Effect of transplantation of BMMSCs on pathological change of gastric precancerous lesions of rats
Zhen-Lv Lin, Guang-Wei Zheng, Lin Zhang, Jian-Tao Zheng, Hui Chen
1Department of Emergency Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, 350005, China
2Department of Gastroenterology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, 350005, China
Effect of transplantation of BMMSCs on pathological change of gastric precancerous lesions of rats
Zhen-Lv Lin1, Guang-Wei Zheng1, Lin Zhang2*, Jian-Tao Zheng2, Hui Chen2
1Department of Emergency Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, 350005, China
2Department of Gastroenterology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, 350005, China
ARTICLE INFO
Article history:
in revised form 20 October 2015
Accepted 3 November 2015
Available online 20 December 2015
Mesenchymal stem cells
Gastric precancerous lesions
Cytokine
Immune regulation
Objective: To build the rat model of gastric precancerous lesions and discuss the effect of transplantation of mesenchymal stem cells (BMMSCs) on the pathological change. Methods: The rat model of gastric precancerous lesions was built using N-methyl-N'-nitro-N'-nitrosoguanidine. After the intravenous transplantation of BMMSCs, the migration and colonization location was then observed, as well as its effect on the related factors of gastric precancerous lesions,including VEGF, IL-10 and IFN-γ. Results: BMMSCs were mainly colonized in the gastric body and gastric antrum, which could be differentiated into the epithelial and interstitial cells. The expression of VEGF in the transplantation group and non-transplantation group was significantly higher than that in the control group (P<0.05); while the expression of VEGF in the transplantation group was significantly higher than that in the non-transplantation group (t=3.88, P<0.001). The expression of serum IL-10 and IFN-γ in the transplantation group and non-transplantation group was significantly higher than that in the control group (P<0.05), while the expression of IL-10 and IFN-γ in the transplantation group was significantly lower than that in the non-transplantation group (t=3.03, P=0.004; t=3.80, P<0.001). Conclusions: BMMSCs can be directionally differentiated into the epithelial and interstitial cells and can also regulate the related growth factors and inflammatory factors to reduce the injury of inflammation, relieve or reverse the process of gastric precancerous lesions.
Document heading doi:10.1016/j.apjtm.2015.11.006
1. Introduction
The gastric precancerous lesions is some kind of pathological change of gastric mucosa that can easily cause cancerization, which usually appears in the chronic atrophic gastritis and shows dysplasia and intestinal metaplasia of mucosa[1,2]. In China, gastric cancer is a common malignant tumor. The cancerization of human cells is a multilevel incremental process, and the phase from gastritis to cancerization is named as the precancerous lesions[3,4]. The probability for the chronic atrophic gastritis to be cancerized isincreased with the longer duration of disease and the aggravation of dysplasia[5,6].
The mesenchymal stem cells (BMMSCs) are the adult stem cells from mesoderm, with the function of self-renewal, which can not only mechanically support the blood stem cells in the bone marrow,but can also secrete many kinds of growth factors to support the hematopoiesis[7]. Because of easy collection and adherent growth,it has become a hot topic of researches in recent years[8,9]. In the researches of gastric associated diseases, BMMSCs are mainly limited to the treatment of gastric injury, which can be differentiated into the gastric epithelial cells to repair injured gastric mucosa[10]. The injury and inflammation of gastric mucosa can promote the release of BMMSCs. In this study, after building the rat model of gastric precancerous lesions, BMMSCs were transplanted into the model through the vein and thus the related intervention mechanism was observed.
2. Materials and Methods
2.1. Laboratory animal
A total of 80 healthy male SPF Wistar rats with the weight range of 180-220 g were mixed, with 5 rats in each cage. The feeding room had the natural light, with the room temperature of about 23 ℃,relative humidity of 45%-55%, good ventilation and ultraviolet radiation.
2.2. Modeling
Six-month rats were randomly divided into two groups, where the 20 rats in control group were fed with clean running water and diet and the 60 rats in model group were fed with 100 mg/L N-methyl-N'-nitro-N'-nitrosoguanidine (MNNG) and diet. 2 months after the beginning of modeling, 2 rats were executed every month to observe the progress of modeling. Preparation of 100 mg/L MNNG solution:5 g MNNG was dissolved in 1 L distilled water to be diluted into 5 mg/mL mother solution, which was then stored in a dark and cool place. Before the modeling, 10 mL mother solution was added into 500 mL distilled water to be diluted into 100 mg/L. It was then put in the dark drinking fountain for the drinking of rats in the model group.
2.3. Transplantation of BMMSCs
The transplantation group: 1 μg/μL CM-dil-labeled BMMSCs were transplanted into 20 rats in the model group through caudal vein. The dosage for each rat was 2 × 106/mL every time, 1 time/ week and 3 times of injection in total. The non-transplantation group: 20 rats in the model group were given with the intravenous injection of normal saline with equal dosage. After injection, all rats were anesthetized by using 10% chloral hydrate. After drawing the blood from the heart, rats were executed. The serum was obtained and stored at -20 ℃ after being centrifuged at 3 000 r/min. For the executed rats, their stomachs were incised and then paved evenly. The samples in the longitudinal strip were collected from the lesions and the section from the pylorus and cardia. Samples were fixed with 10% formaldehyde and then dehydrated using the conventional method. After being embedded with paraffin, they were sliced into pieces with the thickness of 3 μm. 10 rats in the control group were given the injection of BMMSCs solution with equal dosage, while other 10 rats were only treated with normal saline.
2.4. Outcome measures
The immunofluorescence double staining protocol was employed to detect BMMSCs that were transplanted in the sample tissues,where CM-dil-labeled cytoplasm appeared to be red, the nucleus to be blue and the epithelial and interstitial cells that were differentiated from BMMSCs to be green. The immunohistochemical method was adopted to detect the vascular endothelial growth factor (VEGF) in the gastric mucosa tissue. 5 different visual fields were selected for the observation under 400× lens. The cytoplasm that contained the brown particles was regarded as the positive cells. The mean optical density (cumulative optical density/area) of VEGF was calculated. The flow cytometry was employed to detect the expression of serum interleukin-10 (IL-10) and interferon (IFN-γ).
2.5. Statistics
The experimental data were treated with SPSS 20.0. t-test was performed for the comparison between the means of samples, while the one-way analysis of variance for the comparison among multisample comparison and SNK test for the comparison between groups. The rank sum test was performed for the data with the abnormal distribution. P<0.05 was regarded as the statistical significance.
3. Results
3.1. Transplantation and differentiation of BMMSCs
After the transplantation of BMMSCs in the model group, the red fluorescence marker could be observed in the gastric samples of rats. The marker was mainly centralized in the gastric body, with limited distribution in the gastric antrum, but none in the pylorus. The overlapping distribution of red and blue fluorescence markers represented the transplanted BMMSCs (Figure 1 A-B), while the overlapping distribution of red, blue and green fluorescence markers meant that the transplanted BMMSCs could be differentiated into the epithelial and interstitial cells. After the transplantation of BMMSCs in the control group, there was no such red fluorescence marker. According to the observation results, it could be proved that BMMSCs could be transplanted in the gastric body and antrum of rats with gastric precancerous lesions and could be differentiated into the epithelial and interstitial cells.
3.2. Expression of VEGF
The expression of VEGF in each group was shown in Figure 2,which was mainly distributed in the cytoplasm and membrane of gastric mucosa tissue. The expression of VEGF was low in the normal gastric mucosa tissue of control group. The expression of VEGF in the transplantation group and non-transplantation group was significantly higher than that in the control group (P<0.05). The further comparison showed that, the expression of VEGF in the transplantation group was significantly higher than that in the nontransplantation group (t=3.88, P<0.001), as shown in Figure 3.
3.3. Expression of serum cytokine
The expression of serum IL-10 and IFN-γ in the nontransplantation group and transplantation group was significantly higher than that in the control group (P<0.05). The further comparison showed that, the expression of serum IL-10 and IFN-γ in the transplantation group was significantly lower than that in the non-transplantation group (t=3.03, P=0.004; t=3.80, P<0.001), as shown in Table 1.
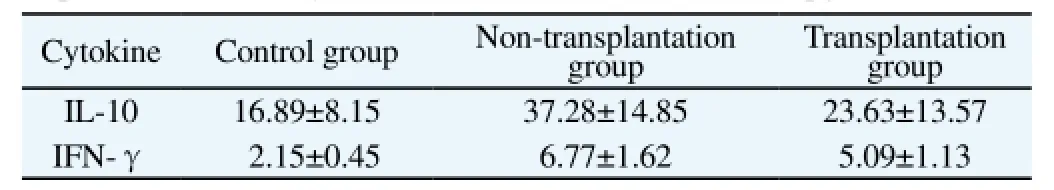
Table 1 Expression of serum cytokines of IL-10 and IFN-γ in rats (pg/mL).
4. Discussion
The gastric precancerous lesions mainly include epithelial metaplasia and dysplasia, which are mainly coexisted in the chronic atrophic gastritis. The gastric atrophy, epithelial metaplasia and dysplasia are the evolution process before cancerization. But not all precancerous lesions will be developed into gastric cancer. So that how to block or reverse such process is a key method to avoid the cancerization[11-13]. In this study, the MNNG method was employed for building the rat model of gastric precancerous lesions. The pathological figure of gastric cancer of rats is similar with the one of human. During the modeling, the incidence of gastric mucosal atrophy, intestinal epithelial metaplasia and dysplasia was up to 95%, with a satisfied success rate[14]. It's found that the gastric precancerous lesions were mainly centralized in the gastric body, with limited distribution in the gastric antrum, but none in the pylorus. Deng Dajun[15] firstly adopted the MNNG method to build the rat model of dysplasia. After the irrigation of 0.4 mg/mL MNNG,it was found that 70% of dysplasia lesions were located in the gastric antrum, which was a bit different from the findings of this study. The reason might be that the dosage of MNNG is different. Besides, it was the modeling of dysplasia in the above literature, while in this study, the occurrence of gastric mucosal atrophy, intestinal epithelial metaplasia and dysplasia would be regarded as the successful modeling, which might lead to different results. But according to the previous researches, the local lesions in the gastric body or gastric antrum can be treated as the ideal model.
BMMSCs are the adult stem cells from mesoderm, with the strong function of self-renewal and differentiation. Under different conditions, they can be differentiated into the mesoderm-originated bone, cardiac muscle and fat cells, as well as the lung, intestines,epithelial and nerve cells across the blastoderm. According to the previous researches, the intravenous injection of BMMSCs could increase the expression of VEGF and its receptor and thus promote the healing of gastric ulcer[16-19]. In this study, it was labeled with CM-dil, which could understand the migration and transplantation in the body. The related literature also reported that the CM-dil within the certain concentration range would not affect the activity and differentiation of BMMSCs[20]. For the observation under the microscope, it was found that the transplantation of BMMSCs could be found in the gastric body and gastric antrum in the transplantation group, but not in the pylorus, which might be related to the coverage of squamous epithelium and insufficient blood supply in the pylorus. The results of fluorescence localization showed that BMMSCs could be differentiated into epithelial and interstitial cells, which might be lated to the directional differentiation of BMMSCs. To be specific, could block the chemotaxis in the inflammatory and injury sites and then back to the chronic inflammation sites, which would be differentiated into the correct phenotypes to repair the inflammatory and injury cells.
The study also found that the transplantation of BMMSCs could increase the expression of VEGF in rats. VEGF is some kind of proangiogenic factor, which can act on the vascular endothelial cells,induce the angiogenesis and maintain the vascular permeability[21]. After the transplantation, BMMSCs could secrete the growth factors such as VEGF, maintain the repairing of gastric mucosa epithelialcells and establish the protection mechanism of mucosa. The expression of VEGF in the transplantation group was significantly higher than that in the non-transplantation group, while the expressions of VEGF in both groups were all significantly higher than that in the healthy rats without the treatment, which indicated that when the gastric mucosa had injury and lesions, the expression of VEGF was increased to enhance the repairing of gastric mucosa and the transplantation of BMMSCs could further induce the release of VEGF to strengthen the protection[22]. The immune factors of IL-10 and IFN-γ belong to the cell immune-related factors. BMMSCs have a certain capacity of immune regulation, which could transplant T-lymphocytes to reduce the harm to the local inflammatory response[23]. In this study, it's found that the expression of serum IL-10 and IFN-γ in rats with the gastric precancerous lesions was significantly higher than that in the healthy rats, which indicated that after the induction, there was strong inflammatory response in the gastric mucosa tissue of rats. The expression of serum IL-10 and IFN-γ in the transplantation group was significantly lower than that in the non-transplantation group, which indicated that the transplanted BMMSCs could inhibit the activation of T-lymphocytes and reduce the release of inflammatory factors.
In conclusion, BMMSCs can not only be directionally differentiated into the gastric epithelial and interstitial cells to protect the gastric mucosa, but also can regulate the expression of related growth factors and inflammatory factors to reduce the damage of inflammatory response and thus relieve or reverse the process of gastric precancerous lesions.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Zhang H, Ding C, Suo Z, Kang Y. Effect of Helicobacter pylori on cyclooxygenase-2 and inducible nitric oxide synthase in patients with gastric precancerous lesions and its clinical significance. Exp Ther Med 2015; 9(6): 2364-2368.PMID: 26136988.
[2] Mansour-Ghanaei F, Joukar F, Rajpout Y, Hasandokht T. Screening of precancerous gastric lesions by serum pepsinogen,gastrin-17,antihelicobacter pylori and anti-CagA antibodies in dyspeptic patients over 50 years old in Guilan Province, north of Iran. Asian Pac J Cancer Prev 2014; 15(18): 7635-7638.PMID: 25292040.
[3] Wang DQ, Ding XP, YinS. Effect of proinflammatory cytokine in the gastric precancerous lesions. World Chin J Digestol 2014; 1: 39-45.
[4] Zhao HJ, Shen LZ. Advances in gastric intestinal metaplasia. Chin J Bases Clin General Surg 2015; 6: 767-771.
[5] Zhao Z, Fang C, Wang F, Shuang J, Wang G, Hua J, et al. Expression and clinical significance of gastric dramatic down-related gene in gastric cancer and precancerous lesions. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014; 30(3): 306-308. PMID: 24606753.
[6] Li P, Ma D, Zhu ST, Tang XD, Zhang ST. Serum peptide mapping in gastric precancerous lesion and cancer. J Dig Dis 2014; 15(5): 239-245. PMID: 24438315.
[7] Bar-Or D, Thomas GW, Rael LT, Gersch ED, Rubinstein P, Brody E. Low molecular weight fraction of commercial human serum albumin induces morphologic and transcriptional changes of bone marrowderived mesenchymal stem cells. Stem Cells Transl Med 2015; 4(8): 945-955. PMID: 26041739.
[8] Qi M, Liu W, Ma Y, Yang Y, Jin Y. Spermidine enhances osteogenic differentiation and inhibits adipogenic differentiation of bone marrowderived mesenchymal stem cells from ovariectomized mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015; 31(6): 787-791. PMID: 26062423.
[9] Pan X, Peng L, Yin G. Downregulation of Annexin A1 by short hairpin RNA inhibits the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Int J Mol Med 2015; 36(2): 406-414. PMID:26063293.
[10] Huang YQ. Advances of mesenchymal stem cells in the treatment of digestive system diseases. World Chin J Digestol 2015; 17: 2688-2696.
[11] Mansour-Ghanaei F, Joukar F, Mojtahedi K, Sokhanvar H, Askari K,Shafaeizadeh A. Does treatment of Helicobacter pylori infection reduce gastric precancerous lesions? Asian Pac J Cancer Prev 2015; 16(4): 1571-1574. PMID: 25743833.
[12] Jiang N, Huang Z, Fan YH, Ni GB, Lv B, Meng LN, et al. Effect of herb therapy on expression of APC, p53 and Ki67 proteins in patients with gastric precancerous lesions. Chin J Trad Chin Med Pharm 2013; 2: 356-360.
[13] Zhou L, Zhao Y, Zhu W, Shen YP. Influencing factors of reverse of gastric precancerous lesions. Chin J Public Health 2013; 8: 1154-1157.
[14] Li CY, Liang AH, Gao SR, Hui LQ, Liu T, Cao CY, et al. Development of gastric precancerous lesion animal model. Chin J Chin Materia Med 2012; 1: 89-93.
[15] Deng DJ, Zhu SX, Chen Q. Modeling of MNNG-induced prostate and gastric cancers of neonatal rats and its application in the study of pathogenesis of gastric cancer. Chin J Pathol 1994; 23(5): 293-295.
[16] Tseng KY, Lin S. Zinc finger factor 521 enhances adipogenic differentiation of mouse multipotent cells and human bone marrow mesenchymal stem cells. Oncotarget 2015; 6(17): 14874-14884. PMID:26008984.
[17] Chen J, Shao Y, Xu G, Lim C, Li J, Xu D, et al. Bone marrow-derived mesenchymal stem cells attenuate phosgene-induced acute lung injury in rats. Inhal Toxicol 2015; 27(5): 254-261. PMID: 25970824.
[18] Liu DL. A study on mechanism of mesenchymal stem cells in the apoptosis of hepatic stellate cells. Chin J Tissue Eng Res 2015; 23: 3639-3643.
[19] Ge ZM, Guo ZH, Gao F. Separation, culture and identification of mesenchymal stem cells of rats. Chin J Clin Res 2015; 7: 836-839.
[20] Weir C, Morel-kopp MC, Gill A, Tinworth K, Ladd L, Hunyor SN, et al. Mesnchymal stem cells: isolation,characterization and in vivo fluorescent dye tracking. Heart Lung Circ 2008; 17: 395-403. PMID: 18396458.
[21] Ou YR, Kang M, Zhou L, Cheng ZY, Tang SL, Yu DH. Infection with L-form of Helicobacter pylori and expressions of MIF, MMP9 and VEGF in gastric carcinoma. J Southern Med Univ 2014; 2: 180-187.
[22] Díaz-Rodríguez P, Gómez-Amoza JL, Landin M. The synergistic effect of VEGF and biomorphic silicon carbides topography on in vivo angiogenesis and human bone marrow derived mesenchymal stem cell differentiation. Biomed Mater 2015; 10(4): 045017. PMID: 26238485.
[23] Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang T, et al. Distribution of Th17 cells and Foxp3-expressing T cells in rumor-infiltrating lymphocytes in patients with uterine cervical cancer. Clin Chim Acta Int J Clin Chem 2012; 413(23-24): 1848-1854. PMID: 22820395.
15 September 2015
Lin Zhang, MD, Department of Gastroenterology, First Affiliated Hospital of Fujian Medical University, No.20 Chazhong Road, Fuzhou,Fujian, 350005, China.
Tel: 13805068799
E-mail: zjtfujian2014@163.com
Foundation project: It was supported by Training Project (2015-ZQN-JC-23) for Middle-aged and Young Backbones of Heath System of Fujian Province.
 Asian Pacific Journal of Tropical Medicine2015年12期
Asian Pacific Journal of Tropical Medicine2015年12期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Immunomodulatory effect of garlic oil extract on Schistosoma mansoni infected mice
- Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae)
- Human ocular dirofilariasis due to Dirofilaria repens in Sri Lanka
- Childhood brucellosis: Review of 317 cases
- Effect of cyclophosphamide on fungal infection in SLE mice detected by fluorescent quantitative PCR
- Therapeutic effect of okra extract on gestational diabetes mellitus rats induced by streptozotocin
