携带GFAP启动子的慢病毒载体介导外源基因在肝星状细胞的特异表达
赵园园1,2姜 燕1,2,3汪海健1,2
(1复旦大学生命科学学院现代人类学教育部重点实验室与遗传工程国家重点实验室 上海 200438;2复旦大学生物医学研究院 上海 200032;3复旦大学化学系 上海 200433)
携带GFAP启动子的慢病毒载体介导外源基因在肝星状细胞的特异表达
赵园园1,2▲姜 燕1,2,3▲汪海健1,2△
(1复旦大学生命科学学院现代人类学教育部重点实验室与遗传工程国家重点实验室 上海 200438;2复旦大学生物医学研究院 上海 200032;3复旦大学化学系 上海 200433)
目的旨在构建包含由GFAP启动子介导的荧光素酶的慢病毒载体,检测体内外靶向肝星状细胞的外源基因表达的特异性和效率。方法通过报告基因实验筛选介导效率较高的GFAP启动子,以CMV启动子作为阳性对照分别介导荧光素酶在小鼠肝星状细胞(JS1)、人肝星状细胞(LX-2)及人肝细胞(L-02)中的表达。通过尾静脉注射将包装的慢病毒注射到四氯化碳诱导的肝纤维化小鼠体内,通过活体成像和Western blot检测慢病毒颗粒在小鼠体内的分布,并通过免疫双荧光染色检测肝星状细胞的特异表达。结果人GFAP启动子比小鼠GFAP启动子驱动基因表达的效率高,而且在LX-2和JS1细胞中,人GFAP启动子介导的荧光素酶的活性及表达显著高于L-02细胞。活体成像显示小鼠腹部有荧光素酶的表达。GFAP启动子介导的荧光素酶可以与肝星状细胞的特异性生物标记蛋白GFAP、Desmin和α-Sma共定位。结论成功构建包含由GFAP启动子介导的荧光素酶的慢病毒载体,在体外细胞系以及小鼠体内肝中均可以实现荧光素酶在肝星状细胞中特异性表达。
肝星状细胞; 慢病毒载体; GFAP启动子; 靶向基因表达; 基因治疗方法
Liver fibrosis is the common feature of most types of chronic liver disease and is characterized by excessive accumulation of extracellular matrix (ECM).The hepatic stellate cell(HSC)is the major cell type implicated in this process[1],it is liver resident perisinusoidal cell distributed throughout the liver and represents 5%-10%of the total number of liver cells in normal liver.When the liver is damaged by a fibrogenic stimulus,the HSCs undergo morphological and functional changes such as proliferation,contraction,chemotaxis and increased expression of fibrillary collagens and cytokines[2],which is called HSC activation.The major treatment strategies include inhibiting HSC activation,eliminating activated HSCs,promoting matrix degradation,and cytokine therapy[3-9].
Using a cell-specific and efficient driver and delivery system for gene transduction is critical for HSC targeted gene therapy in liver fibrosis. Antifibrotic agents often fail to target HSCs in a cell-specific way.Therefore,HSC-specific promoters that drive expression of HSC-specific genes in liver tissue have been employed to overcome this technical issue.Glial fibrillary acidic protein (GFAP)is an intermediate filament protein highly and specifically expressed in astrocytes of the central nervous system(CNS)[10].It's also highly and specifically expressed in HSCs compared to other liver cell types[11-12].Transgenic mice with a herpes simplex virus thymidine kinase gene(HSVTK)driven by the mouse GFAP promoter could specifically deplete HSCs in response to ganciclovir[13].Additionally,the knockdown of TGFβ expression in HSCs with pri-miRNA producing plasmids driven by the GFAP promoter significantly decreased TGFβ1 and collagen mRNA and protein levels[7].Furthermore,a PDGFRβ sh RNA expression plasmid driven by GFAP promoter delivered with the hydrodynamic-based transfection(HBT)method specifically knocked down PDGFRβgene in CCl4induced acute injured rat liver[5].These studies suggest that the GFAP promoter drives HSC-specific exogenous gene expression in vitro and in transgenic mice in vivo.
Although the non-viral mode of gene delivery to liver produces satisfactory transfection efficiency in mice and rats[5,14],it is based on the HBT method,which limits its applications in both experimental and clinical settings.The naked plasmid is transiently expressed in host cells,and repeated administrations are needed to maintain stable expression of the exogenous gene in vivo. The rapid injection of a large volume via the tail vein during HBT usually causes increased venous pressure,hepatic infarction and transient heartdysfunction,and may cause animal death[15]. Alternatively,viral gene transfer vectors such as lentiviral vectors have high transduction efficiency and lead to stable long-term transgene expression. These vectors are also physically less harmful to animals.However,there are potential biohazards,and viral vectors may have increased immunogenicity[16-17].A lentiviral expression vector has been successfully used to deliver transgene expression into both astrocytes in brain and stellate cells in liver[17-19].The integration of cell-specific promoters has been proposed to selectively express transgenes in astrocytes[17]. However,the efficiency and specificity of transgene expression in vivo by lentiviral gene delivery systems using GFAP gene promoters has not yet been evaluated in HSCs.
In this study,we constructed a lentivirus vector with a luciferase gene driven by the GFAP promoter and introduced the lentivirus into mouse liver by intravenous delivery.We then investigated the in vivo expression and activity of luciferase.Our results showed that the human GFAP promoter drives directed HSC-specific expression of luciferase in mouse liver after intravenous delivery,which suggest that the combined GFAP promoter and lentiviral delivery system had potential clinical applications in liver fibrosis gene therapy.
Materials and Methods
Cell linesThe human hepatic stellate cell line LX-2[20]was provided by Professor Lieming Xu at the Institute of Liver Diseases,Shanghai University of Traditional Chinese Medicine.The murine hepatic stellate cell line JS1[21-22]was provided by the Division of Digestive Diseases,Zhongshan Hospital,Fudan University.Human hepatocyte L-02 and 293T cells were purchased from the Cell Bank of Shanghai Institutes of Life Sciences,Chinese Academy of Sciences.All cells were cultured in Dulbecco's Modified Eagle's Medium (Hyclone,China)supplemented with 10%fetal bovine serum(Nelso Biotech,USA).
Construction of reporter plasmids and lentiviral vector driven by GFAP promoter and reporter gene assayWe constructed the reporter gene vector and lentiviral vector driven by GFAP promoter according to a previously described method[23]. The 5′-flanking DNA of human GFAP gene (-2 216 to+45,relative to the transcriptional start site)and mouse gene(-2 123 to+127)was amplified by PCR using the following primers:human forward,5′-CCTCGCTAGCTCACTGCTTACGCCCAGGTC-3′;reverse,5′-CCTCAAGCTTGCGAGCAGCGGAGGTGAT-3′and mouse forward,5′-CCTCGCTAGCTCACTGCTTACGCCCAGGTC-3′;reverse,5′-CCTCAAGCTTGCGAGCAGCGGAGGTGAT-3′.The fragments were cloned into Nhe I and HindⅢsites of p GL3-Basic. The translational initiating ATG(+15 for human and+82 for mouse)was converted to TTG by site-directed mutagenesis.The novel reporter plasmids driven by human or mouse GFAP promoters were verified by sequencing and were named pGL3-h GFAP-p-Luci and p GL3-mGFAP-p-Luci,respectively.The fragment containing human GFAP promoter and luciferase cDNA was amplified by PCR using p GL3-hGFAP-p-Luci plasmid as a template with the following primer sequences:5′-CCTCACTAGTTCACTGCTTACGCCCAGGTC-3′and 5′-GGAGCTGACTGGGTTGAAG-3′.The fragment was then inserted into the lentiviral vector p CDH-GFP(System Biosciences,USA)at Spe I-Bam H I sites and was named p CDH-h GFAP-p-Luci.The positive control was pCDH-CMV-Luci,which has luciferase expression driven by a CMV promoter.The construct was made by cleaving luciferase cDNA from the p GL3-Basic plasmid using Nhe I and Bam H I endonucleases.The fragment was then cloned into p CDH-GFP at the same sites.LX-2 and JS1 cells were cultured in 24-well plates at the density of 1×105cells/well.After 24 hours of culture,the reporter plasmids p GL3-h GFAP-p-Luci,p GL3-mGFAP-p-Luci,a negative controlp GL3-basic and a positive control pGL3-promoter (luciferase driven by SV-40 promoter)were transfected into cells at 0.8μg/well.These plasmids were co-transfected with the internal control p RL-CMV(luciferase driven by CMV promoter)at 8 ng/well into the cells using Lipofectamine 2000(Invitrogen,USA)at 1μL/ well.Cell extracts were prepared with 1×passive lysis buffer for 20 min at room-temperature,and luciferase activity was measured using a Dual-Luciferase Reporter Assay System(Promega,USA)on a Veritas Microplate Luminometer (Turner Biosystems,USA)48 hours after transfection.The transcriptional activity of the GFAP promoter was evaluated in terms of the fold change of luciferase activity of each construct relative to the p GL3-basic vector.All samples were measured in four replicates with three independent experiments.
Construction of GFAP promoter driven luciferase expression lentivirus and cell infectionThe luciferase expression vectors(pCDH-h GFAP-p-Luci,pCDH-CMV-Luci and pCDH-GFP)and the packaging vectors psPAX2 and pSD were cotransfected into 293T cells using Trans-EZ (SunBio,China).The culture supernatants were collected and centrifuged at 2 000×g for 5 min at room temperature to pellet cell debris.The supernatants were then filtered through a 0.45μm filter.A portion of the viral particles was aliquoted and stored at-80℃until ready for cell infection. The remaining filtered supernatants were subjected to ultracentrifugation at 25 000×g for 2 hours.The viral particles were dissolved with PBS and stored at-80℃for intravenous tail injection.The titer of the viral particles was measured by a Quick Titer kit(Cell Biolabs,USA)according to the manufacturer′s instructions.L-02,JS1 and LX-2 cells were infected with lentiviral particles(p CDHGFAP-p-Luci,p CDH-CMV-p-Luci and p CDHGFP)once per day for three days.Cell extracts were prepared to measure luciferase expression and activity when more than 90%of cells appeared green by fluorescence microscopy.The luciferase expression was detected by Western blot using a luciferase primary antibody(sc-32896,Santa Cruz Biotech,USA)and antibody(Abmart,M20010,China)forβ-actin(internal control).The luciferase activity was measured with a single-luciferase reporter assay system.The number of cells in each sample was counted using a cell counter (Cellometer auto T4,Nexelom,USA).The relative luciferase activity was calculated as the luciferase activity per total cell number.
Transduction of mouse liver by GFAP promoter driven luciferase expression lentivirus particlesAll 4-week-old male BalB/C mice weighing approximately(14±1)g were provided by the Laboratory Animal Center for Shanghai Medical College of Fudan University.The animals were housed in a sterile animal facility with a 12-hour dark/light cycle and had free access to food and water.Eleven mice were treated with CCl4intraperitoneal injection to induce liver fibrosis. CCl4was diluted with olive oil(1∶4,volume fraction),and the injection volume was 5μL/g body weight for the first dose and 2.5μL/g body weight for the subsequent injections every 3 days. Five mice were injected with olive oil as a vehicle control.At 4 weeks after administration,two mice were randomly selected from each group and sacrificed.The liver tissues were prepared for Sirius red staining.The remaining mice in the CCl4treatment group and control group were randomly divided into three groups and then treated with two successive daily intravenous tails injections with p CDH-GFAP-p-Luci,negative control pCDH-GFP or positive control p CDH-CMV-p-Luci.The virus titer for each group was measured,and the injection volume was 2×107TU/mouse.The animals were then used in experiments 10 days after the second intravenous injection.The protocols for animal experiments were approved by the Animal Ethics Committees of Shanghai Medical College,Fudan University(Permit Number:20130227-068).All animals received humane care,and all efforts weremade to minimize suffering.
Detection of in vivo expression of luciferase and GFP with bioluminescence imaging and Western blotThe overall expression pattern of luciferase from lentivirus particles was profiled by in vivo bioluminescence imaging with the IVIS imaging system(IVIS Spectrum,Xenogen,USA).The mice were administered D-luciferin(150 mg/kg,Promega,USA)by intraperitoneal injection prior to imaging.The mice were then anesthetized by isoflurane inhalation and imaged according to the manufacturer's instructions.The total proteins extracted from mouse livers and kidneys were subjected to SDS-PAGE electrophoresis and Western blot for GFP detection.The protein concentrations were quantified using a BCA Protein Assay Kit(p0011,Beyotime,China).The loading quantity of each protein sample was 20μg. After SDS-PAGE electrophoresis,one part of each panel was stained with Coomassie brilliant blue. The remaining panels were subjected to Western blot for GFP expression using a primary antibody (M20004S,Abmart,USA).
Immunofluorescence assayFrozen liver tissue sections were fixed in 5%paraformaldehyde for 30 min at 37℃and then blocked,rinsed,and incubated with primary antibody.The sections were then incubated with fluorescently labeled secondary antibody.The sections were observed under a fluorescence microscope.The positive fluorescent expression areas were estimated with Image Pro Plus software(Bethesda,MD).The primary antibodies were anti-Desmin rabbit antibody(P17661,Cell Signaling,USA),anti-α-Sma rabbit antibody(ab5696,Abcam,UK),antiluciferase rabbit(sc-32896,Santa Cruz Biotech,USA)and mouse antibodies sc-74548(Santa Cruz Biotech,USA),and anti-GFAP mouse antibody (AM 1870 b,Abgent,USA).The secondary antibodies were Cy3-labeled goat anti-rabbit IgG (p0183,Beyotime,China)and dylight 405-labeled goat anti-mouse Ig G(115-475-003,Jackson Immuno Research,USA).
Results and Discussion
Human GFAP promoter stronger than mouse oneThe myofibroblastic transdifferentiation of HSCs is a central event in liver fibrogenesis. Substantial progress has been made in the development of gene transduction methods for HSC targeted gene therapy against liver fibrosis. These therapies deliver efficient and specific gene transduction targeting to HSCs.Although both human and mouse GFAP promoters have been used as HSC-specific expression drivers[13,23],it is unclear which promoter has the best activity.We first compared the luciferase activity driven by human and mouse GFAP promoters using a reporter gene assay.A comparison of human(LX-2)and mouse(JS1)HSC cell lines indicated that the luciferase activity driven by the human GFAP promoter is significantly stronger than mouse promoter(Fig 1).
The human GFAP promoter has three regions,A,B and D,with several sequences homologous to biding sites of known transcription factors and the B regions has the most activity[24].
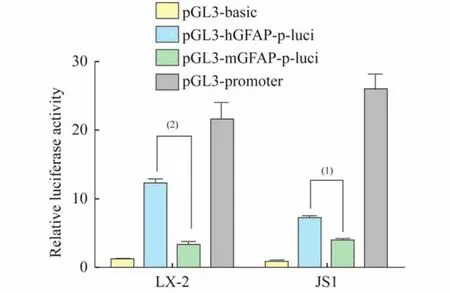
Fig 1 Transcriptional activities of human and mouse GFAP promoters
The comparison of B region of human andmouse GFAP promoter with the documented binding sites of these transcription factors showed that human GFAP promoter has more homologous sequences,which might account for the observation that human GFAP promoter shows much more cis-acting capability even though in different species.Therefore,we selected the human GFAP promoter as the HSC-specific expression diver.We then cloned the sequence and luciferase cDNA into the p CDH-GFP lentiviral vector and used the vector in our subsequent in vitro and in vivo experiments.
HSCs targeted expression driven by GFAP promoter in vitroWe then evaluated the specificity of HSC targeted expression driven by the human GFAP promoter.We compared the expression and activity of luciferase transduced by this driver in the lentivirus delivery vector in mouse(JS1)and human(LX-2)HSCs and human hepatocytes(L-02).The cells were infected with lentiviral particles(pCDH-h GFAP-p-Luci,pCDHCMV-p-Luci and pCDH-GFP)prepared from cotransfection of the hGFAP-promoter-driving luciferase expression vectors and packaging vectors.The luciferase expression level and activity were detected separately by Western blot and single-luciferase reporter assays.Although the driving activity of the GFAP promoter(pCDH-hGFAP-p-Luci)was less than the CMV promoter (pCDH-CMV-Luci)that served as a common positive control across cell lines,it is clear that the expression level of luciferase in JS1 and LX-2 cells driven by the human GFAP promoter was significantly higher than the very weak expression in L-02 cells(Fig 2A,2B).Furthermore,the luciferase activity driven by the GFAP promoter in JS1 and LX-2 cells was significantly greater than in L-02 cells(Fig 2C).These results suggested that a lentivirus delivery system using the human GFAP promoter could specifically target gene expression in human and mouse HSCs in vitro.
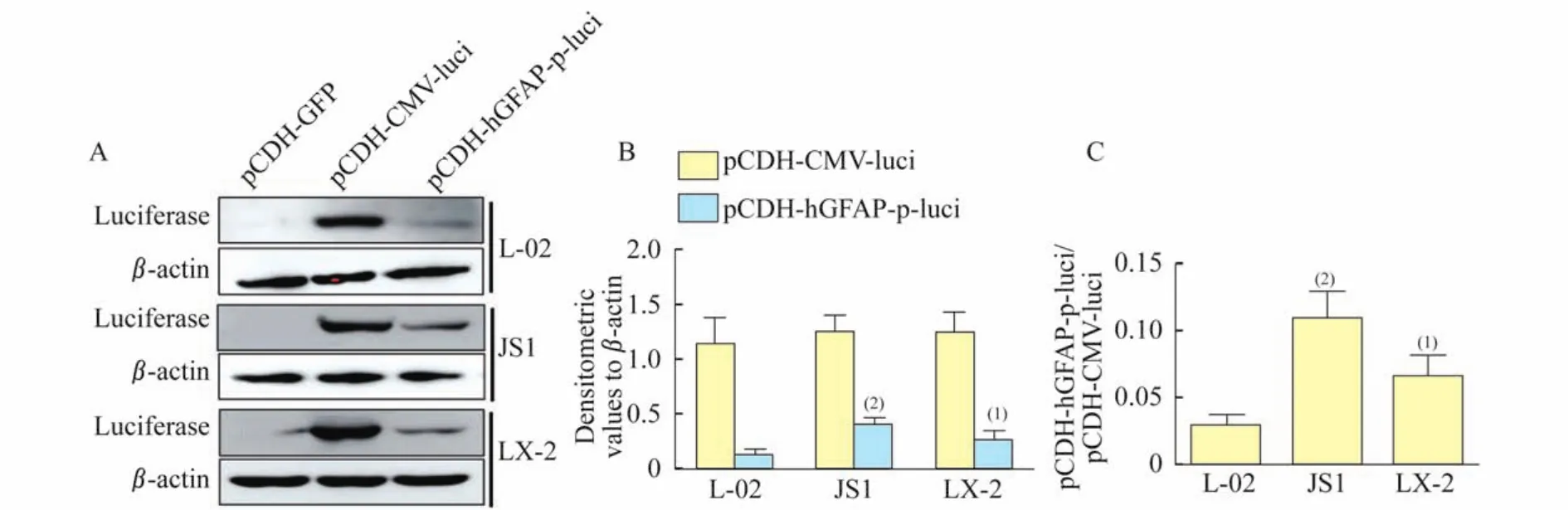
Fig 2 The expression and activity of luciferase driven by human GFAP promoter
The distribution of lentivirus driven by GFAP promoter in the CCL4induced liver fibrosis mouse modelWe further evaluated the in vivo performance of the constructed GFAP promoter driven luciferase expression lentivirus in the liver fibrosis mouse model.This model was induced with an intraperitoneal injection of CCl4.The analysis of Sirius red-stained paraffin embedded sections of liver tissue showed that the mice treated with CCl4displayed more and stronger red staining in the portal area compared with normal control mice. This result confirms that CCl4administration inmice stimulated HSCs activation and induced liver fibrosis(Fig 3A).We next characterized the distribution of lentivirus particles in vivo after intravenous delivery in the liver of fibrotic mice. We injected three types of lentiviral particles by the tail vein.We used the p CDH-GFAP-p-Luci particles,pCDH-GFP particles as a negative control,and p CDH-CMV-p-Luci particles as a positive control.The in vivo imaging showed that the animals with p CDH-CMV-luci(luciferase driven by CMV promoter)had more luciferase activity than the negative control(p CDH-GFP)(Fig 3B).The mouse injected with pCDH-h GFAP-p-Luci driven by the GFAP promoter did not manifest detectable luciferase activity.There could be two possible explanations for this unexpected observation.First,the CMV promoter is among the few viral and cellular regulatory elements with the strongest activity[25].The luciferase driven by pCDH-CMV-luci in all three cell lines showed higher expression and activity than those constructs driven by pCDH-GFAP-p-luci(Fig 2). Second,the subpopulation size of HSCs in normal liver(approximately 5%-10%)or in CCl4-induced liver fibrosis mouse models(approximately 20%)(Fig 3A)may be different.These cells respond to GFAP promoters by driving luciferase expression as its specific target.This cell population is smaller than cell populations in which the CMV promoter could drive ubiquitous gene expression[26].The in vivo imaging also showed that the distribution of luciferase activity driven by the CMV promoter was mostly concentrated in the abdomen.Fibrosis can affect many organs including liver and kidney in the abdomen.Therefore,we measured the quantity of lentivirus particles in liver and kidney by comparing the expression GFP,which was used as the reference indicator forbackground expression of all three pCDH lentiviral expression vectors.
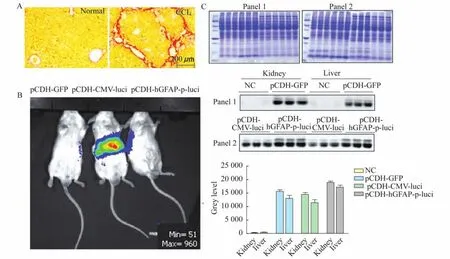
A:The paraffin embedded sections from normal and CCl4-induced liver injured mice were subjected to Sirius red staining to validate the liver fibrosis mouse model.B:The distribution of luciferase activity in mice transduced with pCDH-GFP,pCDH-CMV-luci and pCDH-GFAP-p-luci at 2×107TU/mouse were profiled with bioluminescence in vivo imaging using an IVIS imaging system.C:Coomassie brilliant blue staining was used to confirm the comparable quantities of loading proteins from different samples.The expression level of GFPin Western blot was measured by gray level in Image J software.
The Coomassie brilliant blue staining result quantitatively validated the electrophoresis analysis for the protein samples from both tissues.The Western blot results showed that GFP was expressed in both liver and kidney at comparable levels in mice treated with all three lentivirus particles(Fig 3C).
HSCs targeted expression driven by GFAP promotion in vivoIt is well known that there are two main fibroblastic cell populations involved in liver fibrogenesis.These cell types include hepatic stellate cells and portal fibroblasts.The hepatic stellate cells are activated and involved in repair processes in CCl4treatment models of liver injury.The portal fibroblasts are involved in portal fibrosis induced by bile duct ligation[27-28].We profiled the expression of lentivirus particles in liver and assayed the co-localization of luciferase driven by GFAP promoter and HSC-specific markers such as GFAP,Desmin andα-Sma to evaluate the specific expression of luciferase driven by the GFAP promoter in the lentiviral delivery system.GFAP is specifically expressed in HSCs-derived myofibroblasts in liver.Desmin is a common marker for both quiescent and activated HSCs,andα-Sma is expressed in activated HSCs[29].The distribution of lentivirus particles in liver was illustrated by GFP fluorescence because the expression of GFP was driven by a CMV promoter in these vectors and was similarly distributed in these tissues(Fig 4,panel M,N,O and P).

Fig 4 The distribution of lentivirus particles in mice liver tissue.
The luciferase-positive cells were authenticated by detecting HSC marker proteins by dualimmuno fluorescence.As shown in Fig 4 panel K(merged form panel C and G)and panel L(merged from panel D and H),cells co-expressing both luciferase(red fluorescence)driven by GFAPpromoter and the HSC marker GFAP(blue fluorescence)were mostly distributed in the portal area(panel K)or the sections injured by CCl4(white dots in liver surface by naked eye,panel L). The cells expressing luciferase driven by the CMV promoter were globally distributed in liver tissues including the portal area and the hepatic lobules area(Fig 4,panel F).We explored the colocalization of luciferase driven by the GFAP promoter and HSC markers of Desmin andα-Sma (both in red fluorescence).The results showed that cells co-expressing luciferase and Desmin were present in the portal(shown as yellow arrow)and injured areas(shown as white arrow,Fig 5A). Furthermore,Desmin was expressed in the portal area(quiescent HSCs location)of normal liver and in fibrotic areas(activated HSCs location)in liver fibrosis tissue.The blue fluorescence showed that luciferase-positive cells in the portal area were relatively weaker than those in the CCl4-injured area.This finding indicated that exogenous luciferase expression driven by the GFAP promoter reflected the expression level of endogenous GFAP.Additionally,the gene transduction and targeted expression was higher in activated HSCs than in quiescent cells.We observed that approximately 80%ofα-Sma(activated HSCs marker)-expressing cells were superimposed with luciferase positive cells(Fig 5B),which clearly demonstrated that the human GFAP promoter in the lentiviral delivery system could specifically target luciferase expression in HSCs of fibrotic liver in vivo.
The positive control non-specific CMV promoter drove extensive expression of luciferase in whole liver tissue.However,the GFAP promoter directed HSC-specific expression.This approach avoids disturbing normal physiologic status of other cells that may be affected by exogenous gene expression.However,we should also acknowledge that the promoter activity of the GFAP driver is weaker than the CMV promoter. Thus,GFAP may not be able to provide sufficient transactivation when high levels of exogenous gene expression are needed.Therefore,the application of this HSC targeted gene transduction and expression system should be put in an appropriate context.
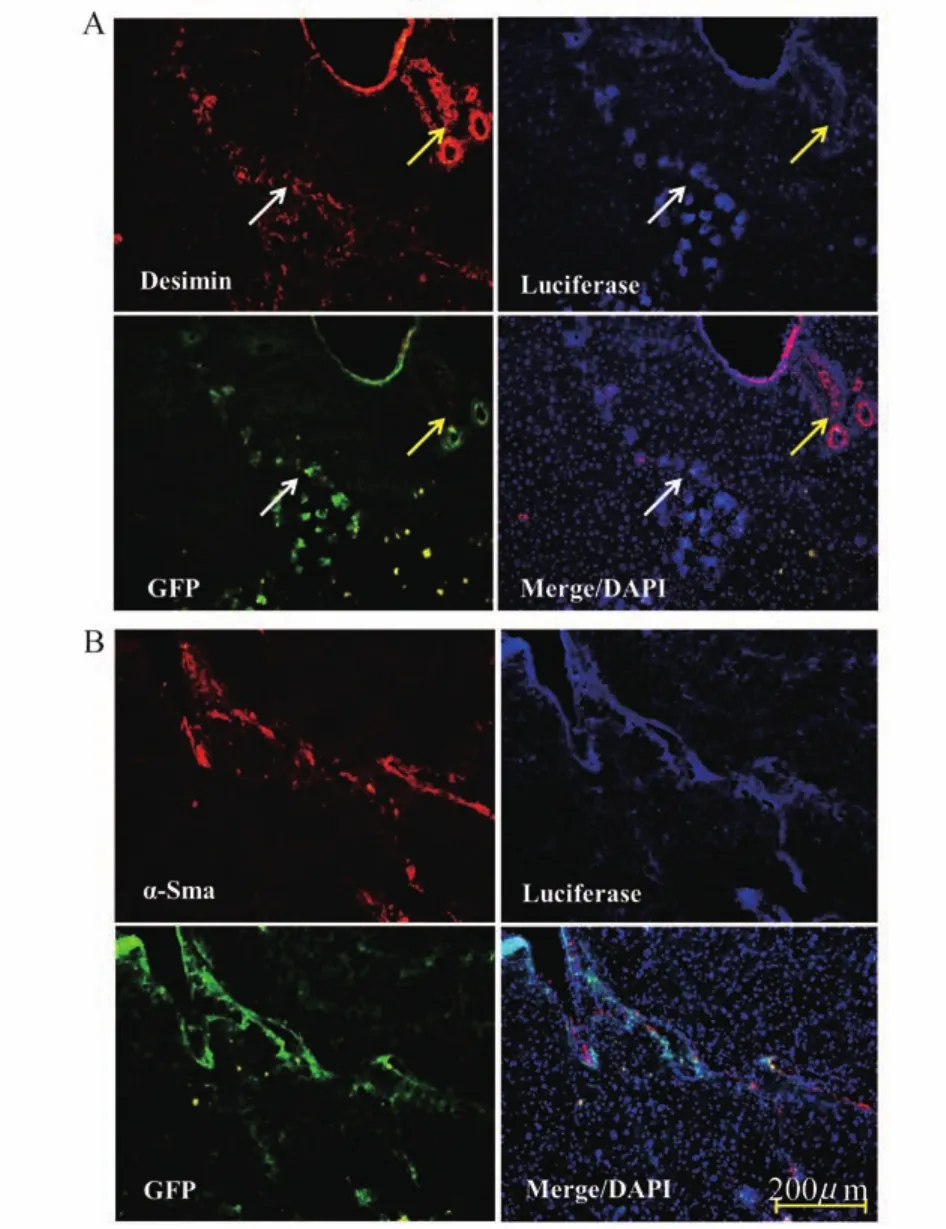
Fig 5 HSCs targeted luciferase expression driven by GFAP promoter
In summary,we combined HSC-specific GFAP promoter with a lentivirus vector and evaluated the specificity and efficiency of HSC gene transduction and targeted expression in vitro and in vivo. Additionally,we verified that the human GFAP promoter drives directed HSC-specific luciferase expression in mouse liver after intravenous delivery of lentivirus.Our results indicate that the GFAP promoter-driven lentiviral vector is a useful tool for gene manipulation in mechanistic investigationsand has potential clinical applications in liver fibrosis gene therapy.
[1]Puche JE,Saiman Y,Friedman SL.Hepatic stellate cells and liver fibrosis[J].Compr Physiol,2013,3(4):1473 -1492.
[2]Senoo H,Yoshikawa K,Morii M,et al.Hepatic stellate cell(vitamin A-storing cell)and its relative-past,present and future[J].Cell Biol Int,2010,34(12):1247-1272.
[3]Friedman SL,Sheppard D,Duffield JS,et al.Therapy for fibrotic diseases:nearing the starting line[J].Sci Transl Med,2013,5(167):167sr1.
[4]Hellemans K,Michalik L,Dittie A,et al.Peroxisome proliferator-activated receptor-beta signaling contributes to enhanced proliferation of hepatic stellate cells[J]. Gastroenterology,2003,124(1):184-201.
[5]Chen SW,Chen YX,Zhang XR,et al.Targeted inhibition of platelet-derived growth factor receptor-beta subunit in hepatic stellate cells ameliorates hepatic fibrosis in rats [J].Gene Ther,2008,15(21):1424-1435.
[6]Baghy K,Iozzo RV,Kovalszky I.Decorin-TGFbeta axis in hepatic fibrosis and cirrhosis[J].J Histochem Cytochem,2012,60(4):262-268.
[7]Yang N,Mahato RI.GFAP promoter-driven RNA interference on TGF-beta1 to treat liver fibrosis[J]. Pharm Res,2011,28(4):752-761.
[8]Zhang DW,Zhao YX,Wei D,et al.HAb18G/CD147 promotes activation of hepatic stellate cells and is a target for antibody therapy of liver fibrosis[J].J Hepatol,2012,57(6):1283-1291.
[9]Ogawa T,Iizuka M,Sekiya Y,et al.Suppression of type I collagen production by microRNA-29b in cultured human stellate cells[J].Biochem Biophys Res Commun,2010,391(1):316-321.
[10]Bignami A,Dahl D.The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocytespecific protein(GFA)in mammalian and submammalian vertebrates[J].Neuropathol Appl Neurobiol,1976,2 (2):99-110.
[11]Maubach G,Lim MC,Zhang CY,et al.GFAP promoter directs lacZ expression specifically in a rat hepatic stellate cell line[J].World J Gastroenterol,2006,12(5):723 -730.
[12]Russo FP,Alison MR,Bigger BW,et al.The bone marrow functionally contributes to liver fibrosis[J]. Gastroenterology,2006,130(6):1807-1821.
[13]Puche JE,Lee YA,Jiao J,et al.A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice[J].Hepatology,2013,57 (1):339-350.
[14]Wolff JA,Budker V.The mechanism of naked DNA uptake and expression[J].Adv Genet,2005,54:3-20.
[15]Zhang G,Gao X,Song YK,et al.Hydroporation as the mechanism of hydrodynamic delivery[J].Gene Ther,2004,11(8):675-682.
[16]Azzam T,Domb AJ.Current developments in gene transfection agents[J].Curr Drug Deliv,2004,1(2):165 -193.
[17]Delzor A,Escartin C,Deglon N.Lentiviral vectors:a powerful tool to target astrocytes in vivo[J].Curr Drug Targets,2013,14(11):1336-1346.
[18]Clement S,Pascarella S,Conzelmann S,et al.The hepatitis C virus core protein indirectly induces alphasmooth muscle actin expression in hepatic stellate cells via interleukin-8[J].J Hepatol,2010,52(5):635-643.
[19]Guo CJ,Pan Q,Jiang B,et al.Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells[J].Apoptosis,2009,14(11):1331-1340.
[20]Xu L,Hui AY,Albanis E,et al.Human hepatic stellate cell lines,LX-1 and LX-2:new tools for analysis of hepatic fibrosis[J].Gut,2005,54(1):142-151.
[21]Guo J,Loke J,Zheng F,et al.Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of toll-like receptor 4 to hepatic stellate cell responses[J]. Hepatology,2009,49(3):960-968.
[22]Hernandez-Gea V,Ghiassi-Nejad Z,Rozenfeld R,et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues[J].Gastroenterology,2012,142(4):938-946.
[23]Brenner M,Kisseberth WC,Su Y,et al.GFAP promoter directs astrocyte-specific expression in transgenic mice [J].J Neurosci,1994,14(3 Pt 1):1030-1037.
[24]Besnard F,Brenner M,Nakatani Y,et al.Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein[J].J Biol Chem,1991,266(28):18877-18883.
[25]Zarrin AA,Malkin L,Fong I,et al.Comparison of CMV,RSV,SV40 viral and Vlambda 1 cellular promoters in B and T lymphoid and non-lymphoid cell lines[J].Biochim Biophys Acta,1999,1446(1-2):135-139.
[26]Cassiman D,Libbrecht L,Desmet V,et al.Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers[J].J Hepatol,2002,36(2):200-209.
[27]Kinnman N,Francoz C,Barbu V,et al.The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis [J].Lab Invest,2003,83(2):163-173.
[28]Tuchweber B,Desmouliere A,Bochaton-Piallat ML,et al. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat[J].Lab Invest,1996,74(1):265-278.
[29]Desmouliere A,Darby IA,Gabbiani G.Normal and pathologic soft tissue remodeling:role of the myofibroblast,with special emphasis on liver and kidney fibrosis[J].Lab Invest,2003,83(12):1689-1707.
Lentiviral vector containing GFAP promoter directs targeted expression of exogenous genes in hepatic stellate cells
ZHAO Yuan-yuan1,2▲, JIANG Yan1,2,3▲, WANG Hai-jian1,2△
(1Ministry of Education Key Laboratory of Contemporary Anthropology and State Key Laboratory of Genetic Engineering,School of Life Sciences,Fudan University,Shanghai 200438,China;2Institutes of Biomedical Sciences,Fudan University,Shanghai 200032,China;3Department of Chemistry,Fudan University,Shanghai 200433,China)
ObjectiveTo construct a lentivirus vector containing a luciferase gene driven by GFAP promoter,and to evaluate the specificity and efficiency of hepatic stellate cells(HSCs)targeted gene expression in vitro and in vivo.MethodsWe constructed lentiviral vectors driving the luciferase gene with the human GFAP promoter or CMV promoter,and then transfected these recombinant lentivirus particles into mouse(JS1)and human(LX-2)HSC lines and a human hepatocyte cell line (L-02).We also transferred the viral vectors by intravenous delivery into mice with CCl4-inducedliver fibrosis.We profiled the distribution of lentivirus expression in mice using in vivo imaging and Western blot.We then characterized the HSC-specific expression with double staining immunofluorescence.ResultsThe human GFAP promoter showed higher promoter activity compared with mouse GFAP promoter,and it could drive luciferase expression in HSCs but not in hepatocytes.In vivo imaging showed that lentivirus distributed in mouse epigastrium and hypogastrium between liver and kidney.The luciferase driven by the GFAP promoter in liver co-localized with HSC specific markers including GFAP,Desmin andα-Sma.ConclusionsA lentivirus vector containing GFAP promoter was constructed successfully,which can specifically target luciferase expression both in these cell lines and in HSCs of mice liver in vivo.
hepatic stellate cells; lentiviral vector; GFAP promoter; targeted gene expression;gene therapy method
Q 782
A
10.3969/j.issn.1672-8467.2015.05.016
2015-03-23;编辑:张秀峰)
国家科技重大专项(2012ZX10002-011),国家自然科学基金(81172093,81372526,31301050)
▲Co-first authors
△Corresponding author E-mail:haijianwang@fudan.edu.cn
*This work was supperted by the National Science and Technology Major Project(2012ZX10002-011)and National Natural Science Foundation of China(81172093,81372526,31301050).

