纳米团簇Au19Pd和Au19Pt催化解离N2O
俞炜铃 左会文 陆春海 李 奕 章永凡 陈文凯,*
(1福州大学化学系,福州350116;2成都理工大学核技术与自动化工程学院,成都610059)
纳米团簇Au19Pd和Au19Pt催化解离N2O
俞炜铃1左会文1陆春海2,*李 奕1章永凡1陈文凯1,*
(1福州大学化学系,福州350116;2成都理工大学核技术与自动化工程学院,成都610059)
采用密度泛函理论研究Au-Pd和Au-Pt纳米团簇催化解离N2O.首先根据计算得到Au19Pd和Au19Pt团簇的最优构型(杂原子均位于团簇的表面).以Au19Pd催化解离N2O为例研究催化解离的反应机理.对此主要考虑两个反应机理,分别是Eley-Rideal(ER)和Langmuir-Hinshelwood(LH).第一个机理中N2O解离的能垒是1.118 eV,并且放热0.371 eV.N2分子脱附后,表面剩余的氧原子沿着ER路径消除需要克服的能垒是1.920 eV,这比反应沿着LH路径的能垒高0.251 eV.此外根据LH机理,氧原子在表面的吸附能是-3.203 eV,而氧原子在表面转移所需的能垒是0.113 eV,这表明氧原子十分容易在团簇表面转移,从而促进氧气分子的生成.因此,LH为最优反应路径.为了比较Au19Pd和Au19Pt对N2O解离的活性,根据最优的反应路径来研究Au19Pt催化解离N2O,得到作为铂族元素的铂和钯对N2O的解离有催化活性,尤其是钯.同时,将团簇与文献中的Au-Pd合金相比较,得到这两种团簇对N2O解离有较高的活性,尤其是Au19Pd团簇.再者,O2的脱附不再是影响反应的主要原因,这可以进一步提高团簇解离N2O的活性.
纳米团簇;催化活性;N2O解离;反应机理
1 Introduction
Recently,metallic nanocluster with unique shape and size exhibits unusual physical and chemical properties.They have many applications in the fields of magnetic,optical,and electronic materials,catalysts,and drug delivery and so on,which have been of great interest and intensely researched.1-24Therefore morphology of nanocluster can control reactivity and selectivity. Furthermore,multimetallic cluster composed of two or more elements possesses activity,selectivity,and stability,in which there exist structural,ligand,or electronic effects.25,26It is found that bimetallic nanoclusters have been particularly attractive because of the improvement of catalytic properties.27-30And also bimetallic catalysts have been investigated to obtain the relationship between the metal structure and catalytic activity.31Note that it is elusive for catalytic origin of gold or gold-based bimetallic catalysts with nanosize because of the lack of understanding on the nanoscale core-surface property correlation.However,it is interesting to find that the gold-based bimetallic materials of nanoscale exhibit synergistic effect,which are the protection from poisonous species adsorption and change for electronic band structure to alter the adsorption ability of surface.
In the magical world of gold clusters,there is a gold cluster containing 20 gold atoms(Au20),which is enough chemical inert and a highly stable cluster with a tetrahedral pyramidal structure via ab initio DFT-based calculation32-34and experimental studies like far-infrared vibrational spectroscopy and photoelectron spectroscopy.33There is a large energy gap between the highest occupied molecular orbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)for Au20cluster,which is superior to that of C60to manifest that Au20cluster should be a chemical inert and stable cluster.35Meanwhile there are some literature on interaction between a pyridine molecule and surface ofAu20cluster36and catalytic activity ofAu20cluster with an anion to CO oxidation,37which indicate that the Au20cluster is also a good catalyst.Furthermore,multimetallic nanoclusters intimately alloyed are of high interest due to their optical and catalytic properties.38-40In particular,these bimetallic gold clusters have more widely catalytic applications.And also some literature has reported the electronic properties and geometries of gold clusters with the doped transition metal or alkali atoms.41-52
TheAu-Pd bimetallic alloys have been widely used as catalysts because of their superior catalytic activities in many reactions,24,53-70like selective oxidation for alcohols,62-67synthesis for vinyl acetate,60,61direct synthesis for H2O2on the surface of the Au-Pd bimetallic alloy,53-59selective oxidation for alkene,68generating benzene via coupling acetylene,24and oxidation for primary carbon-hydrogen bond.69And also palladium serves as a catalyst for CO oxidation and Suzuki reaction in the form of monometal and bimetal.71-73Moreover,the platinum has many applications including CO/NOxoxidation,syngas reformation,and petroleum refinement as an excellent catalysts.The catalytic activities of Au-Pt nanoparticles are superior to those nanoclusters containing gold or platinum alone,which is indicated by recent theoretical studies.74Therefore Pt-doped gold clusters have attracted the special attention because of many potential applications in catalysis.75
Furthermore,the catalytic decomposition of nitrous oxide has been attracting much attention to propulsion systems for satellites76and environmental protection.77Meanwhile,Rh-,78-88Ir-,76,89-91and Pd-supported catalysts78-88viewed as platinum group metal catalysts possess effective catalytic activities for this reaction. However,the platinum group metals have the strong adsorption toward oxygen.It is cumbersome for desorption of the oxygen molecule on the metal surface,which is usually viewed as a ratedetermining step in the process of N2O decomposition,92-95while desorption of O2on the gold can be more easily.96And also the recent study presents that the presence of gold could have no obvious influence on the catalytic activity of platinum in any significant way.97
Therefore the single gold atom on the cluster can be substituted with the palladium or platinum atom in the Au20cluster.To investigate the catalytic activities of the doped gold clusters for N2O decomposition,we have studied the reaction mechanism of N2O dissociation on the cluster.Note that the mechanisms of the nitrous oxide dissociation on the surface of metal cluster have been simply indicated as follows:98,99

Here,the step 1 means that nitrous oxide on the active site of the cluster decomposes to generate N2molecule leaving from the cluster and oxygen atom on the cluster.Step 2 presents the two adjacent oxygen atoms on the surface close to each other and then are desorbed by oxygen molecule.O(a)means the oxygen atom on the catalyst.Both of the step 1 and step 2 would be described by the LH mechanism.Meanwhile the step 3 shows the oxygen atom adsorbed on the cluster is removed by the ER mechanism.100Dandl and Emig101presented that the ER mechanism could be more inclined at the lower temperature while the LH mechanism might be more inclined at the higher temperature according to the kinetics simulation.
In the present work,we want to obtain the most optimized geometries via structural and thermodynamic analyses in orderto investigate their catalytic activities for N2O decomposition. There are two reaction pathways for N2O decomposition on the cluster to be considered:ER and LH,respectively.And we select Au19Pd cluster as a model cluster to investigate the reaction mechanism via DFT-based calculation.The catalytic activity of the Au19Pt cluster for N2O decomposition has been studied in comparison with the Au19Pd as a function of the optimized reaction channel,which indicates how the different heteroatom located at the same site on the cluster influences the activity of the cluster in this reaction.Finally,comparison between this work and the study on the periodic systems presents the influence of the geometry on the catalytic activity of the cluster in this reaction.
2 Computational method
To investigate catalytic activities of Au19Pd and Au19Pt clusters for N2O molecule decomposition,the program package Dmol3of Materials Studio of Accelrys Inc has been performed to optimize geometries and search transition states.Generalized gradient approximation(GGA)with exchange-correlation functional proposed by Perdew,Burke,and Ernzerhof(PBE)was performed. The DFT semicore pseudopotential was employed for core electrons of gold,palladium,and platinum atoms,but the N and O atoms were treated with all-electron basis set.Meanwhile the double-numerical basis with polarization functions(DNP)has also been employed in the calculation.The Fermi smearing method for a window size was set as 0.005 hartree,and it was 0.45 nm for orbital cutoff range.The energy,maximum displacement,and maximum force for convergence tolerance were 10-5hartree, 0.0005 nm,and 0.02 hartree·nm-1,respectively.Every atom of the cluster was relaxed in the calculation.The transition states were determined by the complete linear synchronous transit and quadratic synchronous transit methods.Every transition state structure has a single imaginary frequency,which corresponds to the reaction pathway.
In order to investigate and compare the stability between Au19Pd and Au19Pt clusters,we have calculated the bind energy (BE)and the interaction energy(IE)of the heteroatom with the Au19cluster.
The bind energy forAu19Pd orAu19Pt cluster was calculated via
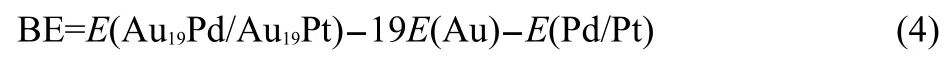
Meanwhile the interaction energy of the heteroatom with the Au19cluster was obtained by performing

Here,E(Au19Pd/Au19Pt),E(Au),E(Pd/Pt),and E(Au19)mean the energies for Au19Pd or Au19Pt,gold atom,palladium or platinum atom,andAu19clusters,respectively.
And then we calculated adsorption energy using following equation

where Eadsis the adsorption energy for the system,Esystemmeans the total energy of the substrate and absorbate,the Eclusterand Eabsorbatepresent the energies of cluster and absorbate,respectively.
3 Result and discussions
3.1 Investigation for structural properties of the Au19Pd and Au19Pt clusters

Fig.1 Optimized geometries ofAu19Pd andAu19Pt clusters with heteroatom located at(a)vertex(V),(b)edge(E),and(c)surface(S)
The heteroatom can be on the vertex(V),edge(E),or surface (S)of the cluster,as shown in Fig.1.It is noted that the radii of palladium and platinum atoms are 0.137 and 0.139 nm,respectively,which are close to the radius of gold atom,0.144 nm.Fig.1 presents that there is no obvious deformation for the geometries of Au19Pd and Au19Pt clusters in comparison with the Au20cluster with a tetrahedral structure after optimization.Furthermore,we have calculated the bond distances between the heteroatom andthe nearest adjacent gold atom.And also we have obtained the corresponding bond distances on the Au20cluster.These bond distances are compiled in Table 1.The letter X means the gold atom on the Au20cluster and heteroatom on the clusters shown in Fig.1,respectively.And also the letter in the bracket in the first column indicates the position of atom on the cluster,in which the E1 and E2 present the gold atom on the edge without and with the heteroatom,respectively.It is found that there is no obvious elongated or shorter for these bond distances,which indicate that the both of the palladium and platinum can not make configurations obvious deformations.
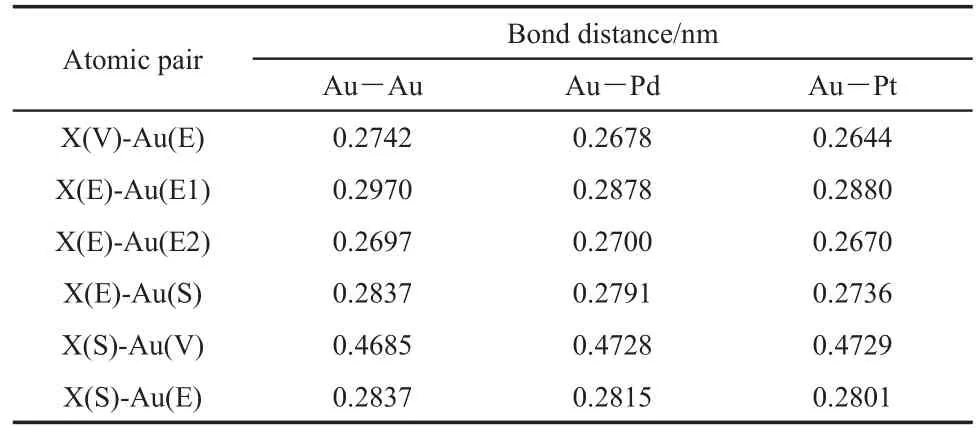
Table1 Bond distances between the X atom and its nearest adjacent gold atoms
Moreover we have studied the thermodynamic stability of these clusters shown in Fig.1 as functions of the bind energy and interaction energy via the DFT-based calculation.These results are compiled in the Table 2.It is noted that the more negative value indicates that the system may be more optimized.Therefore both of the palladium and platinum can make cluster stable via thermodynamic analysis.Furthermore,the heteroatom locates at the surface of the cluster to obtain the more optimized geometry, especially the platinum atom,which is also demonstrated by the recent study.102It is found that the heteroatom is on the surface of the cluster and surrounded by gold atoms,which is similar to the surface of the Au3Pd(111)alloy in the previous study.103The catalytic activity of theAu-Pd alloy for N2O decomposition has been investigated via DFT-based calculation,in which the palladium atom is also isolated by the gold atoms.103We have investigated catalytic activities of these two clusters with the heteroatom on the surface for N2O decomposition.

Table2 Binding energy for every atom and the interaction energy for the gold and heteroatoms with the correspondingAu19clusters
3.2 Investigation for N2O decomposition on the Au19Pd and Au19Pt clusters
It is found that the platinum group metal(PGM)catalysts are effective for N2O decomposition.82,104-108Therefore both of the palladium and platinum atoms on the clusters are considered as active sites in this study.The adsorption of N2O on the cluster is an elementary step.In the previous study,N2O is weakly and tiltedly on the active site via terminal N atom.100,103,109We have obtained the optimized adsorption geometries for N2O on the clusters via DFT-based calculation,as shown in Fig.2,which are the most stable.
The bond distances of N—N and N—O in the N2O molecule are 0.1142 and 0.1195 nm before adsorption.They are slightly elongated after N2O adsorption on the clusters,as shown in Fig.2, indicating that N2O can be activated.And the adsorption energies for N2O on the Au19Pd and Au19Pt cluster are-0.176 and-0.087 eV,respectively,suggesting that they may be physical adsorption. Furthermore,it is found that the d orbital of heteroatom and p orbital of N2O molecule can play major roles for the adsorption of the N2O on the active site in this study.Therefore the partial density of states(PDOSs)for the N2O molecule,Pd and Pt before and after adsorption are obtained,as shown in Fig.3,to better understand the interaction between the N2O molecule and heteroatom on the surface of the cluster.The p orbital of N2O molecule shifts to the lower energy region and there is a wide range near the HOMO,which overlaps with the d orbital of the heteroatom,after adsorption,suggesting the charge transfer between heteroatom and the absorbate so that it can slightly activate N2O molecule in the reaction.Moreover the different heteroatom has no obvious influence on the adsorption of the N2O on the cluster in this study.
Fig.4 presents the first N2O decomposition on theAu19Pd cluster on the basis of the reaction mechanism.It is found that the adsorption energy is-0.176 eV for N2O on the Au19Pd cluster,but adsorption energies of N2O on the Pd(111)and Au3Pd(111) are-0.120 and-0.080 eV,respectively.The energy barrier and the value of reaction energy for the first N2O decomposition on the Au19Pd cluster are 1.118 and-0.371 eV,respectively,which are larger than those on the Pd(111),but less than those on theAu3Pd (111).And also the imaginary frequency of the transition state in the energy profile is-505.62i cm-1.It is noted that the palladium atom is isolated by gold atoms on surface of the Au19Pd cluster, which is similar to the surface of Au3Pd(111).Therefore we haveobtained that Pd is the active site in this reaction,but the gold can weaken the catalytic activity of substrate for the first N2O decomposition in this study.However,the structure of the substrate is changed so that it can improve the catalytic activity.Finally,we have obtained that the Au19Pd cluster has better catalytic activity for the first N2O decomposition.

Fig.3 Partial density of states(PDOSs)for N2O,Pd,and Pt before and after adsorption,in which the adsorption systems are as a function of the corresponding configurations in Fig.2
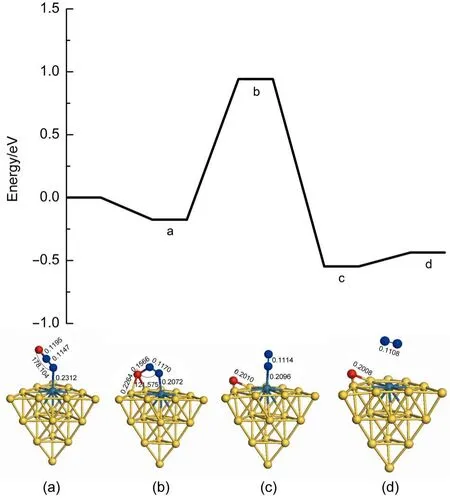
Fig.4 Energy profile for the first N2O decomposition on theAu19Pd cluster

Fig.5 Energy profile for the first N2O decomposition on theAu19Pt cluster
Furthermore,the palladium has been substituted with the platinum atom on the surface of the cluster.Then we can compare catalytic activity between the Au19Pd and Au19Pt clusters for N2O decomposition.Therefore Fig.5 has been obtained.The adsorption energy of N2O on the platinum atom on the surface of cluster is-0.087 eV,which is more weakly than that on the Au19Pd cluster with the adsorption energy of-0.176 eV.However,the energy barrier of N2O decomposition on the platinum atom on the cluster is 0.989 eV,which is slightly lower than that on theAu19Pd cluster with the energy barrier of 1.118 eV.The reactions on theAu19Pt andAu19Pd clusters are exothermic by 0.303 and 0.371 eV, respectively.And also the N2desorption energies on these two substrates are 0.258 and 0.110 eV,respectively.Therefore platinum atom possesses effective catalytic activity for the first N2O decomposition as well as palladium atom on the surface of the cluster.However,N2O adsorption and N2desorption on Au19Pt cluster are more difficult than those on the Au19Pd cluster,which can weaken the catalytic activity of cluster.Thus the Au19Pd has a little bit better catalytic activity than Au19Pt cluster for the first N2O decomposition.Meanwhile,an atom on the cluster can be changed to enhance the catalytic activity.
There are two reaction pathways to clear residual oxygen atom on the cluster after the first N2O decomposition and N2desorption, which are the ER and LH reaction channels,respectively.The Au19Pd cluster is similar to the Au19Pt cluster for the influence on the reaction pathway.Therefore we can select the Au19Pd as a model to investigate the reaction mechanism after the first N2O decomposition and N2desorption.Fig.6 indicates that the second N2O on the palladium reacts with the residual oxygen atom to generate N2and O2molecules along the ER pathway,in which the energy barrier is 1.920 eV and the reaction energy is 0.454 eV. However,the second N2O can continue decomposing to leave oxygen atom on the cluster.Therefore oxygen atoms on the cluster are removed by the LH pathway,as shown in Figs.7 and 8.The adsorption energies of single oxygen atom and two adjacent oxygen atoms on the cluster are-3.203 and-6.173 eV,respectively, indicating that oxygen is very easily on the heteroatom and these two oxygen atoms are prone to be close to each other.There is a low energy barrier of 0.113 eV and it is endothermic by 0.099 eV in the oxygen atom transferring on the cluster after N2desorption, suggesting that the surface of the cluster benefits the diffusion of the oxygen atom.It is found that the formation of oxygen molecule has an energy barrier of 1.669 eV and is exothermic by 0.324 eV,which are less difficult than the elimination of the residual oxygen atoms along the ER pathway.Therefore the residual oxygen atoms on the cluster can be more prone to be desorbed by the LH pathway.
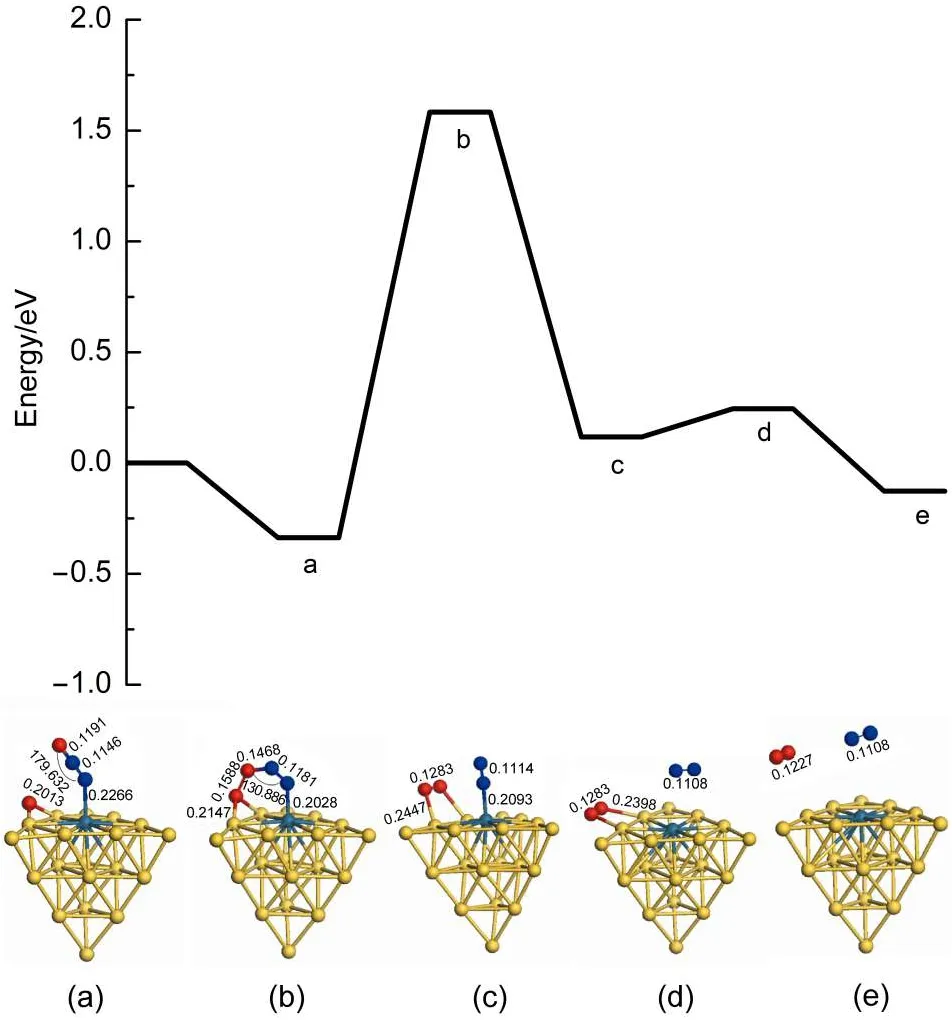
Fig.6 Energy profile for the elimination of residual oxygen atom on theAu19Pd cluster along the ER reaction pathway
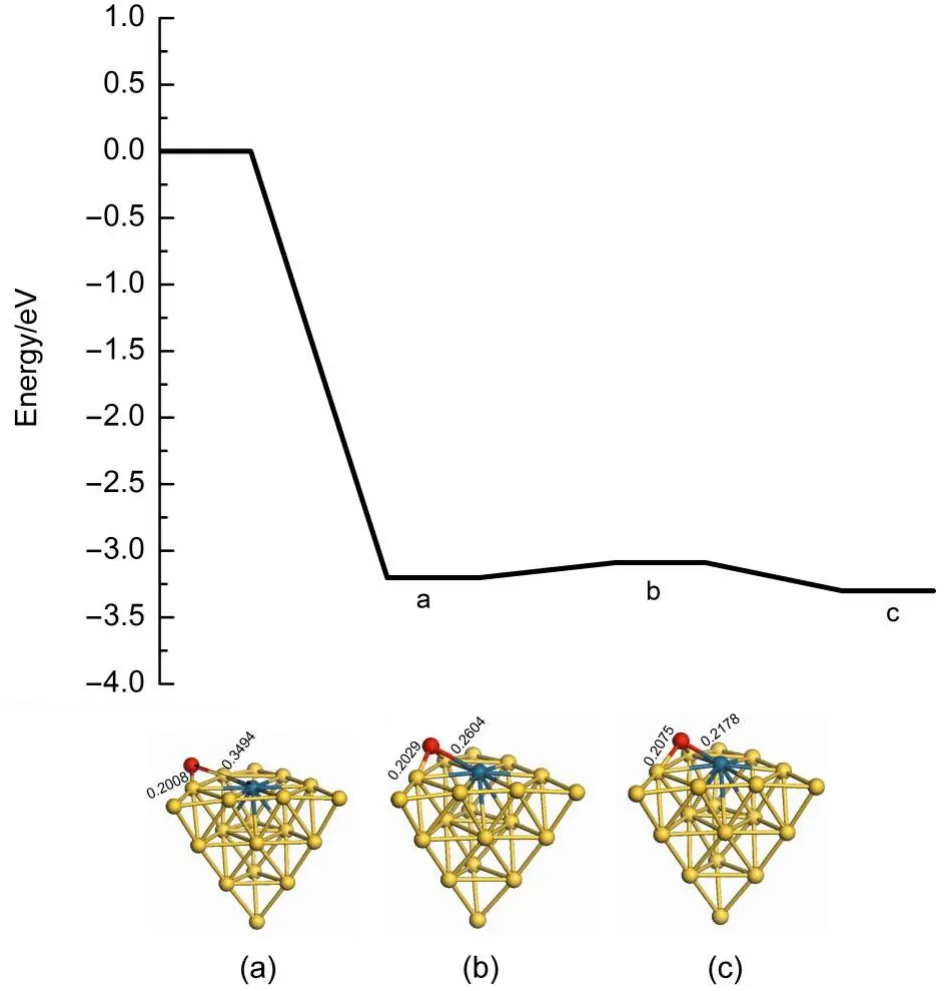
Fig.7 Energy profile for the diffusion of the oxygen atom on the Au19Pd cluster after N2desorption

Fig.8 Energy profile for the formation of oxygen molecule on the Au19Pd cluster along the LH reaction pathway
It is noted that oxygen atom diffusion on the Pd(111)has anenergy barrier of 0.540 eV and is endothermic by 0.230 eV,as illustrated in the Table 3.Then the elimination of the residual oxygen atoms on the Pd(111)has an energy barrier of 1.960 eV and is endothermic by 0.860 eV along the LH pathway,which illustrates that the formation of the oxygen molecule on the palladium should be more difficult.Furthermore,Table 3 presents the oxygen atom is more easily transferring on the surface ofAu3Pd (111)and elimination of two adjacent oxygen atoms on the surface has a low energy barrier of 0.500 eV.However,the adsorption energy of two adjacent oxygen atoms is 1.110 eV,indicating that the gold benefits the formation of the oxygen molecule,but hinders the adsorption of the oxygen.Moreover it is interesting to find that O2desorption,which used to be thought to be a ratelimiting step,becomes more easily in this study,indicating that the existence of the gold can promote desorption of O2molecule to improve the catalytic activity of cluster for N2O decomposition. Therefore Au19Pd cluster also possesses high catalytic activity in this reaction process,which can promote the adsorption of the oxygen,the formation,and desorption of the oxygen molecule. We have found the importance of the structure of the catalyst in the reaction further.
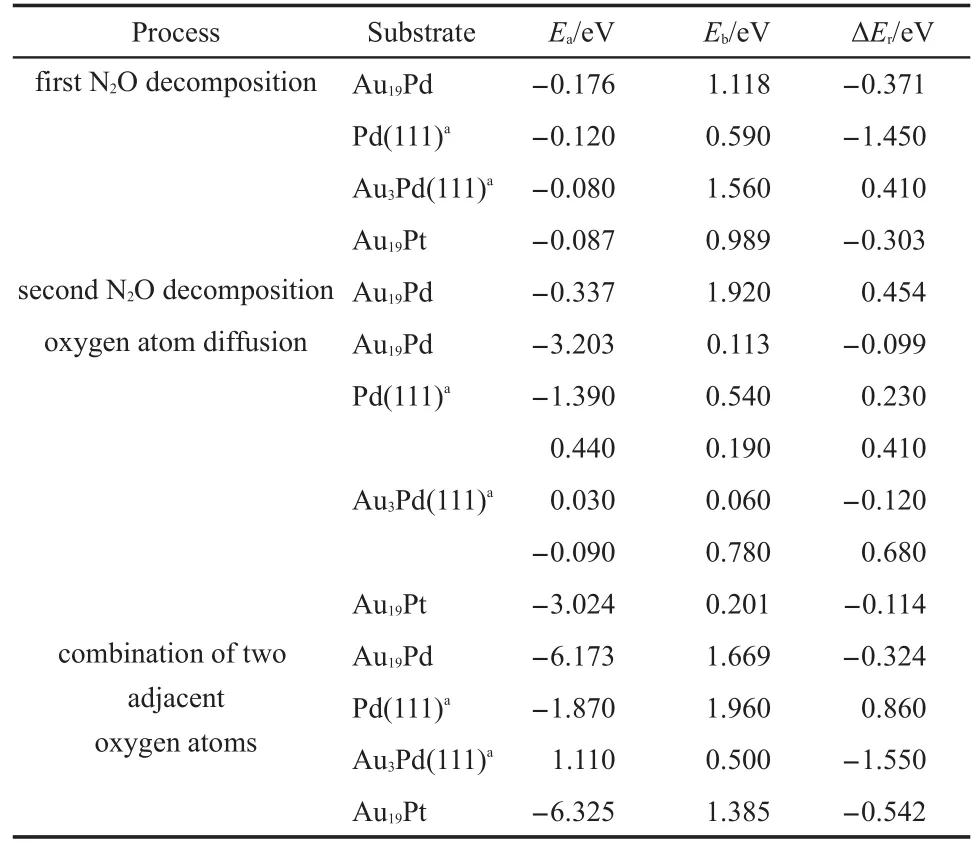
Table3 Comparison of the adsorption energy(Ea),energy barrier(Eb),and reaction energy(ΔEr)of N2O decomposition on theAu19Pd andAu19Pt cluster with literature values

Fig.9 Energy profile for the diffusion of the oxygen atom on the Au19Pt cluster after N2molecule desorption

Fig.10 Energy profile for the formation of oxygen molecule on the Au19Pt cluster along the LH reaction pathway
We have compared catalytic activity between the Au19Pt and Au19Pd clusters for the elimination of oxygen atoms along the LH pathway.Here,Fig.9 presents the transfer of oxygen atom on the Au19Pt cluster surface after N2desorption.The adsorption energy of oxygen atom on the surface is-3.024 eV,signifying that the Au19Pt cluster is very favourable for oxygen atom adsorption. Then energy barrier and reaction energy are 0.201 and-0.114 eV for the oxygen atom transferring on the surface,respectively, suggesting that this reaction process is prone to proceed.Then the formation of oxygen molecule on the Au19Pt cluster is shown in Fig.10.The energy barrier for formation of oxygen molecule on theAu19Pt cluster is 1.385 eV,which is 0.284 eV lower than 1.669 eV on the Au19Pd cluster.And also the reaction energy is-0.542 eV,which is 0.218 eV lower than-0.324 eV on theAu19Pd cluster. Therefore Au19Pt cluster has higher activity than Au19Pd cluster in this reaction process.However,O2desorption energy on theAu19Pt cluster is 0.603 eV,which is 0.335 eV higher than 0.268 eV on the Au19Pd cluster so that it can weaken the activity of the Au19Pt cluster.Finally,it has obtained that they have similar activity inthis reaction process.
4 Conclusions
In summary,the heteroatom is located at the surface of the cluster to obtain more optimized configuration on the basis of the structural and thermodynamic analyses via DFT-based calculation. And also it is found that the LH is a superior reaction pathway for N2O decomposition on the Au19Pd and Au19Pt clusters.The palladium has effective activity for N2O decomposition,but hinders the formation and desorption of the oxygen molecule in comparison with the investigation of the N2O dissociation on the Pd (111)via DFT-based calculation,which weakens the catalytic activity.Gold on the Au3Pd(111)promotes the formation of oxygen molecule on the surface,but makes the adsorption of the oxygen atoms become more difficult,which obviously lowers the activity of the catalyst.In this study,bimetallic clusters with the heteroatoms on the surfaces have high catalytic activities in a complete process of N2O decomposition and improve the O2desorption,which used to be thought to be a rate-limiting step,especially for Au19Pd cluster.Therefore the change of the geometry and the single atom on the cluster can present different activities. Finally,it is hoped that these could provide clue for further explanation on the influence of the geometry for catalytic activity.
(1)Burda,C.;Chen,X.;Narayanan,R.;El-Sayed,M.A.Chem. Rev.2005,105,1025.doi:10.1021/cr030063a
(2)Ghosh,S.K.;Pal,T.Chem.Rev.2007,107,4797.doi:10.1021/ cr0680282
(3)Jin,R.;Cao,Y.;Mirkin,C.A.;Kelly,K.;Schatz,G.C.;Zheng, J.Science2001,294,1901.doi:10.1126/science.1066541
(4)Sun,Y.;Xia,Y.Science2002,298,2176.doi:10.1126/ science.1077229
(5)Narayanan,R.;El-Sayed,M.A.J.Am.Chem.Soc.2004,126, 7194.doi:10.1021/ja0486061
(6)Tian,N.;Zhou,Z.Y.;Sun,S.G.;Ding,Y.;Wang,Z.L.Science2007,316,732.doi:10.1126/science.1140484
(7)Warren,S.C.;Messina,L.C.;Slaughter,L.S.;Kamperman, M.;Zhou,Q.;Gruner,S.M.;DiSalvo,F.J.;Wiesner,U.Science2008,320,1748.doi:10.1126/science.1159950
(8)Sulman,E.;Matveeva,V.;Doluda,V.;Nicoshvili,L.;Bronstein, L.;Valetsky,P.;Tsvetkova,I.Top Catal.2006,39,187.doi: 10.1007/s11244-006-0056-z
(9)Ozin,G.A.Adv.Mater.1992,4,612.
(10)Cahn,R.W.Nature1992,359,591.doi:10.1038/359591a0
(11)Hayashi,C.Phys.Today1987,12,44.
(12)Gleiter,H.Prog.Mater.Sci.1989,33,223.doi:10.1016/0079-6425(89)90001-7
(13)Fendler,J.H.Chem.Rev.1987,87,877.doi:10.1021/ cr00081a002
(14)Henglein,A.Chem.Rev.1989,89,1861.
(15)Schimid,G.Clusters and Colloids:from Theory to Application; Wiley-VCH:New York,1994.
(16)Toshima,N.;Yonezawa,T.New J.Chem.1998,22,1179.doi: 10.1039/a805753b
(17)Beecroft,L.L.;Ober,C.K.Chem.Mater.1997,9,1302.doi: 10.1021/cm960441a
(18)Siegel,R.MRS Bulletin1989,14,66.
(19)Schmid,G.Chem.Rev.1992,92,1709.doi:10.1021/ cr00016a002
(20)Kamat,P.V.Chem.Rev.1993,93,267.doi:10.1021/ cr00017a013
(21)Lewis,L.N.Chem.Rev.1993,93,2693.doi:10.1021/ cr00024a006
(22)Gates,B.Chem.Rev.1995,95,511.doi:10.1021/cr00035a003
(23)Brus,L.J.Phys.Chem.1986,90,2555.doi:10.1021/ j100403a003
(24)Lee,A.F.;Baddeley,C.J.;Hardacre,C.;Ormerod,R.M.; Lambert,R.M.;Schmid,G.;West,H.J.Phys.Chem.1995,99, 6096.doi:10.1021/j100016a053
(25)Ponec,V.;Sachtler,W.J.Catal.1972,24,250.doi:10.1016/ 0021-9517(72)90069-3
(26)Sinfelt,J.H.;Carter,J.;Yates,D.J.Catal.1972,24,283.doi: 10.1016/0021-9517(72)90072-3
(27)Toshima,N.;Yonezawa,T.;Kushihashi,K.J.Chem.Soc.1993,89,2537.
(28)Toshima,N.;Harada,M.;Yamazaki,Y.;Asakura,K.J.Phys. Chem.1992,96,9927.doi:10.1021/j100203a064
(29)Harada,M.;Asakura,K.;Toshima,N.J.Phys.Chem.1993,97, 5103.doi:10.1021/j100121a042
(30)Wang,Y.;Toshima,N.J.Phys.Chem.B1997,101,5301.doi: 10.1021/jp9704224
(31)Sinfelt,J.H.Accounts Chem.Res.1987,20,134.doi:10.1021/ ar00136a002
(32)Li,J.;Li,X.;Zhai,H.J.;Wang,L.S.Science2003,299, 864.doi:10.1126/science.1079879
(33)Gruene,P.;Rayner,D.M.;Redlich,B.;van der Meer,A.F.; Lyon,J.T.;Meijer,G.;Fielicke,A.Science2008,321,674.doi: 10.1126/science.1161166
(34)Wang,J.;Wang,G.;Zhao,J.Chem.Phys.Lett.2003,380, 716.doi:10.1016/j.cplett.2003.09.062
(35)Kryachko,E.;Remacle,F.Int.J.Quantum Chem.2007,107, 2922.
(36)Aikens,C.M.;Schatz,G.C.J.Phys.Chem.A2006,110, 13317.doi:10.1021/jp065206m
(37)Molina,L.;Hammer,B.J.Catal.2005,233,399.doi:10.1016/j. jcat.2005.04.037
(38)Jonah,C.D.;Rao,B.M.Radiation Chemistry:Present Status and Future Trends;Elsevier:Amsterdam,2001.
(39)Sinfelt,J.H.Bimetallic Catalysts:Discoveries,Concepts,and Applications;Wiley:New York,1983.
(40)Belloni,J.;Mostafavi,M.;Remita,H.;Marignier,J.L.; Delcourt,M.O.New J.Chem.1998,22,1239.doi:10.1039/a801445k
(41)Pyykkö,P.;Runeberg,N.Angew.Chem.2002,114,2278.doi: 10.1002/1521-3757(20020617)114:12<2278::AID-ANGE2278>3.0.CO;2-F
(42)Häkkinen,H.;Abbet,S.;Sanchez,A.;Heiz,U.;Landman,U. Angew.Chem.Int.Edit.2003,42,1297.doi:10.1002/ anie.200390334
(43)Janssens,E.;Tanaka,H.;Neukermans,S.;Silverans,R.E.; Lievens,P.Phys.Rev.B2004,69,085402.doi:10.1103/ PhysRevB.69.085402
(44)Torres,M.;Fernández,E.;Balbás,L.Phys.Rev.B2005,71, 155412.doi:10.1103/PhysRevB.71.155412
(45)Yuan,D.;Wang,Y.;Zeng,Z.J.Chem.Phys.2005,122, 114310.doi:10.1063/1.1862239
(46)Majumder,C.;Kandalam,A.K.;Jena,P.Phys.Rev.B2006,74, 205437.doi:10.1103/PhysRevB.74.205437
(47)Walter,M.;Häkkinen,H.Phys.Chem.Chem.Phys.2006,8, 5407.doi:10.1039/b612221c
(48)Gao,Y.;Bulusu,S.;Zeng,X.C.ChemPhysChem2006,7,2275.
(49)Wang,L.M.;Bulusu,S.;Zhai,H.J.;Zeng,X.C.;Wang,L.S. Angew.Chem.Int.Edit.2007,46,2915.
(50)Wang,L.M.;Bulusu,S.;Huang,W.;Pal,R.;Wang,L.S.;Zeng, X.C.J.Am.Chem.Soc.2007,129,15136.doi:10.1021/ ja077465a
(51)Fa,W.;Dong,J.J.Chem.Phys.2008,128,144307.doi: 10.1063/1.2897917
(52)Zorriasatein,S.;Joshi,K.;Kanhere,D.G.J.Chem.Phys.2008,128,184314.doi:10.1063/1.2913153
(53)Edwards,J.K.;Solsona,B.;Ntainjua,E.;Carley,A.F.;Herzing, A.A.;Kiely,C.J.;Hutchings,G.J.Science2009,323, 1037.doi:10.1126/science.1168980
(54)Bernardotto,G.;Menegazzo,F.;Pinna,F.;Signoretto,M.; Cruciani,G.;Strukul,G.Appl.Catal.A2009,358,129.doi: 10.1016/j.apcata.2009.02.010
(55)Menegazzo,F.;Burti,P.;Signoretto,M.;Manzoli,M.;Vankova, S.;Boccuzzi,F.;Pinna,F.;Strukul,G.J.Catal.2008,257, 369.doi:10.1016/j.jcat.2008.05.019
(56)Ishihara,T.;Hata,Y.;Nomura,Y.;Kaneko,K.;Matsumoto,H. Chem.Lett.2007,36,878.doi:10.1246/cl.2007.878
(57)Solsona,B.E.;Edwards,J.K.;Landon,P.;Carley,A.F.; Herzing,A.;Kiely,C.J.;Hutchings,G.J.Chem.Mater.2006,18,2689.doi:10.1021/cm052633o
(58)Edwards,J.K.;Solsona,B.E.;Landon,P.;Carley,A.F.; Herzing,A.;Kiely,C.J.;Hutchings,G.J.J.Catal.2005,236, 69.doi:10.1016/j.jcat.2005.09.015
(59)Landon,P.;Collier,P.J.;Papworth,A.J.;Kiely,C.J.; Hutchings,G.J.Chem.Commun.2002,2058.
(60)Pohl,M.M.;Radnik,J.;Schneider,M.;Bentrup,U.;Linke,D.; Brückner,A.;Ferguson,E.J.Catal.2009,262,314.doi: 10.1016/j.jcat.2009.01.008
(61)Chen,M.;Kumar,D.;Yi,C.W.;Goodman,D.W.Science2005,310,291.doi:10.1126/science.1115800
(62)Marx,S.;Baiker,A.J.Phys.Chem.C2009,113,6191.doi: 10.1021/jp808362m
(63)Dimitratos,N.;Lopez-Sanchez,J.A.;Anthonykutty,J.M.; Brett,G.;Carley,A.F.;Tiruvalam,R.C.;Herzing,A.A.;Kiely, C.J.;Knight,D.W.;Hutchings,G.J.Phys.Chem.Chem.Phys.2009,11,4952.doi:10.1039/b904317a
(64)Wang,D.;Villa,A.;Porta,F.;Prati,L.;Su,D.J.Phys.Chem.C2008,112,8617.
(65)Wang,D.;Villa,A.;Porta,F.;Su,D.;Prati,L.Chem.Commun.2006,1956.
(66)Enache,D.I.;Edwards,J.K.;Landon,P.;Solsona-Espriu,B.; Carley,A.F.;Herzing,A.A.;Watanabe,M.;Kiely,C.J.; Knight,D.W.;Hutchings,G.J.Science2006,311,362.doi: 10.1126/science.1120560
(67)Dimitratos,N.;Villa,A.;Wang,D.;Porta,F.;Su,D.;Prati,L.J. Catal.2006,244,113.doi:10.1016/j.jcat.2006.08.019
(68)Wang,X.;Venkataramanan,N.S.;Kawanami,H.;Ikushima,Y. Green Chem.2007,9,1352.doi:10.1039/b703458j
(69)Kesavan,L.;Tiruvalam,R.;Ab Rahim,M.H.;Bin Saiman,M. I.;Enache,D.I.;Jenkins,R.L.;Dimitratos,N.;Lopez-Sanchez, J.A.;Taylor,S.H.;Knight,D.W.Science2011,331,195.
(70)Liu,P.;Nørskov,J.K.Phys.Chem.Chem.Phys.2001,3, 3814.doi:10.1039/b103525h
(71)Mueller,U.;Schubert,M.;Yaghi,O.;Ertl,G.;Knözinger,H.; Schüth,F.;Weitkamp,J.Wiley VCH2008,1,247.
(72)Kaya,S.;Üner,D.Turk J.Chem.2008,32,645.
(73)Miyaura,N.;Suzuki,A.Chem.Rev.1995,95,2457.doi: 10.1021/cr00039a007
(74)Bond,G.C.Platinum Metals Review2007,51,63.doi:10.1595/ 147106707X187353
(75)Bernhardt,T.;Heiz,U.;Landman,U.Chemical and Catalytic Properties of Size-Selected Free and Supported Clusters; Springer:Berlin Heidelberg,2007.
(76)Zhu,S.;Wang,X.;Wang,A.;Cong,Y.;Zhang,T.Chem. Commun.2007,1695.
(77)Ravishankara,A.;Daniel,J.S.;Portmann,R.W.Science2009,326,123.doi:10.1126/science.1176985
(78)Beyer,H.;Emmerich,J.;Chatziapostolou,K.;Köhler,K.Appl. Catal.A2011,391,411.doi:10.1016/j.apcata.2010.03.060
(79)Haber,J.;Nattich,M.;Machej,T.Appl.Catal.B2008,77, 278.doi:10.1016/j.apcatb.2007.07.028
(80)Suárez,S.;Yates,M.;Petre,A.;Martín,J.;Avila,P.;Blanco,J. Appl.Catal.B2006,64,302.doi:10.1016/j.apcatb.2005.12.006
(81)Xu,X.;Xu,H.;Kapteijn,F.;Moulijn,J.Appl.Catal.B2004,53,265.doi:10.1016/j.apcatb.2004.04.023
(82)Li,Y.;Armor,J.N.Appl.Catal.B1992,1,L21.
(83)Doi,K.;Wu,Y.Y.;Takeda,R.;Matsunami,A.;Arai,N.; Tagawa,T.;Goto,S.Appl.Catal.B2001,35,43.doi:10.1016/ S0926-3373(01)00231-4
(84)Centi,G.;Perathoner,S.;Vazzana,F.;Marella,M.;Tomaselli,M.;Mantegazza,M.Adv.Environ Res.2000,4,325.doi: 10.1016/S1093-0191(00)00032-0
(85)Paul,D.K.;Marten,C.D.;Yates,J.T.Langmuir1999,15, 4508.doi:10.1021/la980672r
(86)Yuzaki,K.;Yarimizu,T.;Ito,S.I.;Kunimori,K.Catal.Lett.1997,47,173.doi:10.1023/A:1019017407609
(87)Oi,J.;Obuchi,A.;Bamwenda,G.R.;Ogata,A.;Yagita,H.; Kushiyama,S.;Mizuno,K.Appl.Catal.B1997,12,277.doi: 10.1016/S0926-3373(96)00079-3
(88)Dann,T.W.;Schulz,K.H.;Mann,M.;Collings,M.Appl.Catal. B1995,6,1.doi:10.1016/0926-3373(95)00006-2
(89)Zhu,S.;Wang,X.;Wang,A.;Zhang,T.Catal.Today2008,131, 339.doi:10.1016/j.cattod.2007.10.093
(90)Ohnishi,C.;Iwamoto,S.;Inoue,M.Chem.Eng.Sci.2008,63, 5076.doi:10.1016/j.ces.2007.08.011
(91)Boissel,V.;Tahir,S.;Koh,C.A.Appl.Catal.B2006,64, 234.doi:10.1016/j.apcatb.2005.12.001
(92)Nobukawa,T.;Yoshida,M.;Kameoka,S.;Ito,S.I.;Tomishige, K.;Kunimori,K.Catal.Today2004,93,791.
(93)Tanaka,S.I.;Yuzaki,K.;Ito,S.I.;Kameoka,S.;Kunimori,K. J.Catal.2001,200,203.doi:10.1006/jcat.2001.3197
(94)Uetsuka,H.;Aoyagi,K.;Tanaka,S.;Yuzaki,K.;Ito,S.; Kameoka,S.;Kunimori,K.Catal.Lett.2000,66,87.doi: 10.1023/A:1019066732528
(95)Tanaka,S.I.;Yuzaki,K.;Ito,S.I.;Uetsuka,H.;Kameoka,S.; Kunimori,K.Catal.Today2000,63,413.doi:10.1016/S0920-5861(00)00486-7
(96)German,E.;Efremenko,I.J.Mol.Struct.-Theochem2004,711, 159.doi:10.1016/j.theochem.2004.10.001
(97)Mihut,C.;Descorme,C.;Duprez,D.;Amiridis,M.D.J.Catal.2002,212,125.doi:10.1006/jcat.2002.3770
(98)Kapteijn,F.;Rodriguez-Mirasol,J.;Moulijn,J.A.Appl.Catal. B1996,9,25.doi:10.1016/0926-3373(96)90072-7
(99)Yamashita,T.;Vannice,A.J.Catal.1996,161,254.doi: 10.1006/jcat.1996.0183
(100)Leglise,J.;Petunchi,J.O.;Hall,W.K.J.Catal.1984,86, 392.doi:10.1016/0021-9517(84)90384-1
(101)Dandl,H.;Emig,G.Appl.Catal.A1998,168,261.doi: 10.1016/S0926-860X(97)00357-8
(102)Mondal,K.;Banerjee,A.;Ghanty,T.K.J.Phys.Chem.C2014,118,11935.
(103)Wei,X.;Yang,X.F.;Wang,A.Q.;Li,L.;Liu,X.Y.;Zhang,T.; Mou,C.Y.;Li,J.J.Phys.Chem.C2012,116,6222.doi: 10.1021/jp210555s
(104)Tateishi,Y.;Tsuneyuki,T.;Furukawa,H.;Kagawa,S.; Moriguchi,I.;Kanmura,Y.;Teraoka,Y.Catal.Today2008,139,59.doi:10.1016/j.cattod.2008.08.008
(105)Dacquin,J.P.;Dujardin,C.;Granger,P.Catal.Today2008,137,390.doi:10.1016/j.cattod.2008.01.016
(106)Yoshida,H.;Tsuruta,T.;Yazawa,Y.;Hattori,T.Appl.Catal.A2007,325,50.doi:10.1016/j.apcata.2007.03.001
(107)Christoforou,S.;Efthimiadis,E.;Vasalos,I.Catal.Lett.2002,79,137.doi:10.1023/A:1015360425678
(108)Gunasekaran,N.;Rajadurai,S.;Carberry,J.Catal.Lett.1995,35,373.doi:10.1007/BF00807194
(109)Burch,R.;Daniells,S.;Breen,J.;Hu,P.J.Catal.2004,224, 252.doi:10.1016/j.jcat.2004.03.027
Nitrous Oxide Decomposition Catalyzed by Au19Pd and Au19Pt Clusters
YU Wei-Ling1ZUO Hui-Wen1LU Chun-Hai2,*LI Yi1ZHANG Yong-Fan1CHEN Wen-Kai1,*
(1Department of Chemistry,Fuzhou University,Fuzhou 350116,P.R.China;2College of Nuclear Technology and Automation Engineering,Chengdu University of Technology,Chengdu 610059,P.R.China)
The catalytic decomposition of N2O using Au19Pd and Au19Pt clusters as catalysts with optimized geometries was studied using density functional theory(DFT).The optimized geometries of the Au19Pd and Au19Pt clusters were obtained as a function of structural and thermodynamic analyses,in which the heteroatoms are on the surfaces of the clusters.We selected theAu19Pd cluster as a model cluster to investigate the reaction mechanism of N2O decomposition.There are two reaction pathways to be considered:Eley-Rideal(ER)and Langmuir-Hinshelwood(LH).We found that the first N2O decomposition needs to surmount an energy barrier of 1.118 eV,and is exothermic by 0.371 eV.The elimination of the residual oxygen atom on the surface has an energy barrier of 1.920 eV along the ER pathway after N2desorption,which is higher than that along the LH channel(1.669 eV).The adsorption energy of the oxygen atom on the surface is-3.203 eV,and the oxygen atom diffusion on the surface needs to surmount an energy barrier of 0.113 eV along the LH pathway.This indicates that the oxygen atom is prone to transfer on the cluster to promote the generation of the O2molecule, and therefore the LH is the optimized reaction pathway.We investigated the catalytic activity of Au19Pt for N2O decomposition along the LH pathway in comparison with theAu19Pd cluster.Both platinum and palladium havecatalytic activities for N2O decomposition,especially the palladium in this study.Comparison between this work and the theoretical study on periodic systems shows that these two clusters can be used as better catalysts for N2O decomposition,especially theAu19Pd cluster.Furthermore,the O2desorption is no longer the main barrier to the reaction,which further enhances the catalytic activities of these two clusters for N2O decomposition.©Editorial office ofActa Physico-Chimica Sinica
Nanocluster;Catalytic activity;N2O decomposition;Reaction mechanism
O641
10.3866/PKU.WHXB201501191www.whxb.pku.edu.cn
Received:November 13,2014;Revised:January 19,2015;Published on Web:January 19,2015.
∗Corresponding authors.LU Chun-Hai,Email:luchhi@126.com;Tel:+86-28-84078773.CHEN Wen-Kai,Email:wkchen@fzu.edu.cn;
Tel:+86-591-22866162.
The project was supported by the National Natural Science Foundation of China(21203027)and Fujian Provincial Natural Science Foundation, China(2012J01041).
国家自然科学基金(21203027)和福建省自然科学基金(2012J01041)资助项目

