Laccase Immobilized on Mesoporous Silica Materials and Its Corrosion Inhibition Performance in Circulating Cooling Water System
Liu Fang; Lü Yucui; Zhong Huiyun; Zhang Shuang; Fan Fengtao; Zhao Chaocheng
(College of Chemical Engineering, China University of Petroleum, Qingdao 266580)
Laccase Immobilized on Mesoporous Silica Materials and Its Corrosion Inhibition Performance in Circulating Cooling Water System
Liu Fang; Lü Yucui; Zhong Huiyun; Zhang Shuang; Fan Fengtao; Zhao Chaocheng
(College of Chemical Engineering, China University of Petroleum, Qingdao 266580)
Mesoporous SiO2microspheres were synthesized using the sol-gel method and were characterized by TEM, FT-IR and BET techniques. The diameter of the microspheres is about 100—150 nm, and the average mesopore diameter is 2.55 nm, while the specific surface area is 1 088.9 m2/g. Mesoporous SiO2microspheres adsorb glutaraldehyde and immobilize laccase by means of the aldehyde group in glutaral which can react with the amidogen of laccase. The immobilization conditions were optimized at a glutaraldehyde concentration of 0.75%, a crosslinking time of 8 h, a laccase concentration of 0.04 L/L and an immobilization time of 10 h. When diesel leakage concentration was 80 mg/L, the highest corrosion inhibition efficiency of immobilized laccase reached 49.23%, which was slightly lower than the corrosion inhibition efficiency of free laccase (59%). The diesel degradation ratio could reach up to 45%. It has been proved that the immobilized laccase could degrade diesel to inhibit corrosion.
diesel leakage; circulating cooling water; mesoporous SiO2microspheres; laccase immobilization; corrosion inhibition
1 Introduction
Recently, at the petroleum refineries in China, the corrosion problem of heat exchanger pipeline in circulating cooling water system has become more and more serious especially in the presence of diesel leakage. The relation between diesel leakage and corrosion has been studied by Lu Xianhui, et al[1]. Plenty of petroleum hydrocarbons were detected in the circulating cooling water system because of the diesel spill, which has a great impact on the normal unit operation. Currently, significant scientific and technological measures have been taken to alleviate the effect of diesel on corrosion through adding novel inhibitors, decreasing the pollutants emission and displacing the system[2]. The latter can intensify the contradiction between water shortage and wastewater discharge. The enzyme corrosion inhibitor is a kind of novel and environmentally friendly agents[1]. Thanks to the catalyst property of enzymes, along with their high selectivity and specificity, high activity and moderate reaction conditions, they have received extensive attention in the field of wastewater treatment.
Laccases have been extensively studied in the last decades, since their wide specific requirement for the reducing substrates makes these enzymes particularly useful for a wide variety of industrial applications[3]. Laccases are cuproproteins belonging to a small group of named blue oxidase enzymes (Figure 1). Laccase is a phenol oxidase that can catalyze the oxidation of several aromatic and inorganic substances (particularly phenols) with the concomitant reduction of oxygen to water[4-5]. In general, the laccases exhibit four neighboring copper atoms, which are classified into three types: viz., the copper type I, the copper type II and the copper type III, which are differentiated by specific characteristic properties that allow them to play an important role in the catalytic mechanism of enzyme[6]. Laccase can oxidize many substrates: such as phenolic dyes[7-9], phenols[10-15], bisphenol A[16-17], benzopyrenes[18], and organophosphorus compounds[19]. Our previous research showed that laccase has good corrosion inhibition performance in circulating cooling water system[1].

Figure 1 Pictorial model of laccase (copper centers)
Practical use of enzymes (such as laccase) may be hampered by some peculiar properties of enzymatic proteins such as their non-reusability, high sensitivity to several denaturating agents and presence of adverse sensory or toxicological effects. Many of these undesirable constraints can be removed by using immobilized enzymes. This approach is proved to be more advantageous to catalysis than the use of free enzymes.
Several techniques are applied to immobilize enzymes on solid supports. They are mainly based on chemical and physical mechanisms[20]. Immobilization methods mainly include:① enzyme attachment to matrix by adsorption process, ② covalent bonds between enzyme and matrix, ③cross-linking by multifunctional bond between enzyme and matrix, and ④ entrapment of enzyme molecules within a porous hollow fiber.[21]
Owing to the small pore size and non-open pore hindrance, the immobilized enzymes usually show lower activity than free enzymes. Furthermore, the non-uniform pore sizes of most silica gel supports make biochemical processes less reproducible. Unlike the sol-gel silica, the mesoporous silica (MPS) materials provide tunable and uniform pore system, functionalizable surfaces, and restricted nanospaces for enzyme immobilization. MPSs have caused extensive concern ever since they were synthesized and characterized by Mobil researchers in 1992.[22]Recently, many research groups have immobilized enzymes on MPS which showed improvements in enzyme stability, catalytic activity, products specificity, and resistance to extreme environmental conditions[23-24]. In addition, confined enzymes in the nanospaces of MPS provide a good model to understand the enzyme action inside the cell.
In this paper, a simple and typical route of sol-gel method for synthesizing MPS spheres was presented and laccase immobilization was developed on the basis of MPSs. Effects of glutaraldehyde concentration, crosslinking time, laccase concentration and immobilization time on the relative activity were investigated. The properties of immobilized laccase such as pH-activity and temperatureactivity profiles were evaluated. The affinity changes, exhibited by kinetic parameters, were also tested. Finally, the corrosion inhibition efficiency and diesel removal ratio achieved by the immobilized laccase were investigated in a laboratory-scale circulating cooling water system contaminated by diesel leakage.
2 Materials and Methods
2.1 Materials
Chemicals used for synthesis of MPSs included tetraethyl orthosilicate (TEOS, >98%) used as the precursor, and cetyltrimethylammonium bromide (CTAB, 95%) used as the template. All solvents were purchased from the Sinopharm Chemical Reagent Co., Ltd. and were used without further purification. Laccase from white-rot fungi was purchased from the Pangtong Bio-tech Limited Liability Co. (Nanning, China). All other chemicals were of analytically pure grade and were used as received without further purification.
2.2 Preparation and characterization of mesoporous silica materials
The mesoporous silica materials were synthesized following the typical method based on the Stober process[25-26]. CTAB (1.5 g) was dissolved in a mixture containing distilled water (300 mL) and anhydrous ethanol (75 mL) and aqueous ammonia (13 mL) in a 500 mL polypropylene reactor. After adding TEOS (5 mL), the mixture was stirred for 2 h. After 12 h, the resulting white powder was filtered off and washed with 50 mL of distilled water and 50 mL of ethanol. After being dried at 90 ℃ for 10 h, the sample was subject to grinding. The resulting solid was heated to 550 ℃ for 5 h by applying a heating rate of 1 ℃/min.
Nitrogen sorption isotherms were obtained using a Quanta-chrome Nova 4000e instrument at -196 ℃ to determine the BET-surface area, pore volume and pore size. Transmission electron micrographs (TEM) were taken using a JEOL 2100 electron microscope operating at 200 kV. Thesamples for TEM analysis were prepared by suspending a small amount of solid in acetone using sonication in an ultrasonication water bath for 5 min. A drop of this suspension was then dispersed onto a holey carbon film on a copper grid, followed by drying at room temperature. The infrared spectra (IR) of samples were analyzed using a NICOLET impact 410 spectrometer.
2.3 Immobilization of laccase on mesoporous silica particles
The immobilization experiment was carried out by adding 0.15 g of mesoporous silica particles into 10 mL of glutaraldehyde solution. The mixture was shaken at a rate of 100 r/min for a certain time at room temperature. Then, the glutaraldehyde activated composite particles were subject to washing sequentially with distilled water, acetic acid solution (0.1 mol/L, 100 mL) and acetate buffer (0.1 mol/L, pH=5.5). The resulting particles were dried at 30 ℃.
The glutaraldehyde activated particles (about 0.1 g) were transferred into a sodium succinate buffer (pH= 4.5) containing laccase. The immobilization reaction was carried out at room temperature for 2—12 h. After this reaction period, the laccase-immobilized composite particles were transferred in the EDA solution (5.0 mg/mL) to block the remaining free reactive aldehyde groups.
The effects of glutaraldehyde concentration, glutaraldehyde crosslinking time, laccase concentration and immobilization time on the laccase activity were studied.
The range of glutaraldehyde concentration covered 0.05%, 0.1%, 0.25%, 0.5%, 0.75%, 1% and 1.5%. The range of crosslinking time was 2 h, 4 h, 6 h, 8 h, 12 h and 16 h, respectively. The range of laccase concentration was 0.015, 0.02, 0.025, 0.03, 0.035, 0.04, 0.045, 0.05 and 0.075 L/L, respectively. The range of immobilization time covered 2 h, 4 h, 6 h, 8 h, 10 h and 12 h.
2.4 Laccase activity assay
The activity of free and immobilized laccase was determined spectrophotometrically at 420 nm with 0.5 mmol/L of ABTS as substrate in a 0.1 M sodium citrate buffer (pH 4.0) at 25 ℃[27]. The oxidation of substrate to ABTS+was measured for 5 min using a UV–vis spectrophotometer (UV-2250, Shimadzu Corporation, Japan) (ε=36×10-3M-1cm-1). One unit (U) of laccase activity was defined as the amount of enzyme needed to oxidize 1 μmol of ABTS per minute[23]. In this work, the laccase activity was expressed in terms of the relative activity, which was calculated from the following formula (percentage of the maximum activity obtained):

where R is the relative activity, Aiis the activity of immobilized laccase, and Amaxis the maximum value of Ai.

Figure 2 Schematic illustration of laccase immobilization process
2.5 Laccase reaction dynamics
The Michaelis–Menten kinetic parameters Kmand Vmaxof the free laccase and the immobilized laccase, respectively, were determined by measuring the laccase activity using ABTS as the substrate under different substrate concentrations ranging from 2 to 12 mmol/L. The values of the kinetic parameters were obtained by a non-linear curve of the plot of reaction rate versus the substrate concentration based on the following equation:

where v is the reaction rate, [S] is the substrate concentration, and Vmaxrepresents the maximum rate achieved by the enzyme-catalytic system. The Michaelis constant Kmis the substrate concentration at which the reaction rate is half of Vmaxand is often used as an indication of the enzyme affinity to the substrate.
2.6 Thermal stability and pH stability of free and immobilized laccase
The laccase was immobilized according to the optimal immobilization conditions obtained in the precedingstage. Thermal stability of the free and immobilized laccase was determined by the residual activity of the free and immobilized laccase that had been exposed to six different temperatures (50, 60, 70, 80, 90 and 100 ℃) for 60 min. Then the residual activity of the free and immobilized laccase exposed to 60 ℃ at different time duration was also determined. The residual activity was defined as the fraction of the total activity recovered after the immobilization of laccase on the composite particles compared with the same quantity of free enzyme. A laccase sample was removed from each incubation solution with a subsequent time interval of 30 min and assayed for enzymatic activity. The results were converted to relative activity (percentage of the maximum activity obtained).
The pH stability of the free and immobilized laccase was determined by the residual activity of the free and immobilized laccase that had been exposed to six different pH values (3, 4, 5, 6, 7 and 8) in the sodium succinate buffer (50 mmol/L, pH 4.5) for 60 min.
2.7 Corrosion inhibition and diesel degradation by immobilized laccase
In previous study, it was found that diesel at different concentrations could affect the corrosion rate and the corrosion rate reached a maximum value with the diesel concentration reaching 80 mg/L in the cooling water[1]. In this study the corrosion inhibition performance of the immobilized laccase was investigated at a diesel concentration of 80 mg/L. The corrosion inhibition performance was indicated by the corrosion rate and inhibition efficiency determined according to the rotating coupon corrosion method. A certain concentration of immobilized laccase was added to the cooling water and the corrosion test was carried out according to the method described below. The immobilized laccase concentration ranged from 0.05 g/L to 0.8 g/L. In addition, the diesel degradation ratio at different immobilized laccase concentrations was also studied. The degradation ratio was calculated from the change of diesel concentration before and after adding the immobilized laccase to the cooling water.
2.8 Methods for analysis of circulating cooling water
The turbidity, pH value, calcium ion, magnesium ion, chloride ion, sulfate ion, total iron, total hardness, total alkalinity and petroleum hydrocarbons in circulating cooling water sample were analyzed according to the methods referred to in the reference[28]and the analysis results of circulating cooling water before adding diesel are presented in Table 1. The cooling water quality characteristics were analyzed in a previous research[29].

Table 1 Water quality analysis method and results of circulating cooling water quality
2.9 Corrosion inhibition performance test
The rotating coupon corrosion test device was used for the corrosion inhibition tests. Weight loss measurements were carried out for determining the corrosion inhibition efficiency. Standard carbon steel coupons (50 mm×25 mm× 2 mm) were used in experiments. Prior to the measurement, the coupons were abraded with emery paper, degreased ultrasonically in ethanol and acetone, and dried at room temperature.
The inhibitors were added into the laboratory circulating cooling water. The coupons were immersed in the inhibitorcontained solution for 72 h at 40 ℃ under a rotary speed of 80 rpm. After immersion, the corroded coupons were removed from the solution. The corrosion product layers were scraped off from the coupon surface by a surgical blade, and the residual corrosion products on coupon were then immersed in a mixed solution for 5 min to 10 min. The mixed solution was composed of 500 mL of hydrochloric acid, 20 g of hexamethylenetetramine and 1 L of distilled water. After the corrosion products were com-pletely removed, the coupons were rinsed with distilled water, dried, and then weighed. A blank test without inhibitors was also carried out. The coupons were weighed using an analytical balance with a precision of 0.000 1 g. The weight loss was calculated using the coupon weight obtained before and after the corrosion inhibition tests. The weight loss measurements were normally repeated at least three times to obtain a final average value. The corrosion rate X1and the corrosion inhibition efficiency X2were calculated by using the following equations:
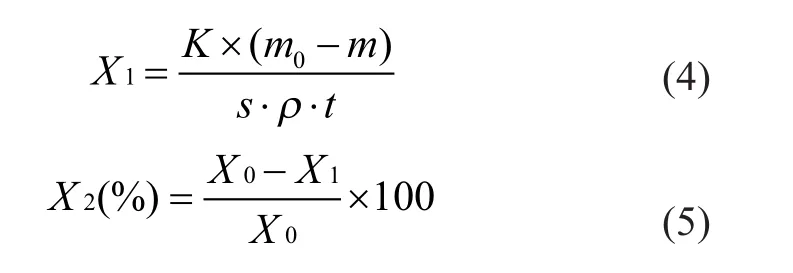
In Equations (4) and (5), m0is the initial weight of coupon (g); m is the coupon weight after removing the corrosion products in the cleaning test (g); s is the coupon surface area (cm2); ρ is the coupon density (g/cm3); t is the test time (h); K is a constant with its value equating to 87600; and X0is the corrosion rate in blank test without inhibitors (mm/a).
3 Results and Discussion
3.1 Characterization of mesoporous silica particles
It can be seen from the TEM image of mesoporous silica particles (Figure 3) that a spherical-like shape with an average size of approximately 100—150 nm was observed for the composite particles. Furthermore, large domains of ordered mesoporous structures are clearly noticed from these images.
The Brunauer-Emmett-Teller (BET) and the Barrett-Joyner-Halenda (BJH) analysis results of mesoporous silica particles are shown in Figure 4. The isotherm curve (Figure 4) displays a typical IV adsorption isotherm according to BDDT classification[30]. The volume adsorbed rose sharply with a P/P0ratio being in the range of 0.2—0.3. This feature was attributed to the capillary condensation of N2molecules in mesoporous channels of particles at low temperature. The hysteresis loops in the isotherm curve indicate that the phenomena of capillary condensation and capillary evaporation occurred not under the same pressure. This might be ascribed to the thermodynamic equilibrium of N2adsorption and desorption and the shapes of mesoporous channels[31]. The BET surface area was 1 088.9 m2/g. The pore size distribution calculated from desorption branches (inset of Figure 4) showed one pore system centered at 2—3 nm with a mean pore diameter of 2.55 nm. The pore size distribution showed a single peak shape and the half peak width was narrow, indicating to an uniform pore size.

Figure 3 TEM image of mesoporous SiO2microspheres of the sample

Figure 4 Nitrogen adsorption-desorption isotherms of mesoporous silica particles and corresponding pore size distribution calculated from the desorption branches

Figure 5 FTIR spectrum of mesoporous silica particles
The FT-IR spectrum of mesoporous silica particles is presented in Figure 5. The band at 3 415 cm-1is assignedto the Si-OH stretching vibrations and OH stretching vibrations, representing the water in the silica particle channels. The peak at 1 639 cm-1is attributed to H2O adsorbed in mesopores. The bands at 1 087 cm-1, 800 cm-1and 464 cm-1belong to the asymmetric stretching vibration, bending vibration and stretching vibration of Si-O-Si bonds, respectively.
3.2 Laccase immobilization
Some factors (such as glutaraldehyde concentration, glutaraldehyde crosslinking time, laccase concentration and immobilization time) all can affect the activity of laccase immobilization. So, these factors were investigated in order to optimize the immobilization process.
3.2.1 Effect of glutaraldehyde concentration
0.15 g of mesoporous silica particles were activated with 10 mL of glutaraldehyde solution. The glutaraldehyde concentration of 0.05 vol%, 0.1 vol%, 0.25 vol%, 0.5 vol%, 0.75 vol%, 1.0 vol% and 1.5 vol% was used, respectively. Then the glutaraldehyde activated particles (about 0.1 g) were transferred into a laccase-containing sodium succinate buffer (20 mL, 0.075 vol%) for immobilization. Figure 6(a) shows the effect of glutaraldehyde concentration on the relative activity of immobilized laccase. The relative activity of immobilized laccase at first increased and then declined with an increasing glutaraldehyde concentration. A maximum relative activity reached 100% at a glutaraldehyde concentration of 0.75 vol%. When the glutaraldehyde concentration was lower than 0.75 vol%, the amount of glutaraldehyde that could react with mesoporous silica particles was small, resulting in a low concentration of laccase immobilized on the mesoporous silica particles. This occurred because covalent bonds were formed between the amino groups of the enzyme and the aldehyde groups of the glutaraldehyde. So a higher glutaraldehyde concentration could lead to higher relative activity of immobilized laccase to some extent. However, excess glutaraldehyde could react with the laccase and cause the alkylation of amino groups, which would weaken the relative activity of immobilized laccase[32]. Furthermore, excess glutaraldehyde could lead to intramolecular crosslinking, which would change the spa-tial configuration and active sites of protein molecules[33]. Therefore, the glutaraldehyde concentration was specified at 0.75 vol% in the following research.
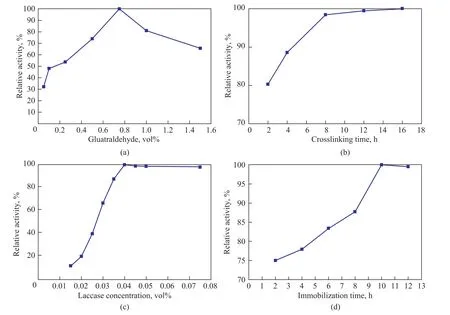
Figure 6 Effect of glutaraldehyde concentration (a), glutaraldehyde crosslinking time (b), laccase concentration (c), immobilization time (d) on relative activity of immobilized laccase
3.2.2 Effect of glutaraldehyde crosslinking time
Figure 6 (b) shows the relative activity of immobilized laccase at a crosslinking time of 2 h, 4h, 6 h, 8h, 12 h and 16 h, respectively. When the crosslinking time was 2 h, the relative activity reached above 80%. Then the relative activity increased with an increasing crosslinking time until a stable value was obtained. The relative activity could rise to 99.3%, when the crosslinking time reached 8 h. With the crosslinking time going by, the enzyme was firmly bound to the supports. As a result, the activity of enzyme also increased. Excess crosslinking time might result in the denaturation of enzyme[34]. So a crosslinking time of 8 h was selected as the optimal value.
3.2.3 Effect of laccase concentration
Figure 6(c) shows the relative activity of immobilized laccase at different laccase concentrations. The relative activity at first increased and then slightly decreased with an increasing laccase concentration. A maximum value was obtained at a laccase concentration of 0.04 vol%. The high laccase concentration could increase the relative activity of immobilized laccase to some extent; however, an excess laccase concentration would lead to the congestion of laccase molecules on the mesoporous silica particles, which could result in the decrease of laccase active sites. Thus the resulting steric constraints could subsequently restrain the dispersion and transmission of the substrate and product, which would be exhibited as the reduction of activity[27]. When the laccase concentration rose to 0.075 vol%, its relative activity fell to 98.3%. Wang, et al. also showed that a similar phenomenon was identified during his study of laccase immobilization on magnetic mesoporous silica nanoparticles as a function of laccase concentration[35].
3.2.4 Effect of immobilization time
The relative activity of the immobilized laccase showed a continued increase and then reached a stable level, when the immobilization time (Figure 6(d)) was specified at 2 h, 4 h, 6 h, 8 h, 10 h and 12 h, respectively. A maximum relative activity of the immobilized laccase was obtained at an immobilization time of 10 h.
3.3 Properties of immobilized laccase
The results in the preceding stage showed that the optimal immobilization conditions covered a glutaraldehyde concentration of 0.75 vol%, a glutaraldehyde crosslinking time of 8 h, a laccase concentration of 0.04 vol% and an immobilization time of 10 h. The activity and stability of the immobilized laccase under the optimal conditions were investigated.
3.3.1 Effect of temperature on the activity of immobilized laccase
Figure 7 shows the change in relative activity of the immobilized laccase with an increasing temperature. In comparison with the free laccase, the immobilized laccase exhibited a signi ficantly broader temperature profile. The immobilized laccase exhibited a maximal activity at 40 ℃, which was higher than that value at 30 ℃ for the free laccase. The improvement in resistance of the immobilized laccase against temperature was probably ascribed to the reduction in molecular mobility and conformational changes by reaction with glutaraldehyde of the supports[36].
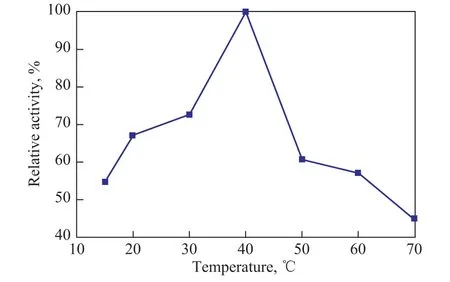
Figure 7 Effect of temperature on the activity of immobilized laccase
3.3.2 Effect of pH value on the activity of immobilized laccase
Figure 8 shows the effect of pH value on the activity of immobilized laccase when the pH value varied from 3.0 to 8.0. The immobilized laccase exhibited a maximal activity at a pH value of 4.0. Besides, the optimal pH value of free laccase was 3.0. This shift of optimal pH value was also reported with regard to the laccase that was immobilized on the magnetic bimodal mesoporouscarbon and on the poly(GMA-MMA-g-4-VP)-Cu(II) beads[27,37]. The reason of the shift was attributed to the influence of carrier microenvironment on the electrostatic interaction. The isoelectric point of silica supporter was 2.5, so the supporter carried negative electric charge under the experimental condition when the pH value was ≥ 3. This pH shift might be caused by the attraction of H+ions by silica supporter that provided negative electric charge, leading to lower pH value in the enzyme microenvironment than that of bulk solution. Thus, the pH value of the outside solution must shift toward the alkaline condition in order to counteract the effect of microenvironment. As a result, the optimal pH value of the immobilized laccase was higher than that of free laccase.

Figure 8 Effect of pH value on the activity of immobilized laccase

Figure 9 Thermal stability of immobilized and free laccase●—Immobilized laccase; ■—Free laccase
3.3.3 Thermal stability and pH stability of immobilized laccase and free laccase
Relative activity of both the free and immobilized laccase as a function of temperature is compared in Figure 9(a). The relative activity of the free and immobilized laccase at 60 ℃ is shown in Figure 9(b). It can be observed that the activity of two enzyme samples was strongly dependent on temperature. Figure 9(a) shows that the activity of both the immobilized and free enzyme decreased with an increasing temperature. But the activity of the immobilized laccase was higher than that of free laccase at the same temperature. According to the activity comparison between the free and the immobilized laccase as a function of incubation time at 60 ℃ (Figure 9(b)), the immobilized laccase exhibited a much slower inactivation rate than the free laccase. The free laccase only retained 1.8% of its initial activity after 3.5 h, while the immobilized laccase still retained 14% of its initial activity after 24 h. The stable form of the immobilized laccase suggests that a suitable environment was provided by the carrier to maintain enzymatic activity against heat, which could be attributed to the mesoporous channels of supports and the binding interaction between the laccase and carrier to keep laccase from injury caused by direct exposure to environmental changes[38].
Effects of pH value on the activity of the free and immobilized laccase were investigated at different pH values ranging from 3 to 8 (Figure 10). The free and immobilized laccase exhibited a maximum activity at pH 4.5 and pH 4, respectively. This shift of optimum pH value was attributed to the electrostatic interaction influenced by the microenvironment of mesoporous silica particles. The immobilized laccase showed broader pH-activity profile than the free laccase, and higher activity values of the immobilized laccase were obtained from pH 3 to pH 8, indicating that immobilization preserved the enzyme activity in a broader pH profile[39].

Figure 10 The stability of immobilized and free laccase at different pH bu0ffer●—Immobilized laccase; ■—Free laccase
3.3.4 Kinetic parameters of the free and immobilization laccase
Kinetic parameters of the immobilized and free laccase, i.e. Kmand Vmax, were estimated from the Lineweaver–Burk plot using ABTS as the substrate. The immobilized laccases on mesoporous silica particles exhibited a higher Kmvalue than that of the free laccase. The obtained Kmand Vmaxvalues were equal to 0.372 mmol/L and 0.001 6 mmol/min for the free enzyme and 0.902 mmol/L and 0.001 4 mmol/min for the immobilized laccase, respectively (Figure 11). This change of Kmvalue indicated to less affinity for the substrate of the immobilized enzyme than that of the free enzyme, which might be caused by the steric hindrance and diffusion limits, resulting in lower accessibility to the active sites of the immobilized laccase due to the conformational changes of the laccase from adsorption into the mesoporous channel of the support[40].

Figure 11 Double reciprocal plot of 1/V0vs. 1/[S] for the immobilized and free laccase involved reactions●—Immobilized laccase; ■—Free laccase
The Michaelis constant in this system, Km, increased about 2.4 times for the immobilized laccase as compared to the free enzyme. The Kmvalue increased 1.8 times by using phenol as the substrate for the kinetic study in the research on immobilization of laccase on polypropylene membrane by covalent bonding[41]. Bayramoglue, et al.[39]reported that the Kmvalues increased 2.3 times after laccase was immobilized onto p(HEMA-g-GMA) film. These reports were in a good agreement with the results from our work.
3.4 The corrosion inhibition effect and the diesel degradation performance of immobilized laccase
The immobilized laccase was added in several beakers with 1 L of circulating water polluted with 80 mg/L of diesel. The range of immobilized laccase concentration was 0.05 g/L, 0.1 g/L, 0.2 g/L, 0.3 g/L, 0.5 g/L, and 0.8 g/L, respectively. Through corrosion tests, the corrosion inhibition efficiency and the corrosion inhibition rate of the immobilized laccase changed as shown by Figure 12.

Figure 12 The corrosion rate of carbon steel and corrosion inhibition efficiency at different immobilized laccase concentrations●—Corrosion rate; ■—Corrosion inhibition rate
With the increase of immobilized laccase concentration, the corrosion rate on the coupon dropped obviously, and correspondingly, the corrosion inhibition rate increased gradually. The corrosion inhibition efficiency was 45.08% when the concentration of laccase was 0.5 g/L. The corrosion inhibition efficiency reached 49.23% when the concentration of laccase increased to 0.8 g/L. The laccase itself had no corrosion inhibition property, the effect of this phenomenon depended on the diesel degradationperformance of the immobilized laccase. Then the diesel degradation rate was investigated.
A different concentration of immobilized laccase was added to the circulating cooling water polluted by diesel leakage. The range of immobilized laccase concentration covered 0, 50, 100, 200, 300, 500, and 800 mg/L. Figure 13 shows the results obtained from the experiments, and, with an increasing immobilized laccase concentration, the diesel degradation ratio increased at first and then became stable. When the immobilized laccase concentration reached 0.8 g/L, the diesel degradation ratio was equal to 45%. The diesel degradation effect of laccase was significant because laccase could degrade phenols, aromatic amines, carboxylic acids and their derivatives through catalytic oxidation. The mechanism of catalytic oxidation of laccase includes two processes involving the electron transfer of Cu2+species[42]. Firstly, the substrate is transformed into the intermediate with free radicals. The reductant in diesel provides electrons to the type I Cu2+species and can be oxidized into free radicals to perform the non-enzyme-reaction, such as disproportionation, or hydroxylation. Secondly, with the presence of oxygen, a reduced laccase is oxidized and oxygen is reduced to water. Decrease in the degradation ratio of diesel after addition of 0.5 g/L of the immobilized laccase to the circulating water could be attributed to the decline of degradation of substrate (eg. phenols), which might occur probably due to the accumulation of the catalytic reaction products, causing inhibition of the catalytic process, as reported by previous studies[37].

Figure 13 The diesel degradation rate versus immobilized laccase concentration
4 Conclusions
1) Mesoporous SiO2microspheres were synthesized by using the sol-gel method. The diameter of the microspheres was about 100—150 nm, and the average mesopore diameter was 2.55 nm, while the specific surface area was equal to 1 088.9 m2/g.
2) The conditions of immobilization were optimized at a glutaraldehyde concentration of 0.75% and a reaction time of 8 h, while laccase at a concentration of 0.04 mL/mL was subject to immobilization for 10 h.
3) Compared with the free laccase, the optimal temperature for the immobilized laccase was 40 ℃, and the optimal pH value for the immobilized laccase was 4. With an increasing temperature, the activity of immobilized laccase decreased more slowly than the case with the free laccase. After being kept at 60 ℃ for 3.5 h, the relative activity of free laccase decreased to 1.8% of its initial value, while after being kept at that temperature for 24 h, the immobilized laccase still retained 14.0% of its initial activity. The immobilized laccase was less affected by changes in pH value as compared to the free laccase. Kmof the immobilized laccase became higher obviously than that of the free laccase, indicating to a decreased affinity of immobilized laccase.
4) When the immobilized laccase concentration was 0.8 g/L in the circulated cooling water polluted by 80 mg/L of diesel leakage, the diesel degradation ratio could reach 45% and the corrosion inhibition efficiency was equal to 49.23 %.
Acknowledgements:The project is supported by the Foundation for Top Talents Program of China University of Petroleum.
[1] Lu X H, Liu F, Lu J J, et al. The biological enzyme corrosion inhibitors in the circulating water system with diesel leak[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(6): 1090-1095 (in Chinese)
[2] Zhong H Y, Liu F, Lu J, et al. Effect of diesel leakage in circulating cooling water system on preponderant bacteria diversity and bactericidal effect of biocides[J]. Environmental Technology, 2014, 36(9): 1147-1159
[3] Rodríguez C S, Toca Herrera J L. Industrial and biotechnological applications of laccases: A review[J]. Biotechnology Advances, 2006, 24(5): 500-513
[4] Durán N, Esposito E. Potential applications of oxidativeenzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review[J]. Applied Catalysis B: Environmental, 2000, 28(2): 83-99
[5] Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition[J]. Biochemistry, 1996, 35(23): 7608-7614
[6] McGuirl M A, Dooley D M. Copper-containing oxidases[J]. Current Opinion in Chemical Biology, 1999, 3(2): 138-144
[7] Bibi I, Bhatti H N, Asgher M. Comparative study of natural and synthetic phenolic compounds as efficient laccase mediators for the transformation of cationic dye[J]. Biochemical Engineering Journal, 2011, 56(3): 225-231
[8] Taha A A, Shwaish I I, Mohammed A H, et al. Production of a laccase from Botrytis cinerea (DSMZ 877) and application for textile phenolic dye decolorization[J]. Energy Procedia, 2013, 36: 862-871
[9] Nazari M, Kashanian S, Rafipour R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 145: 130-138
[10] Rangelov S, Nicell J A. A model of the transient kinetics of laccase-catalyzed oxidation of phenol at micromolar concentrations[J]. Biochemical Engineering Journal, 2015, 99: 1-15
[11] Tavares A P M, Pinho B, Rodriguez O, et al. Biocatalysis in Ionic Liquid: Degradation of Phenol by Laccase[J]. Procedia Engineering, 2012, 42: 226-230
[12] Lante A, Crapisi A, Krastanov A, et al. Biodegradation of phenols by laccase immobilised in a membrane reactor[J]. Process Biochemistry, 2000, 36(1/2): 51-58
[13] Kurniawati S, Nicell J A. Efficacy of mediators for enhancing the laccase-catalyzed oxidation of aqueous phenol[J]. Enzyme and Microbial Technology, 2007, 41(3): 353-361
[14] Wang F, Hu Y, Guo C, et al. Enhanced phenol degradation in coking wastewater by immobilized laccase on magnetic mesoporous silica nanoparticles in a magnetically stabilized fluidized bed[J]. Bioresource Technology, 2012, 110: 120-124
[15] Ko C-H, Chen S-S. Enhanced removal of three phenols by laccase polymerization with MF/UF membranes[J]. Bioresource Technology, 2008, 99(7): 2293-2298
[16] Ivanec-Goranina R, Kulys J, Bachmatova I, et al. Laccasecatalyzed bisphenol A oxidation in the presence of 10-propyl sulfonic acid phenoxazine[J]. Journal of Environmental Sciences, 2015, 30: 135-139
[17] Kim Y-J, Nicell J A. Laccase-catalyzed oxidation of bisphenol A with the aid of additives[J]. Process Biochemistry, 2006, 41(5): 1029-1037
[18] Rama R, Mougin C, Boyer F-D, et al. Biotransformation of bezo[a]pyrene in bench scale reactor using laccase of Pycnoporus cinnabarinus[J]. Biotechnology Letters, 1998, 20(12): 1101-1104
[19] Trovaslet-Leroy M, Jolivalt C, Froment M-T, et al. Application of laccase-mediator system (LMS) for the degradation of organophosphorus compounds[J]. Chemico-Biological Interactions, 2010, 187(1/3): 393-396
[20] OR Z. Immobilized Enzymes[M]. OH, USA: CRC Press, 1974.
[21] Durán N, Rosa M A, D’Annibale A, et al. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review[J]. Enzyme and Microbial Technology, 2002, 31(7): 907-931
[22] Lee C-H, Lin T-S, Mou C-Y. Mesoporous materials for encapsulating enzymes[J]. Nano Today, 2009, 4(2): 165-179
[23] Gascón V, Márquez-Álvarez C, Blanco R M. Efficient retention of laccase by non-covalent immobilization on amino-functionalized ordered mesoporous silica[J]. Applied Catalysis A: General, 2014, 482: 116-126
[24] Fried D I, Schlossbauer A, Bein T. Immobilizing glycopyranose on mesoporous silica via “click-chemistry” for borate adsorption[J]. Microporous and Mesoporous Materials, 2012, 147(1): 5-9
[25] Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range[J]. Journal of Colloid and Interface Science, 1968, 26(1): 62-69
[26] Fang X S. Preparation of mesoporous silica microsphere[J]. Journal of Changchun University of Science and Technology (Natural Science Edition), 2010, 33(2): 90-93 (in Chinese)
[27] Liu Y, Zeng Z, Zeng G, et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds[J]. Bioresource Technology, 2012, 115: 21-26
[28] EPA of China. Analysis Methods for Water and Waste Water[M]. 4th Ed. Beijing: Press of Chinese Environmental Science, 2002
[29] Liu F, Lu X H, Yang W, et al. Optimization of inhibitors compounding and applied conditions in simulated circulating cooling water system[J]. Desalination, 2013, 313: 18-27
[30] Kruk M, Jaroniec M. Gas adsorption characterization of ordered organic−inorganic nanocomposite materials[J]. Chemistry of Materials, 2001, 13(10): 3169-3183
[31] Kruk M, Jaroniec M, Sayari A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements[J]. Langmuir, 1997, 13(23): 6267-6273
[32] Li T Q. Glutaraldehyde[J]. Chemical Reagents, 1988, 10(5): 303-308 (in Chinese)
[33] Yang X F, Chen M. Study on immobilized β-D-fructoranosidase by cross-linking with glutaraldehyde[J]. Soybean Science, 2009, 28(5): 902-905
[34] Guimin L. Enzyme Project[M]. Beijing: Chemical Industry Press, 2003
[35] Wang F, Guo C, Yang L, et al. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance[J]. Bioresource Technology, 2010, 101(23): 8931-8935
[36] Celikbicak O, Bayramoglu G, Yılmaz M, et al. Immobilization of laccase on hairy polymer grafted zeolite particles: Degradation of a model dye and product analysis with MALDI–ToF-MS[J]. Microporous and Mesoporous Materials, 2014, 199: 57-65
[37] Bayramoğlu G, Yilmaz M, Yakup Arica M. Reversible immobilization of laccase to poly(4-vinylpyridine) grafted and Cu(II) chelated magnetic beads: Biodegradation of reactive dyes[J]. Bioresource Technology, 2010, 101(17): 6615-6621
[38] Zhu Y, Kaskel S, Shi J, et al. Immobilization of Trametes versicolor Laccase on Magnetically Separable Mesoporous Silica Spheres[J]. Chemistry of Materials, 2007, 19(26): 6408-6413
[39] Bayramoğlu G, Yakup Arıca M. Immobilization of laccase onto poly(glycidylmethacrylate) brush grafted poly(hydroxyethylmethacrylate) films: Enzymatic oxidation of phenolic compounds[J]. Materials Science and Engineering: C, 2009, 29(6): 1990-1997
[40] Sarı M, Akgöl S, Karataş M, et al. Reversible Immobilization of Catalase by Metal Chelate Affinity Interaction on Magnetic Beads[J]. Industrial & Engineering Chemistry Research, 2006, 45(9): 3036-3043
[41] Georgieva S, Godjevargova T, Portaccio M, et al. Advantages in using non-isothermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera[J]. Journal of Molecular Catalysis B: Enzymatic, 2008, 55(3/4): 177-184
[42] Liu F, Zhang L, Yan X, et al. Effect of diesel on corrosion inhibitors and application of bio-enzyme corrosion inhibitors in the laboratory cooling water system[J]. Corrosion Science, 2015,93(0):293-300
Multiple Confined-Zone-Based Nickel Hydrogenation Catalyst Made by Shanxi Institute of Coal Chemistry
The Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences has designed and prepared a multiple confined-zone-based nickel hydrogenation catalyst by means of the atomic layer deposition (ALD) technology. In comparison with the non-confined-zone based catalyst, the multiple confined-zone-based nickel catalyst possesses an enhanced catalytic reactivity and catalytic stability for hydrogenation of cinnamaldehyde and nitrobenzene.
The research team by means of the ALD technology realizes at first the deposition of NiO nanoparticles on the surface of template (carbon nanospiral or carbon nanotube), followed by deposition of Al2O3nanofilm. The deposited nano-sized materials are then subject to calcination and reduction treatment to become the alumina nanotube-coated multiple confined-zone-based Ni catalyst. This preparation method is versatile and can be used to synthesize other systems of confined-zone-based catalysts to provide an important scientific reference for the design of future high-efficiency nanocatalysts.
date: 2015-06-07; Accepted date: 2015-08-05.
Prof. Liu Fang, Telephone: +86 53286984668; E-mail: liufangfw@163.com.
- 中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol

