Development of Light Cycle Oil (LCO) Hydrocracking Technology over a Commercial W-Ni Based Catalyst
Peng Chong; Yang Xuejing; Fang Xiangchen; Huang Xinlu; Cheng Zhenmin; Zeng Ronghui; Guo Rong
(1. State Key Laboratory of Chemical Engineering, East China University of Science and Technology (ECUST), Shanghai 200237; 2. Fushun Research Institute of Petroleum and Petrochemicals (FRIPP), Sinopec, Fushun 113001)
Development of Light Cycle Oil (LCO) Hydrocracking Technology over a Commercial W-Ni Based Catalyst
Peng Chong1,2; Yang Xuejing1; Fang Xiangchen1,2; Huang Xinlu2; Cheng Zhenmin1; Zeng Ronghui2; Guo Rong2
(1. State Key Laboratory of Chemical Engineering, East China University of Science and Technology (ECUST), Shanghai 200237; 2. Fushun Research Institute of Petroleum and Petrochemicals (FRIPP), Sinopec, Fushun 113001)
Because of its high density and low cetane number, the light cycle oil (LCO) containing heavy aromatics (60%—80%) can hardly be transformed through the conventional hydro-upgrading technology. In this report, a novel LCO hydrocracking technology (FD2G) was proposed for the utilization of LCO to manufacture high value-added products. Through the ingenious combination of hydroprocessing catalyst and the hydrocracking process, the high octane gasoline and the ultra-low sulfur diesel (ULSD) blendstocks were produced simultaneously. The influence of catalyst type, reaction temperature, pressure, respectively, on the research octane number (RON) of produced gasoline was studied in a fixed bed hydrogenation reactor. It indicated that high reaction temperature and medium pressure would favor the production of highoctane gasoline through the conversion of bi-aromatic and tri-aromatic hydrocarbons. The typical results of FD2G technology on commercial units showed that it could produce clean diesel with a sulfur content of less than 10 μg/g and clean gasoline with a research octane number (RON) of up to 92. It would be contributed to the achievement of the maximum profit of a refinery, the FD2G technology could provide a higher economic efficiency than the other diesel quality upgrading technology under the current gasoline and diesel price system.
LCO; hydrocracking; high octane gasoline; ULSD; aromatics
1 Introduction
Generally, LCO is a secondary stream obtained from the catalytic cracking unit (FCC), having a boiling point similar to that of diesel. Its production is increasing in step with the predicted role of the FCC unit for meeting the increasing demand for light olefins and the increasing trend of feeding heavy feedstocks into the FCC unit[1]. However, due to its high content of aromatics, high specific gravity, high sulfur and nitrogen contents as well as low cetane rating[2], LCO is a poor diesel fuel blending component. For instance, the aromatics content of LCO delivered from FCC units varies between 75% and 90%. The relatively high sulfur and nitrogen contents can go up to 1.5 wt% and 750 μg/g, respectively. Typically, its cetane index of LCO ranges from 15 to 25, whereas it can be 40—60 for the straight run distillates produced from the same crude[3]as summarized in Figure 1. In China, LCO amounts to over 33% of the distillate pool and therefore besides desulfurization, the dearomatization process is also needed to transform this stream into a feasible blending component of refinery automotive diesel pool[4]. The disposalof LCO is one of the major concerns to the refiners.

Figure 1 Typical LCO properties and disposition
LCO was reported to be treated either by catalytic cracking[5-6]or hydrogenation[7-21]. The hydrocracking of LCO is obviously of great interest in a bid to maximize the economic profit of a refinery and upgrade the utilization efficiency of the crude oil resource. In recent years, LCO hydrocracking has been studied using model compounds[22], light fractions of LCO[23]and the LCO blended with other refinery streams[24]. Noble metals, such as Pt, Pd and Pt-Pd alloy are reported to be more active for this process. It is revealed that the acidity of the support is of curial importance as well. Zeolite materials with medium acidity showed high conversion, including Hβ, HY and HZSM-5[25]. The effects of pressure, temperature, space velocity on LCO hydrocracking[26-30]were also studied. Nevertheless, these noble metal catalysts are of low stability and are liable to fast deactivation during the hydrocracking of feeds with heteroatomic aromatics containing S and N. Accordingly, these feeds require pretreatment steps (hydrodesulfurization, hydrodenitrogenation, and hydrodemetallization) prior to hydrocracking. Hydrotreating, aromatics saturation, and mild hydrocracking as well as selective ring opening are primary elemental processes for treating LCO. However, aiming to reduce the excessive sulfur compounds, the harsh operating conditions have to be applied. Meanwhile, the aromatic ring is usually over-saturated, which leads to the undesirable decrease of the cetane number as well as the waste of hydrogen resource. The UOP Company has developed a new LCO UnicrackingTMtechnology, which can process LCO to produce gasoline blendstocks with high octane number, and then the LCO-X technology based on the LCO UnicrackingTMtechnology and selective alkyl transfer technology can produce benzene and xylene. However, it has not yet been in practical use in commercial scale. In this paper, we will introduce the LCO hydrocracking technology (FD2G) for the production of a high octane gasoline based on a commercial hydrogenation system based on non-noble metal (W-Ni based) catalysts. This technology was developed in 2002 and then was applied in commercial scale in 2013.
2 Development of the Technology
Figure 2 demonstrates the hydrocarbon distribution of a typical LCO produced using a Middle East vacuum gas oil and residue. Numerous aromatic hydrocarbons are found in LCO, including the majority of polycyclic aromatic hydrocarbons, the di-aromatic content of which reaches >50%. The proportion of polycyclic aromatic hydrocarbons increases when the LCO narrow fractions gradually become heavier, while mono-aromatics content is reduced. The tri-aromatic hydrocarbons are mainly concentrated in the fraction boiling at >290 ℃. The high aromatic content of LCO is undesirable in the diesel fraction because aromatics in LCO can cause high density and poor burning behavior of diesel fuel. Meanwhile, researchers also realize the potential value of polyaromatics in LCO, which can be hydrocracked selectively into mono-aromatics to obtain high-octane gasoline.

Figure 2 Distribution of aromatics in the LCO■—tri-aromatic;■—di-aromatic;■—mono-aromatic
Hydrocracking technology is an excellent choice for transforming the molecular structure of hydrocarbons and upgrading oil quality. One of the characteristics of hydrocracking technology is the ability to control the occurrence of a reaction and the reaction extent by combining catalysts with process technology. The FD2G technology provides a full initiative effect on these characteristics, including preventing mono-aromatics from being hydrogenated into naphthenes by controlling the hydroconversion process, and ultimately transforming heavy aromatics into light aromatics. For example, the ideal reaction pathway of the FD2G technology in the di-aromatics hydrocracking reaction is demonstrated in Figure 3.
The key factors of this technology are the selection of a suitable catalyst and operating conditions. The desired reaction pathway is the transformation of di-aromatic and triaromatic hydrocarbons contained in LCO to monoaromatics with high octane number in the gasoline distil-late, rather than further hydrogenation saturation into low octane naphthenes. To maintain a proper operating run length of the catalyst while obtaining high-octane gasoline is still an issue.

Figure 3 The ideal reaction path of LCO hydrocracking
3 Pilot Verification
Reaction conditions are the main factors that influence the reaction rate. For example, both the reaction temperature and space velocity can affect the conversion and product distribution, while the hydrogen/oil volume ratio can affect the hydrogen partial pressure in the system. The key issue of the FD2G technology is controlling the degree of aromatic hydrogenation saturation using appropriate catalysts combined with the optimal process condition.
Certain differences occur in selecting process parameters between the FD2G and the conventional hydrocracking process in order to maintain the content of light aromatic hydrocarbons in the gasoline product as much as possible. A series of experiments are performed in pilot units to determine the optimized catalysts and operating conditions.
3.1 The flow diagram
A fixed-bed hydrogenation unit is utilized, as shown in Figure 4. All technical parameters are precisely controlled using a distributed control system. Both top and bottom of the reactor are filled with inert particles to maintain the uniform distribution of fluids and to prevent catalyst particles from entering the pipelines. The catalysts are loaded into the hydrofining and cracking reactors with dimensions of D/dp>18, and L/dp>350. (D, L, dp are the inner diameter of the reactor, bed height, and catalyst size, respectively.) Electrolytic hydrogen with a purity of over 99.9 vol% is used in this experiment, and the content of oxygen in hydrogen stream, which is subject to highpressure deoxygenation followed by dehydration using silica gel, is less than 5 μL/L. The tail oil can be recycled back to the feed tank of this unit. Thus, the influence of different process schemes on the reaction can be examined.
3.2 Effects of the catalyst
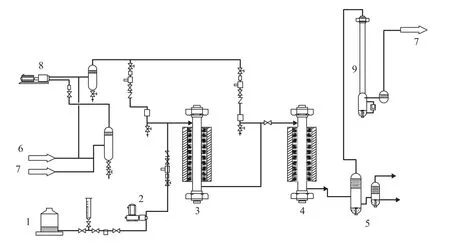
Figure 4 Schematic flow diagram for a single-pass hydrocracking reactor system1—Feed tank; 2—Oil pump; 3—Hydrofining reactor; 4—Hydrocracker; 5—Gas-liquid separator; 6—Fresh hydrogen; 7—Recycle hydrogen; 8—Compressor; 9—Water scrubber
Selecting a hydrocracking catalyst is an important step indeveloping the FD2G technology; and the selected catalyst used in the experiments is a multi-functional catalyst with its active centers capable of performing hydrogenation, cracking and isomerization reactions. A hydrocracking catalyst is usually designed according to the differences in raw materials and the requirements for targeted products. Three different types of hydrocracking catalysts are generally widely applied in industry. For instance, the hydrocracking catalysts with strong acidity and relatively weak hydrogenation activity are used to produce light oil. The hydrocracking catalysts with medium acidity and strong hydrogenation activity are used to produce middle distillates. Single-stage hydrocracking catalysts have appropriate hydrocracking activity, good quality mid-barrel distillates, strong heterogeneous properties, and high selectivity for diesel fuel with good cold flow property.
Theoretical analysis showed that the hydrocracking catalysts for producing light oil were more suitable for the hydrocracking process of light cycle oil. To produce more gasoline with high octane number, light aromatic hydrocarbons should be retained in the gasoline distillate as much as possible. The effects of three different commercial catalysts on LCO hydrogenation process under the same conditions have been studied. The properties of the three commercial catalysts are summarized in Table 1. The LCO is a stream with high sulfur content (1.05%) and low cetane number (about 17) because of its high aromatic content.
Figure 5 shows the results of LCO hydrocracking over different commercial catalysts under the same operating conditions. It shows that a stream of excellent quality gasoline with a high research octane number of 84.4 and an aromatic content of 57.8% could be obtained with catalyst C. By contrast, the research octane number obtained using catalysts A and B only reached 74.5 and 71.6, respectively. Therefore, catalyst C is suitable for LCO hydrocracking because the strong acidic nature and relatively weak hydrogenation activity of this catalyst could retain more light aromatic hydrocarbons in gasoline.
3.3 Effects of pressure
Studies have shown that the reaction pressure greatly affects hydroprocessing reaction and aromatic content. The product slate is directly related to the reaction pressure and hydrogenation saturation of aromatic components, which are more advantageous under a higher pressure[31-35]. Pressure greatly influences the LCO hydrocracking, while 60%—80% of aromatics are found in the feed. Greater differences occur between LCO hydrocracking and conventional hydrocracking processes. Experiments under different reaction pressure are conducted based on experiments 1—3 using catalyst C. The RON of the gasoline distillates along with the change of pressure are shown in Figure 6. It shows that the research octane number of the gasoline distillate increases significantly as the reaction pressure decreases from 13.7 MPa to 6.0 MPa. The reduction in reaction pressure is favorable to LCO hydrocracking for producing excellent high-octane gasoline. However, a decrease in reaction pressure also hampers the overall productivity. According to the general experience of industrial applications of conventional hydrocracking, a low operating pressure affects the service life of catalyst, acceleratescoking, and hinders quality improvement of diesel products. Such hydrocracking requires a comprehensive consideration to determine the proper pressure used in the LCO hydrocracking technology.

Table 1 Properties of the catalyst used

Figure 5 The effects of catalyst on LCO hydrocracking■—RON;●—Aromatics content

Figure 6 The effects of total pressure on the RON of produced gasolineHydrogen /oil volume ratio= 1 200:1; LHSV= 0.8
3.4 Effects of temperature
The reaction of aromatic hydrogenation is a reversible exothermic reaction with the amount of substance reduced (hydrogen consumption). It means that the activation energy of the positive reaction (hydrogenation) is lower than that of the reverse reaction (dehydrogenation). Thus, the acceleration of the dehydrogenation reaction will be more significant than that of hydrogenation reaction, when the reaction temperature increases, which will lead to the reduction of the equilibrium constant as well. In consequence, the conversion rate of aromatics will reach the maximum. The corresponding temperature to the maximum conversion rate is the optimal temperature for hydrogenation reaction. Below this temperature, the hydrogenation of aromatic hydrocarbon is kinetic controlled. Then, the conversion of aromatic hydrocarbon will be greatly thermodynamically hampered, when the temperature is higher than the optimal one. The balance between thermodynamic limitation and the hydrogenation degree of the aromatic hydrocarbons should be carefully evaluated.
Another challenge on the technology development is to control the hydrogenation of aromatics to avoid the excess hydrogenation of aromatic hydrocarbons. Higher reaction temperature is favorable to this technology which is verified by the experiments. As shown in Figure 7, the cracking depth can be significantly improved and the yield of gasoline distillate can increase by increasing the reaction temperature. In consequence, the octane number of gasoline fractions was increased with the increase of the cracking depth.
4 Commercial Application
The LCO hydrocracking technology had been applied in the hydrocracking unit I at the Jinling Petrochemical Company in 2013 and Maoming Petrochemical Company to successfully produce high research octane gasoline and excellent quality diesel. Only the catalyst system was changed, and process conditions were optimized without large equipment modifications. The results showed that this technology could produce gasoline blendstock at a yield of 35%—50% coupled with a high research octane number of 91—94 and a sulfur content of less than 10 μg/g. In addition, the sulfur content of clean diesel blendstock was also less than 10 μg/g, and its cetane number was increased by 10—14 units as compared with the feedstock.
At present, deep desulfurization and aromatic hydrogenation of diesel refining technology (HF), the maximum diesel cetane number technology (MCI) have both been applied in the commercial units. The systematic comparison between the HF technology, MCI technology and the present FD2G technology are summarized in Table 2. The main operating conditions, chemical hydrogen consumption, the production yield and the properties of obtained products were compared under the same quality of thediesel product. The results show that the FD2G technology has a higher economic efficiency than other diesel quality upgrading technologies under the current gasoline and diesel price system.
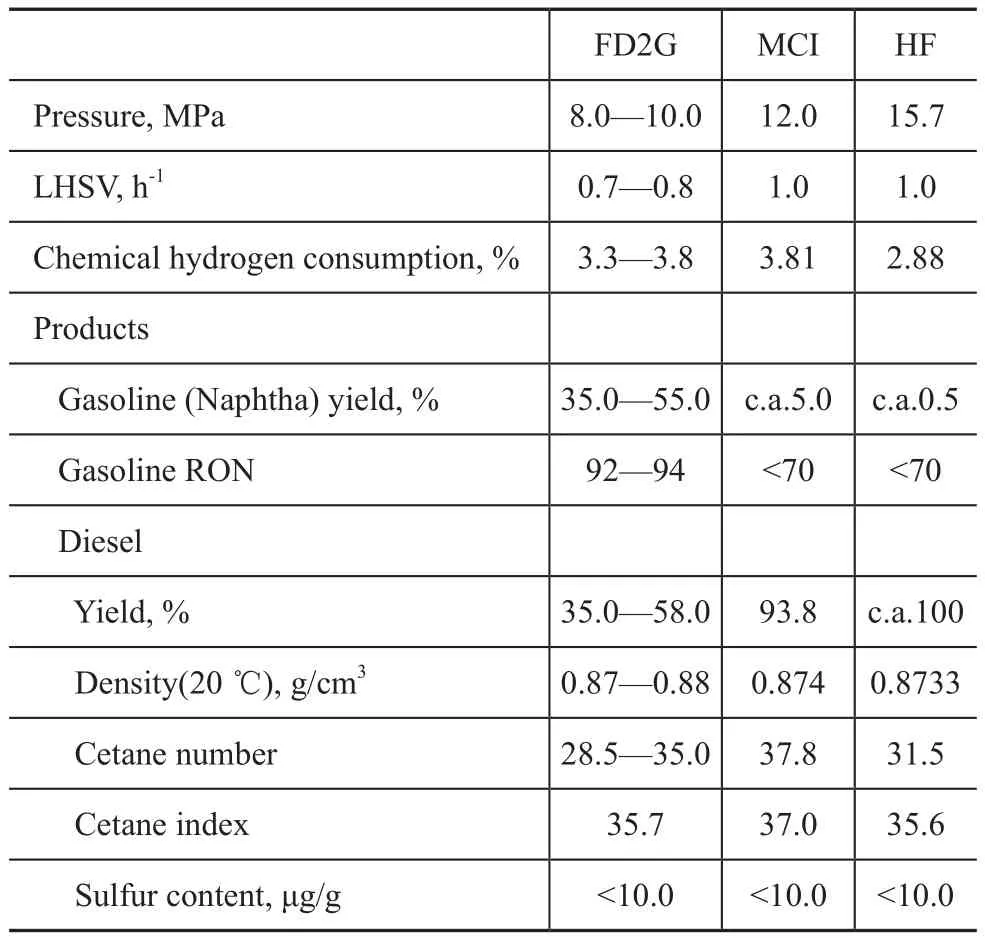
Table 2 The comparison between the HF, MCI and FD2G technology
5 Conclusions
The FD2G technology can effectively convert heavy aromatics in LCO to light aromatics through selective hydrocracking of aromatics, thereby producing high-octane gasoline blendstocks and clean low-sulfur diesel blendstocks.
Typical results of FD2G technology on commercial units show that it could produce clean diesel with a sulfur content of less than 10 μg/g and clean gasoline with a research octane number (RON) of up to 92.
FD2G technology was deemed to be a good choice for refineries abundant in FCC diesel with high aromatics content and are seeking to improve cetane numbers and produce more gasoline and aromatics.
[1] Ren T, Patel M, Blok K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes[J]. Energy, 2006, 31(4): 425-451
[2] Mužíková Z, Procháska F, Pospíšil M. Storage stability of FCC light cycle oil[J]. Fuel, 2010, 89(11): 3534-3539
[3] Rossi R D B, Huovie C, Thakkar V, et al. Maximazing diesel in existing assets[C/CD]. NPRA Annual Meeting, AM-09-33. 2009
[4] Sharafutdinov I, Stratiev D, Shishkova I, et al. Industrial investigation on feasibility to raise near zero sulfur diesel production by increasing fluid catalytic cracking light cycle oil production[J]. Fuel Processing Technology, 2012, 104: 211-218
[5] Gilbert W R, Jr Morgado E, de Abreu M A S, et al. A novel fluid catalytic cracking approach for producing low aromatic LCO [J]. Fuel Processing Technology, 2011, 92(12): 2235-2240
[6] Corma A, Martínez C, Sauvanaud L. New materials as FCC active matrix components for maximizing diesel (light cycle oil, LCO) and minimizing its aromatic content[J]. Catalysis Today, 2007, 127(1/4): 3-16
[7] Yun G-N, Lee Y-K. Dispersion effects of Ni2P catalysts on hydrotreating of light cycle oil[J]. Applied Catalysis B: Environmental. 2014, 150–151: 647-655
[8] Ding L, Zheng Y, Yang H, et al. LCO hydrotreating with Mo-Ni and W-Ni supported on nano- and micro-sized zeolite beta [J]. Applied Catalysis A: General, 2009, 353(1): 17-23
[9] Ding L, Zheng Y, Zhang Z, et al. Hydrotreating of light cycled oil using WNi/Al2O3catalysts containing zeolite beta and/or chemically treated zeolite Y[J]. Journal of Catalysis, 2006, 241(2): 435-445
[10] Wang Y, Shen B, Li J, et al. Interaction of coupled titanium and phosphorus on USY to tune hydrodesulfurization of 4,6-DMDBT and FCC LCO over NiW catalyst [J]. Fuel Processing Technology, 2014, 128(0): 166-175
[11] Dukanović Z, Glišić S B, Čobanin V J, et al. Hydrotreating of straight-run gas oil blended with FCC naphtha and light cycle oil[J]. Fuel Processing Technology. 2013, 106: 160-165
[12] Del Rio D, Bastos R, Sedran U. Commercial additives for sulfur control in FCC gasoline: Overall analysis of their impact on LCO and gasoline[J]. Catalysis Today, 2013, 213: 206-210
[13] B etancourt P, Marrero S, Pinto-Castilla S. V–Ni–Mo sulfide supported on Al2O3: Preparation, characterization and LCO hydrotreating[J]. Fuel Processing Technology, 2013, 114: 21-25
[14] Azizi N, Ali S A, Alhooshani K, et al. Hydrotreating oflight cycle oil over NiMo and CoMo catalysts with different supports [J]. Fuel Processing Technology, 2013, 109: 172-178
[15] Wang L, Shen B, Fang F, et al. Upgrading of light cycle oil via coupled hydrogenation and ring-opening over NiW/ Al2O3-USY catalysts[J]. Catalysis Today, 2010, 158(3/4): 343-347
[16] Calemma V, Giardino R, Ferrari M. Upgrading of LCO by partial hydrogenation of aromatics and ring opening of naphthenes over bi-functional catalysts[J]. Fuel Processing Technology, 2010, 91(7): 770-776
[17] Tailleur R G. Low-emission diesel production by upgrading LCO plus SR diesel fractions [J]. Catalysis Today, 2008, 130(2/4): 492-500
[18] Liu Z, Zheng Y, Wang W, et al. Simulation of hydrotreating of light cycle oil with a system dynamics model [J]. Applied Catalysis A: General, 2008, 339(2): 209-220
[19] Froment G F, Castaneda-Lopez L C, Marin-Rosas C. Kinetic modeling of the hydrotreatment of light cycle oil and heavy gas oil using the structural contributions approach[J]. Catalysis Today, 2008, 130(2–4): 446-454
[20] Ding L, Zheng Y, Zhang Z, et al. Hydrotreating of light cycle oil using WNi catalysts containing hydrothermally and chemically treated zeolite Y. Catalysis Today. 2007, 125(3/4): 229-238
[21] Chen J, Yang H, Ring Z. HDS kinetics study of dibenzothiophenic compounds in LCO[J]. Catalysis Today, 2004, 98(1/2): 227-233
[22] Albertazzi S, Baraldini I, Busca G, et al. Noble metal containing Al/Ce/Mg pillared montmorillonite clay as catalysts in the hydrotreating of LCO fractions [J]. Applied Clay Science, 2005, 29(3/4): 224-234
[23] Laredo G C, Saint-Martin R, Martinez M C, et al. High quality diesel by hydrotreating of atmospheric gas oil/light cycle oil blends [J]. Fuel, 2004, 83(10): 1381-1389
[24] Ancheyta-Juárez J, Rodríguez-Salomón S, Valenzuela-Zapata M A. Experimental evaluation of vacuum gas oil−light cycle oil blends as FCC feedstock[J]. Energy & Fuels, 2001, 15(3): 675-679
[31] Scherzer J, Gruia A J. Hydrocracking Science and Technology[M]. CRC Press, 1996
[32] Liu Lihua, Liu Shuqun. Ni2P-MoS2/γ-Al2O3catalyst for deep hydrodesulfurization via the hydrogenation reaction pathway[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 12-18
[33] Zheng Renyang, Xin Jing, Zhang Runqiang, et al. Influence of hydrotreating depth on properties of LCO[J]. Petroleum Processing and Petrochemicals, 2014, 45(10): 1-7(in Chinese)
[34] Ge Panzhu, Gao Xiaodong, Ren Liang. Hydrogenation performance of 1-methylnaphthalene on different catalysts[J]. Petroleum Processing and Petrochemicals, 2014, 45(6): 36-39(in Chinese)
[35] Liang Jilei, Liu Yunqi, Wang Liqiang, et al. Preparation and application of hydroupgrading catalyst for inferior diesel. Part I. Catalyst preparation method and selective ring-opening of tetralin[J]. Petroleum Processing and Petrochemicals, 2014, 45(7): 36-41(in Chinese)
date: 2015-06-08; Accepted date: 2015-09-02.
Prof. Fang Xiangchen, E-mail: fxc@ ecust.edu.cn.
- 中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol

