negative effect with more looping cycles. The difference in carbonation ratios between the sorbent at 5 and 10 min durations at 650 ℃ is not very clear, which shows a long duration will not bring great effect on the carbonation at 650 ℃ and in 0.18% SO2. Sulfation that occurred simultaneously with the carbonation of CaO is responsible for the degradation of the sorbent for CO2capture. 680 ℃ is the optimal carbonation temperature among the three temperatures. However, the sulfation ratio is also the highest at this temperature. A probable cause for this is that the effect of temperature on carbonation is stronger than that of sulfation for this kind of sorbent. The negative effect will be increased with more SO2, and the decrease in the carbonation capability of the sorbent by SO2is not proportional to the increase of the SO2concentration in flue gases.
[1]Lasheras A, Ströhle J, Galloy A, et al. Carbonate looping process simulation using a 1D fluidized bed model for the carbonator[J].InternationalJournalofGreenhouseGasControl, 2011, 5(4): 686-693.
[2]Zhao M, Andrew I M, Harris T. Review of techno-economic models for the retrofitting of conventional pulverised-coal power plants for post-combustion capture (PCC) of CO2[J].EnergyandEnvironmentalScience, 2013, 6(1): 25-40.
[3]MacKenzie A, Granatstein D L, Anthony E J, et al. Economics of CO2capture using the calcium cycle with a pressurized fluidized bed combustor[J].EnergyandFuels, 2007, 21(2): 920-926.
[4]Martínez I, Murillo R, Grasa G, et al. Integration of a Ca looping system for CO2capture in existing power plants[J].AicheJournal, 2011, 57(9): 2599-2607.
[5]Ariasa B, Diegoa M E, Abanades J C. Demonstration of steady state CO2capture in a 1.7 MWth calcium looping pilot[J].InternationalJournalofGreenhouseGasControl, 2013, 18: 237-245.
[6]Myöhänen K, Hyppänen T, Pikkarainen T, et al. Near zero CO2emissions in coal firing with oxyfuel CFB boiler[J].ChemicalEngineeringandTechnology, 2009, 32(3): 355-363.
[7]Krishnan S V, Sotirchos S V. Effective diffusivity changes during calcination, carbonation, recalcination, and sulfation of limestones[J].ChemicalEngineeringScience, 1994, 49(8): 1195-1208.
[8]Stanmore B R, Gilot P. Review-calcination and carbonation of limestone during thermal cycling for CO2sequestration[J].FuelProcessTechnology, 2005, 86(16): 1707-1743.
[9]Cheng L M, Chen B, Liu N, et al. Effect of characteristic of sorbents on their sulfur capture capability at a fluidized bed condition[J].Fuel, 2004, 83(7/8): 925-932.
[10]Laursen K, Duo W, Grace J R, et al. Sulfation and reactivation characteristics of nine limestones[J].Fuel, 2000, 79(2): 153-163.
[11]Ryu H J, Grace J R, Lim C J. Simultaneous CO2/SO2characteristics of three limestones in a fluidized-bed reactor[J].EnergyandFuels, 2006, 20(4): 1621-1628.
[12]Sun P, Grace J R, Lim C J, et al. Removal of CO2by calcium-based sorbents in the presence of SO2[J].EnergyandFuels, 2007, 21(1): 163-170.
[13]Sun P, Grace J R, Lim C J, et al. Simultaneous CO2and SO2capture at fluidized bed combustion temperatures[C]//18thInternationalConferenceonFluidizedBedCombustion. Toronto, Canada, 2005:22-25.
[14]Basinas P, Wu Yinghai, Grammelis P, et al. Effect of pressure and gas concentration on CO2and SO2capture performance of limestones[J].Fuel, 2014, 122: 236-246.
[15]Coppola A, Scala F, Salatino P, et al. Fluidized bed calcium looping cycles for CO2capture under oxy-firing calcinations conditions: Part 1. Assessment of six limestones[J].ChemicalEngineeringJournal, 2013, 231: 537-543.
[16]Coppola A, Montagnaro F, Salatino P, et al. Fluidized bed calcium looping: the effect of SO2 on sorbent attrition and CO2capture capacity[J].ChemicalEngineeringJournal, 2012, 207/208: 445-449.
[17]Ridha F N, Manovic V, Macchi A, et al. The effect of SO2on CO2capture by CaO-based pellets prepared with a kaolin derived Al(OH)3binder[J].AppliedEnergy, 2012, 92: 415-420.
碳酸化过程中硫化反应对钙基吸收剂循环捕集CO2的影响
王春波1刘洪才1陈 亮1Lufei Jia2Yewen Tan2
(1华北电力大学能源动力与机械工程学院, 保定 071003)
(2CanmetENERGY, Natural Resources Canada, 1 Haanel Drive, Ottawa, Ontario, Canada K1A 1M1)
采用2种不同特性的加拿大石灰石来研究钙基吸收剂循环煅烧碳酸化捕集CO2过程中硫化反应对碳酸化反应的影响.当反应气氛中有SO2时,每次循环中钙的碳酸化转化率都要低于没有SO2的循环,而且钙的硫酸化转化率几乎随着循环次数线性增长.在650 ℃, 0.18%SO2的环境中,对5 和10 min这2种碳酸化时间而言,前4次循环的碳酸化转化率几乎没有区别.与碳酸化过程同时发生的间接硫化反应是导致吸收剂捕集CO2能力下降的原因,而碳酸化时间并不是影响吸收剂脱碳的主要影响因素.在所测试的3个温度中,680 ℃是最佳碳酸化温度,在此温度下碳酸化转化率最高,而此温度下硫化转化率最大,则可能是由于温度对碳酸化和硫化反应速率的作用不同而造成的.碳酸化转化率随SO2浓度的增加而减小,但两者之间并不是线性关系.
钙基吸收剂;碳酸化;硫化;循环;CO2捕集
TK16
Foundation items:The National Natural Science Foundation of China (No.51276064), the Natural Science Foundation of Beijing City (No.3132028).
:Wang Chunbo, Liu Hongcai, Chen Liang, et al.Effect of sulfation during carbonation on CO2capture in calcium looping cycle[J].Journal of Southeast University (English Edition),2015,31(2):215-219.
10.3969/j.issn.1003-7985.2015.02.010
10.3969/j.issn.1003-7985.2015.02.010
Received 2015-01-07.
Biography:Wang Chunbo (1973—), male, doctor, professor, hdwchb@126.com.
 Journal of Southeast University(English Edition)2015年2期
Journal of Southeast University(English Edition)2015年2期
- Journal of Southeast University(English Edition)的其它文章
- Adaptive modulation in MIMO optical wireless communication systems
- An improving energy efficiency cooperation algorithm based on Nash bargaining solution in selfish user cooperative networks
- Performance analysis of an O2/CO2 power plantbased on chemical looping air separation
- Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
- A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
- Applicability of Markov chain-based stochastic modelfor bubbling fluidized beds
