Electrochemical Performance of SnO2/Graphite Nanocomposites as Anode Material for Lithium-Ion Batteries
BAI Xue-jun(白雪君),WANG Biao(王 彪),2*,CHENG Xu(程 旭),JIANG Jian-ming(江建明)
1 College of Material Science and Engineering,Donghua University,Shanghai 201620,China
2 State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China
Electrochemical Performance of SnO2/Graphite Nanocomposites as Anode Material for Lithium-Ion Batteries
BAI Xue-jun(白雪君)1,WANG Biao(王 彪)1,2*,CHENG Xu(程 旭)1,JIANG Jian-ming(江建明)1
1 College of Material Science and Engineering,Donghua University,Shanghai 201620,China
2 State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China
SnO2/graphite nanocompositeswith differentSnO2contents were successfully prepared by a co-precipitation method.The nanocomposites,used as the anode material for lithium-ion batteries(LIBs),were characterized by X-ray diffraction(XRD),thermogravimetric analysis(TGA),and transmission electron microscopy(TEM).The SnO2particles had the average size of about 15 nm and their distribution on graphite matrix much depended on the contents of SnO2in the nanocomposites.The galvanostatic charge-discharge cycles were used to investigate the effects of SnO2contents on the electrochemical performance of these composites.The results show that the initial specific capacities increase with the SnO2contents.However,the cyclic stabilities are determined by the distribution of SnO2particles in composites.For 55% by weight SnO2/graphite composites,the initial specific capacity is 740 mAh g-1and 70%of the initial specific capacity(518 mAh·g-1)can still be retained after 50 charge-discharge cycles.
tin oxide;graphite;anode material;lithium-ion batteries (LIBs)
Introduction
Due to the reasonably low potential for Li+insertion and high capacities, SnO2appears to be alternative to the commercial graphite anode for the lithium-ion batteries(LIBs).However,its capacity fading under charge-discharge cycles remains a key issue for practical application.Such material deficiency is mainly caused by a large initial irreversible capacity because of the formation of Li2O and the strong volume changes(about 200%)during Li+insertion and extraction[1],which leads to mechanical failure and pulverization of the anode films.
Promising approaches to overcome these deterrents are to create SnO2anode with nanoscale structures or/and to prepare SnO2-based materials with composite structures.Various types of SnO2nanostructure materials such as nanoparticles[2],hollow sphere[3-5],nanowires[6-8],and nanotubes[9-10]have been reported with some improved cycleability.Although smaller particles and special nanostructures are considered to moderate volume changes and thereby improve batteries'performances,these nanostructure materials are still unable to address fully the cycleability issue associated with the application of SnO2.Recently,composites consisting of and carbonaceous matrices,such as amorphous carbon[11-14],graphite[15-17],CNTs[18-20],and graphene[21-24]have attracted much attention.In these cases,carbon components havebeen found effectivefor improving cycleability,where carbon functions as physical buffering layer for the large volume change of SnO2during electrochemicalreaction with Li+. Using graphite (the commercial anode material)as the matrix to prepare the SnO2- based composite anode materials for LIBs is an effective way to suppress the volume effects of SnO2.Because the volume expansion of graphite on lithium intercalation is only about 9% which can partly accommodate the volume changes of SnO2.However,in SnO2/graphite nanocomposites,both components are contributed to the overall capacities,and the detailed influence of content ratio of SnO2/graphite composites on electrochemical performence as lithium battery anode materials is still unclear.The contribution of distribution structure of SnO2nanoparticles on graphite to the cyclic stability is still lacking.In this paper,SnO2/graphite composites with different SnO2contents have been prepared.The effects of SnO2content and distribution on the electrochemical performance of these composites have been discussed in detail.
1 Experimental
1.1 Material synthesis
At first,graphite(Aladdin,China,99.95%)was stirred in a mixed acid solution of HNO3and HCl(1∶3 v/v)for 24 h to activate the surface of graphite,then washed with distilled water and dried by the vacuum freeze-drying method.The precursors were prepared by a co-precipitation method.The typical procedure to prepare S-6 was as follows.The activated graphite (0.1 g)was dispersed in 0.25 M SnCl4·5H2O(Aladdin,China,AR)aqueous solution(10 mL).Then under vigorous stirring,0.25 M NaOH aqueous solution(60 mL)was added dropwise.After 2 h of stirring,the precursors were washed several time with distilled water and dried at 80℃ for 12 h in a vacuum oven.At last,the precursors were calcined at 500℃ for 2 h in air to obtain the SnO2/graphite composites.Other samples (S-1 to S-5)were prepared by using the similar method as S-6.The only difference was the weight of activated graphite.
1.2 Structural characterization
The composites were characterized by X-ray diffraction (XRD,Cu Ka,λ=1.54056 Å,D/max-2550Pc,Rigaku).Precise SnO2contents in composites were determined using thermogravimetric analysis(TGA,209 F1 Iris,Netzsh),from room temperature to 900℃ at a heating rate of 10℃/min in air.The morphology was characterized by transmission electron microscopy(TEM,2100F,JEOL).
1.3 Electrochemical measurement
Electrochemical performances were evaluated by assembling two-electrrode coin-type(CR2016)half cells in argon filled glovebox.The cells consisted of SnO2/graphite composites,acetylene black and poly(vinylidene fluoride)8∶1∶1(w/w)as the working eletrodes,Li metal foil as counter electrodes,and Celgard 2400(DuPont,USA)as sepatator.The electrolyte was 1.0 M LiPF6in a mixture of ethylene carbonate/diethyl carbonate 1∶1(w/w).The cells were cycledat a current density of 100 mA·g-1between 0.01 and 2.0 V using Battery Tester(CT2001A,LAND).
2 Results and Discussion
SnO2/graphite composites with six kinds of SnO2contents (sample S-1 to S-6,see Table 1)were prepared and the SnO2contents(%by weight)were determined by TGA[25].TEM images of these composites are shown in Fig.1.All the prepared SnO2particles are about 15 nm and the particle-size distributions are very narrow.The density of the particles on graphite increases with the SnO2contents.The high-resolution TEM(HRTEM)image(insert in Fig.1(d))displays the lattice fringe with a spacing of 0.33 nm,corresponding to the (110)face of tetragonal SnO2,which confirms the presence ofSnO2on graphite. However, there are significant differences between the six samples in terms of particles distributions on graphite.The nanoparticles with lower SnO2contents(S-1 to S-3)are slightly aggregated because of the buffer effects of graphite.When the SnO2contents increase to 55% by weight or 73% by weight(S-4 and S-5),the particles form a network structure.When the SnO2contents increase to 80% by weight(S-6),heavy aggregation of particles can be observed.
The XRD patterns of SnO2/graphite composites with different SnO2contents are presented in Fig.2.All reflection peaks are in accordance with a tetragonal SnO2(JCPDS No.41-1445)and a hexagonal graphite(JCPDS No.12-0212).No significant changes of reflection peaks for the samples with various SnO2contents are detected.XRD confirmed the coexisting ofphases oftetragonalSnO2and hexagonal graphite.
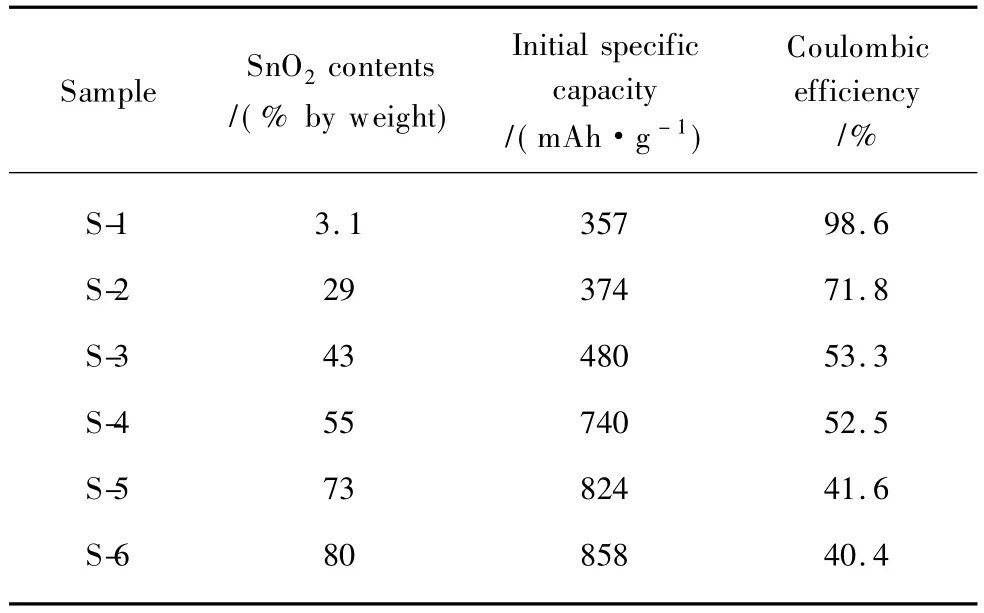
Table 1 SnO2contents,initial specific capacity,and coulombic efficiency of the SnO2/graphite composites
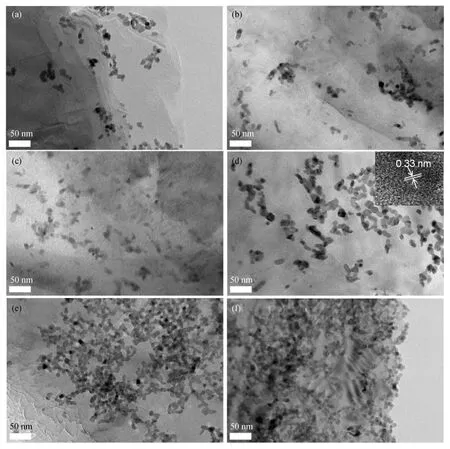
Fig.1 TEM images of various SnO2/graphite composites:(a)S-1,(b)S-2,(c)S-3,(d)S-4 with an inset showing the HRTEM image,(e)S-5,and(f)S-6
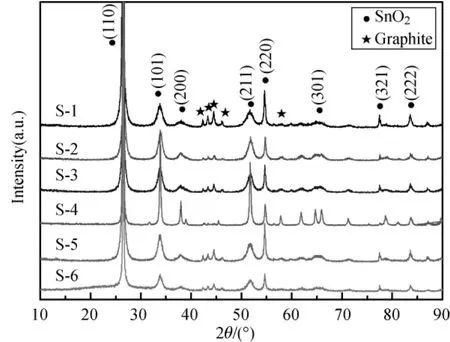
Fig.2 XRD patterns ofSnO2/graphitecomposites with different SnO2contents
The initial specific capacity and coulombic efficienty of SnO2/graphite composites were measured between 0.01 and 2.0 V,in 100 mA·g-1current density.The results are listed in Table 1.The initial specific capacity increases with SnO2contents,while the coulombic efficiency decreases obviously owing to the reduction of SnO2and the formation of solid electrolyte interfae(SEI)layers on graphite and Sn surface (formulas(1)-(4))[17].This phenomenon can also be reflected on the difference in the specific charge/discharge profiles of these composites(Fig.3).With the SnO2contents increasing,the plateaus at about 0.8 V in initial discharge curve become more apparent which indicates more Li2O formation.While the plateaus disappear in the 5th discharge curve,indicating the irreversible reactions mainly exist in the first several cycles.

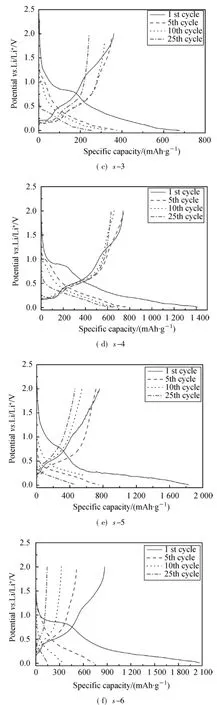
Fig.3 Charge-discharge profiles of SnO2/graphite composites(the voltage is between 0.01 and 2.0 V and the current density is 100 mA·g-1)
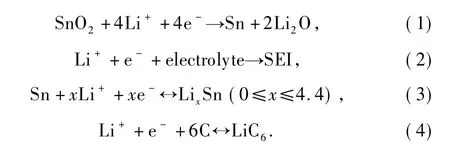
Although the initial specific capacity increases with the SnO2contents,the cycle performance does not show the same tendency.Figure 4 shows the cycling performances of SnO2/ graphite composites.The 55% by weightSnO2/graphite composite(S-4) presents much better cycleability which maintains the capacity of 518 mAh·g-1until the 50th cycle.The sample(S-1)with very low SnO2content(3.1% by weight)shows more stable cycle-life performance because of the main attribution of the graphite.Other samples show lower cycle performances than that of the S-4 sample,even though S-5 and S-6 have higher SnO2contents in the composites.In these cases,the cycleability of graphite was assumingly the same[16]and the size and size-distribution of SnO2particles were similar for the six samples,so distribution state of SnO2on graphite could be the main factor to affact the cycle-life performance of the composites.In the several previous reports,the reversible capacities of SnO2are soon decreased if the Sn particles (reduction products,see formula(1)are aggregated during the alloying and dealloying of Li with Sn(formula(3)).In this work,the graphite not only increases the conductivity of the composites but alsoserves as a physical buffer layer to accommodate the volume change of Sn in the lithiation and delithiation process[23].When SnO2contents are relative low,the stack densities of SnO2nanoparticles are small,as S-2 and S-3.These seperated nanoparticles are submitted to dramatic volume change by lithium insertion and extraction and they shed from graphite which become electrically disconnected,leading to a decrease of the reversible capacity[26].With the increase of SnO2contents,SnO2nanoparticles trend to form a network and this network bounds to graphite tightly which could effectively and rapidly transport electrons between SnO2and graphite.The relatively loose SnO2network of S-4 also retards the aggregation of Sn during the Sn alloying to accommodate with volume change.Therefore S-4 shows a better cycle performance.When SnO2contents are higher than 73%(as S-5 and S-6),the SnO2nanoparticles distribute too densely and aggregate seriously which cause severe aggregation of Sn during charge-discharge process,leading to a rapid fading of capacity.Distribution structure ofSnO2on graphite is another important factor affecting the cycle-life performance of these composites which has been ignored previously.

Fig.4 Cycling performances of SnO2/graphite composites(the voltage is between 0.01 and 2.0 V and the current density is 100 mA·g-1)
Figure 5 shows the SnO2contributions in sample S-4 assuming constant graphite capacity of 300 mAh· g-1.Capacity of SnO2is calculated according to the following equation[16]:

where C is the capacity,M is mass fraction.The calculated initial specific capacity of SnO2is 1100 mAh·g-1,which is much higher than SnO2theoretical capacity(782 mAh·g-1).And it maintains 696 mAh·g-1until the 50th cycle,which is still near the theoretical capacity.This phenomenon indicates that sample S-4 is not simply an intimate physical mixture of the two components.There may be a special synergistic effect in the SnO2/graphite composites.The mechanism of this effect is still being investigated in our lab.
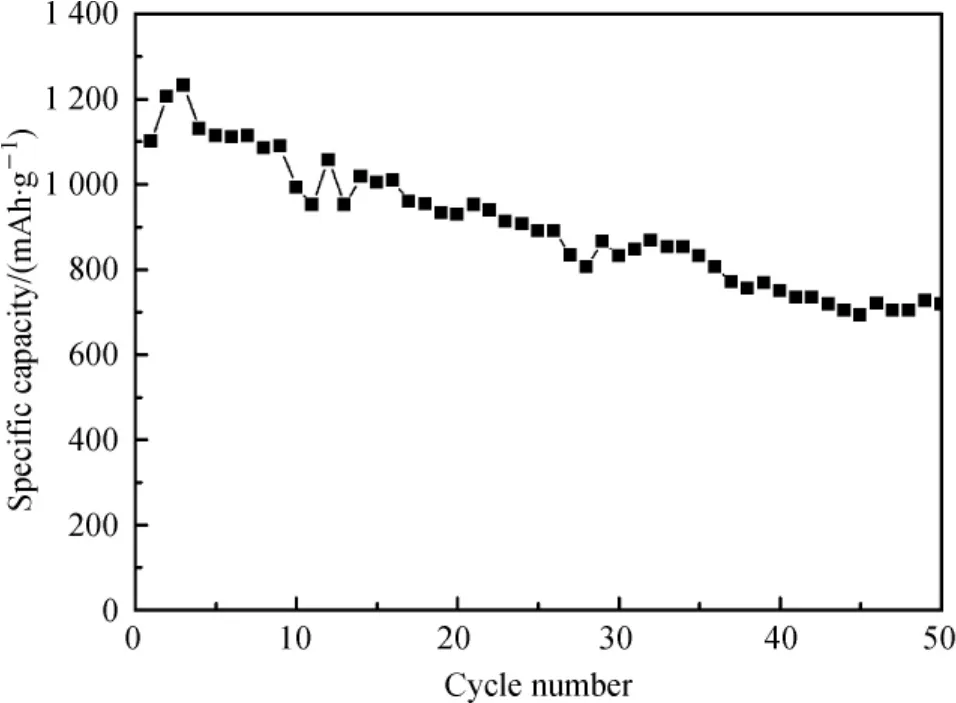
Fig.5 SnO2contributions in sample S-4 assuming constant graphite capacity of 300 mAh·g-1(the voltage is between 0.01 and 2.0 V and the current density is 100 mA·g-1)
3 Conclusions
SnO2/graphite composites have been successfully prepared by a co-precipitation method.And 55% by weight SnO2/ graphite sample with a network structure exhibits better cyclic stability,which could guide researchers to design a special distribution structure of SnO2on graphite to improve the cyclelife performance of these composites.Based on the calculation results of capacities of these composites with the physical mixture law,the capacity of SnO2is near the theoretical capacity.
[1]Huang J Y,Zhong L,Wang C M,et al.In situ Observation of the ElectrochemicalLithiation ofa Single SnO2Nanowire Electrode[J].Science,2010,330(6010):1515-1520.
[2]Kim S,Lee H,Park C M,et al.Synthesis of Tin Oxide Nanoparticle Film by Cathodic Electrodeposition[J].Journal of Nanoscience and Nanotechnology,2012,12(2):1616-1619.
[3]Lou X W,Yuan C L,Archer L A.Shell-by-Shell Synthesis of Tin Oxide Hollow Colloids with Nanoarchitectured Walls:Cavity Size Tuning and Functionalization[J].Small,2007,3(2):261-265.
[4]Lee Y,Jo M R,Song K,et al.Hollow Sn-SnO2Nanocrystal/ Graphite Composites and Their Lithium Storage Properties[J].ACS Applied Materials&Interfaces,2012,4(7):3459-3464.
[5]Liu R Q,Li N,Li D Y,et al.Template-Free Synthesis of SnO2Hollow Microspheres as Anode Material for Lithium-Ion Battery[J].Materials Letters,2012,73:1-3.
[6]Han Y,Wu X,Ma Y,et al.Porous SnO2Nanowire Bundles for Photocatalyst and Li Ion Battery Applications [J].CrystEngComm,2011,13(10):3506-3510.
[7]Lei D,Zhang M,Hao Q,et al.Morphology Effect on the Performances of SnO2Nanorod Arrays as Anodes for Li-Ion Batteries[J].Materials Letters,2011,65(8):1154-1156.
[8]Zhang L Q,Liu X H,Perng Y C,et al.Direct Observation of Sn Crystal Growth During the Lithiation and Delithiation Processes of SnO2Nanowires[J].Micron,2012,43(11):1127-1133.
[9]Wang H E,Xi L J,Ma R G,et al.Microwave-Assisted Hydrothermal Synthesis of Porous SnO2Nanotubes and Their Lithium Ion Storage Properties[J].Journal of Solid State Chemistry,2012,190:104-110.
[10]Wu F D,Wu M H,Wang Y.Antimony-Doped Tin Oxide Nanotubes for High Capacity Lithium Storage [J].Electrochemistry Communications,2011,13(5):433-436.
[11]Lou X W,Deng D,Lee J Y,et al.Preparation of SnO2/Carbon Composite Hollow Spheres and Their Lithium Storage Properties[J].Chemistry of Materials,2008,20(20):6562-6566.
[12]Lou X W,Li C M,Archer L A.Designed Synthesis of Coaxial SnO2@Carbon Hollow NanospheresforHighly Reversible Lithium Storage[J].Advanced Materials,2009,21(24):2536-2539.
[13]Liu J,Li W,Manthiram A.Dense Core-Shell Structured SnO2/C Composites as High Performance Anodes for Lithium Ion Batteries[J].Chemical Communications,2010,46(9):1437-1439.
[14]Li Y,Zhu S M,Liu Q L,et al.Carbon-coated SnO2@C with Hierarchically Porous Structures and Graphite Layers Inside for a High-Performance Lithium-ion Battery[J].Journal of Materials Chemistry,2012,22(6):2766-2773.
[15]Kilby K T,Jiao S Q,Fray D J.Current Efficiency Studies for Graphite and SnO2-Based Anodes for the Electro-Deoxidation of Metal Oxides[J].Electrochimica Acta,2010,55(23):7126-7133.
[16]Wang Y,Lee J Y.Microwave-Assisted Synthesis of SnO2-Graphite Nanocomposites for Li-Ion Battery Applications[J].Journal of Power Sources,2005,144(1):220-225.
[17]Kim J G,Nam S H,Lee S H,et al.SnO2Nanorod-Planted Graphite:An Effective Nanostructure Configuration for Reversible Lithium Ion Storage[J].ACS Applied Materials&Interfaces,2011,3(3):828-835.
[18]Chen Y J,Zhu C L,Xue X Y,et al.High Capacity and Excellent Cycling Stability of Single-Walled Carbon Nanotube/SnO2Core-Shell Structures as Li-Insertion Materials[J].Applied Physics Letters,2008,92(22):3301.
[19]Zhang H X,Feng C,Zhai Y C,et al.Cross-Stacked Carbon Nanotube Sheets Uniformly Loaded with SnO2Nanoparticles:a Novel Binder-Free and High-Capacity Anode Material for Lithium-Ion Batteries[J].Advanced Materials,2009,21(22):2299-2304.
[20]Wang Z,Chen G,Xia D.Coating of Multi-walled Carbon Nanotube with SnO2Films of Controlled Thickness and Its Application for Li-Ion Battery[J].Journal of Power Sources,2008,184(2):432-436.
[21]Paek S M,Yoo E J,Honma I.Enhanced Cyclic Performance and Lithium Storage Capacity of SnO2/Graphene Nanoporous Electrodes with Three-Dimensionally Delaminated Flexible Structure[J].Nano Letters,2009,9(1):72-75.
[22]Yao J,Shen X P,Wang B,et al.In situ Chemical Synthesis of SnO2-Graphene Nanocomposite as Anode Materials for Lithium-Ion Batteries[J].Electrochemistry Communications,2009,11 (10):1849-1852.
[23] Wang X Y,Zhou X F,Yao K,et al.A SnO2/Graphene Composite as a High Stability Electrode for Lithium Ion Batteries[J].Carbon,2011,49(1):133-139.
[24]Ding S J,Luan D Y,Boey F Y C,et al.SnO2Nanosheets Grown on Graphene Sheets with Enhanced Lithium Storage Properties[J].Chemical Communications,2011,47(25): 7155-7157.
[25]Xu C H,Sun J,Gao L.Direct Growth of Monodisperse SnO2Nanorods on Graphene as High Capacity Anode Materials for Lithium Ion Batteries[J].Journal of Materials Chemistry,2012,22(3):975-979.
[26]Billaud D,Balan L,Schneider R,et al.The Influence of the Synthesis Conditions of Graphite/Tin Nanoparticle Materials on Their Electrode Electrochemical Performance in Li-Ion Battery Anodes[J].Carbon,2006,44(12):2508-2515.
TM 912.9
A
1672-5220(2015)03-0379-05
date:2013-09-02
s:the Scientific Research Foundation for the Returned Overseas Chinese Scholars;the Shanghai Leading Academic Discipline Project,China(No.B603);the Programme of Introducing Talents of Discipline to Universities,China(No.111-2-04)
*Correspondence should be addressed to WANG Biao,E-mail:wbiao2000@dhu.edu.cn
 Journal of Donghua University(English Edition)2015年3期
Journal of Donghua University(English Edition)2015年3期
- Journal of Donghua University(English Edition)的其它文章
- Effect of Sludge Retention Time on the Fate of Proteins and Polysaccharides in AAO Process
- Compressive Strength Estimation for the Fiber-Reinforced Polymer(FRP)-Confined Concrete Columns with Different Shapes Using Artificial Neural Networks
- Force-Based Quadrilateral Plate Bending Element for Plate Using Large Increment Method
- Effects of 1,7-Bromine Substitution at Bay Area on Self-assembly Behavior and Photo Physical Properties of Perylene Diimide
- Theoretical and Experimental Analyses of Poisson Ratios for Plain-Woven Fabrics
- Dynamic Engaging Characteristics of Wet Clutch in Automatic Transmission
