Three Dinuclear Zinc Coordination Polymers Constructed by 2,2′-Thiobis(benzoic acid)and Bipyridine Ligands
HU ShengZHOU Chang-Xia
(School of Chemical Engineering and Light Industry,Guangdong University of Technology,Guangzhou 510006,China)
Three Dinuclear Zinc Coordination Polymers Constructed by 2,2′-Thiobis(benzoic acid)and Bipyridine Ligands
HU Sheng*ZHOU Chang-Xia
(School of Chemical Engineering and Light Industry,Guangdong University of Technology,Guangzhou 510006,China)
Three complexes,namely[Zn2(tba)2(bpy)]n(1),[Zn2(tba)2(bpe)]n(2)and[Zn(tba)(bpp)]n(3),(tba=2,2′-thiobis(benzoic acid),bpy=4,4′-bipyridine,bpe=1,2-bis(4-pyridyl)ethylene,bpp=1,3-bis(4-pyridyl)propane)have been synthesized by methods of hydrothermal reaction and their crystal structures were determined.The ligand tba was generated via in situ hydrolyzation of 2-(2-cyanophenylthio)benzoic acid.Complex 1 has dinuclear Zn(Ⅱ) units,which are linked by tba and bpy to generate a two-dimensional(4,4)net.Complex 2 has a three-dimensional structure based on[Zn2(tba)2]nsub-chains,which are further connected by the bpe ligands to form a diamond topological network.Complex 3 possesses interesting 2D→2D polycatenation of(4,4)networks.The structure versatility indicates that the coordination vector of tba and auxiliary bipyridine ligands play a crucial role in modulating the dinuclear zinc coordination polymers of 1~3.The photoluminescence properties of 1~3 in the solid state are also investigated.CCDC:1449480,1;1449481,2;1449482,3.
dinuclear zinc;photoluminescence;crystal structure;coordination polymer;polycatenated structure
0 Introduction
The crystal engineering of coordinated polymers is of great current interest for structural novelty as well as for their potential application as functional materials[1-4].The design of coordination polymers is highly influenced by factors such as the coordination nature of the metal ion,the structural features of theligand,the reaction condition and other possible influences,which provides a possible approach to the controlled assembly of coordinated polymers with tunable properties[5-6].Among the first consideration is the structural features of organic ligands.A mixedligand system[7-8]consisting of two types ligands provides more variability to construct fantastic structures.One of the most fruitful choices is the combination of variouscarboxylicacidsandneutralpyridinecontaining auxiliary ligands[9-10],where the carboxylic acid ligands balance the positive charge of the metal centers and develop secondary building units,while the auxiliary ligands increase the dimensionality or supply the additional structure versatility.So one primary ligand should to be elaborately selected in the mixed-ligand system.
Dicarboxylateligandshaverealizedvarious coordinated polymers with specific topologies.So far, a great number of architectures based on dicarboxylate ligands,such as terephthalic acid[11-12]and 1,4-cyclohexanedicarboxylate[13-14]have been reported.In contrast,diaryl sulfides represent an important class of thio compounds and the diaryl sulfides containing carboxylate donor groups,for instance,2,2′-thiobis (benzoic acid)(tba),has not been subjected to detailed study.The tba ligand,which is the closest analogue of 1,1′-Biphenyl-2,2′-dicarboxylic acid[15-16],presents two carboxylic groups and an interesting sulfur group.In the presence of carboxylate donor groups at the ortho position,the sulfur is difficult to be coordinated. However,the additional angle of the thio group in tba skeleton is expected to modulate and influence the orientation relationship and coordination abilities of the two carboxylate groups.So it can be applied as a configurationally asymmetric bridging ligand leading to more interesting coordination polymers that cannot be achieved with other carboxylate ligands.To the best of our knowledge,the tba ligand has seldom been used in transition-metal coordination polymers,since only a few crystal structures with transition metals have been documented so far[17].Moreover,the 2,2′-thiobis(benzoic acid)ligand in this work was generated via in situ hydrolyzation of 2-(2-cyanophenylthio) benzoic acid.Organic nitriles and their carboxylates derivatives have been widely used for hydrothermal synthesis of coordination polymers[18].Notably,these kinds of in situ ligand reactions,relatively straight forward in the context of reaction chemistry,have become an important non-conventional approach in the crystal engineering of coordination polymers.
The secondary ligands chosen are classical long pillars[19]4,4′-bipyridine(bpy),1,2-bis(4-pyridyl) ethylene(bpe)and 1,3-bis(4-pyridyl)propane(bpp).In general,long ligands will lead to larger voids that may result in multi-fold interpenetrating or entanglement structures[20].Although the three ligands all contain two peripheral pyridines symmetrically,their separation lengths and steric conformations are different,which may offer different link modes.In this research,three dinuclear zinc coordination polymers,[Zn2(tba)2(bpy)]n(1)with(4,4)net,[Zn2(tba)2(bpe)]n(2)with threedimensionalnetworkand[Zn(tba)(bpp)]n(3)with polycatenated structure,were obtained as their single crystals.The details of structures show the cooperative effect of the tba and bipyridine ligands on the conformations of frameworks.The control experiments were carried out by not using the auxiliary bipyridine ligands above and had failed to obtain any singlecrystal product under identical conditions,presumable because the bipyridine ligands play vital roles in stabilizingthesolidstructures.Thesolid-state luminescence spectrums of 1~3 display strong light green emission band at room temperature.
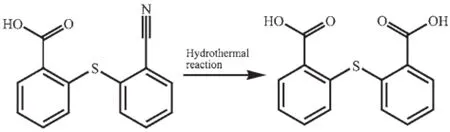
Scheme 1In situ hydrolyzation of 2-(2-cyanophenylthio) benzoic acid
1 Experimental
1.1 Materials and measurements
2-(2-cyanophenylthio)benzoicacidandother chemicals were obtained from commercial sources and were used without further purification.The C,H andN microanalyses were obtained with an Elementar Vario-ELCHNSelementalanalyzer.TheFT-IR spectra were recorded in the range 4 000~400 cm-1on a Bio-Rad FTS-7 spectrometer.X-ray powder diffraction (XRD)patterns for complexes 1~3 were measured at 293 K on a Rigaku D/max-IIIA diffratometer(Cu Kα, λ=0.154 056 nm,3°~60°with a step of 0.1°·s-1. Calculated diffraction patterns of 1~3 were generated with the Mercury program,available free of charge at http://www.iucr.org.Emission/excitation spectra were measured on an Edinburgh FLS 980 fluorescence spectrophotometer.
1.2 Synthesis of[Zn2(tba)2(bpy)]n(1)
Zn(NO3)2·6H2O(0.297 g,1.0 mmol)was dissolved in H2O(6 mL)at ambient temperature,followed by the addition of 2-(2-cyanophenylthio)benzoic acid(0.128 g,0.5 mmol),4,4′-bipyridine(0.039 g,0.25 mmol), NaOH(0.04 g,1.0 mmol)in H2O(8 mL)with vigorous stirring in a 25 mL Parr Teflon-lined stainless steel vessel.The mixture was heated for 3 days at 175℃and then cooled to room temperature at a rate of 10℃·h-1. After cooling to room temperature,colourless blocklike crystals were obtained in the yield of 55%(based on2-(2-cyanophenylthio)benzoicacid).Elemental analysis Calcd.for C38H24N2O8S2Zn2(%):C,54.89;H, 2.91;N,3.37.Found(%):C,54.96;H,2.90;N,3.36. IR(KBr,cm-1):3 435w,3 057w,1 638s,1 607s,1 541s, 1 492w,1 464m,1 281w,1 222m,1 157w,1 072w, 1 040w,851w,813m,745s,716m,645w,491w.
1.3 Synthesis of[Zn2(tba)2(bpe)]n(2)
The synthesis of complex 2 was similar as 1 by using 1,2-bis(4-pyridyl)ethylene(0.091 g,0.5 mmol)in place of 4,4′-bipyridine(0.039 g,0.25 mmol).Colourless block-like crystals were obtained in the yield of 60%(based on 2-(2-cyanophenylthio)benzoic acid). Elemental analysis Calcd.for C40H26N2O8S2Zn2(%):C, 56.02;H,3.06;N,3.27.Found(%):C,56.08;H,3.09; N,3.25.IR data(KBr,cm-1):3 435w,3 050w,1 605s, 1 555m,1 433m,1 406s,1 281w,1 210w,1 153w,1 067 w,1 034w,844w,801w,756m,656w,558w,472w.
1.4 Synthesis of[Zn(tba)(bpp)]n(3)
The synthesis of complex 3 was similar as 2 by using 1,3-bis(4-pyridyl)propane(0.099 g,0.5 mmol)in place of 1,2-bis(4-pyridyl)ethylene(0.091 g,0.5 mmol). Colourless block-like crystals were obtained in the yield of 50%(based on 2-(2-cyanophenylthio)benzoic acid).Elemental analysis Calcd.for C27H22N2O4SZn(%): C,60.51;H,4.14;N,5.23.Found(%):C,60.56;H, 4.16;N,5.20.IR data(KBr,cm-1):3 452w,3 056w, 2 936w,2 863w,1 616s,1 507w,1 460w,1 432s,1 363s, 1 281w,1 227w,1 149w,1 062w,1 033m,843m,803m, 752s,709w,654w,623w,587w,497w,460w.
1.5 Crystal structure determination
Single crystals of complexes 1~3 suitable for X-ray analysis were obtained directly from the above syntheses.The diffraction data were recorded on a Bruker Smart Apex CCD diffractometer with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 296 K.Data processing were accomplished by use of the program SAINT[21];an absorption correction based on symmetry equivalent reflections was applied using the SADABS program[22].The structures were solved by direct methods and refined by the full-matrix leastsquares method on F2with the SHELXTL program package[23].All non-hydrogen atoms were refined with anisotropic displacement parameters.All the hydrogen atoms were generated geometrically and refined isotropically using the riding model.The crystallographic details and selected bond lengths and bond angles are provided in Tables 1 and 2,respectively.
CCDC:1449480,1;1449481,2;1449482;3.
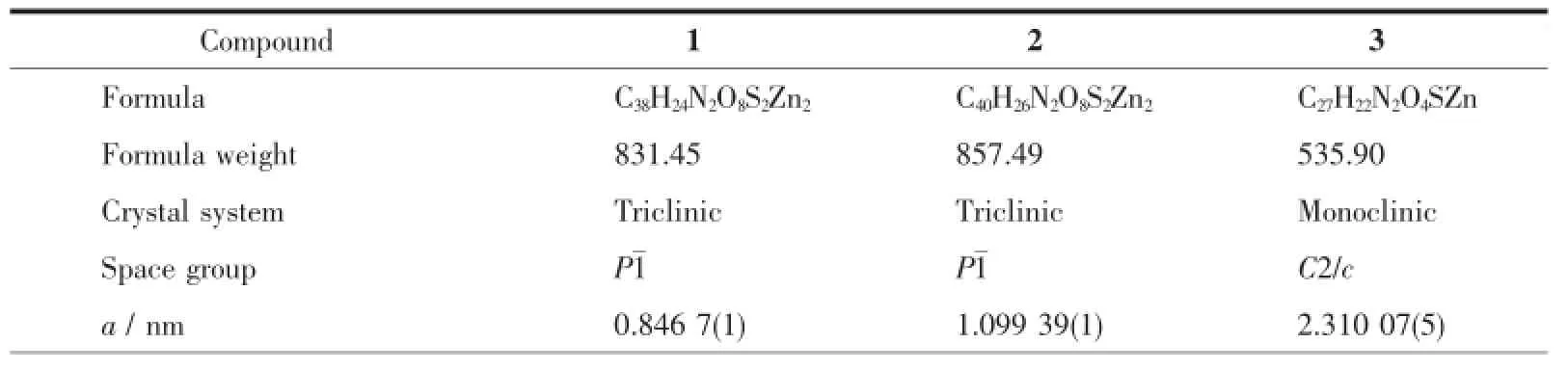
Table 1Crystal data and structure refinement details for 1~3
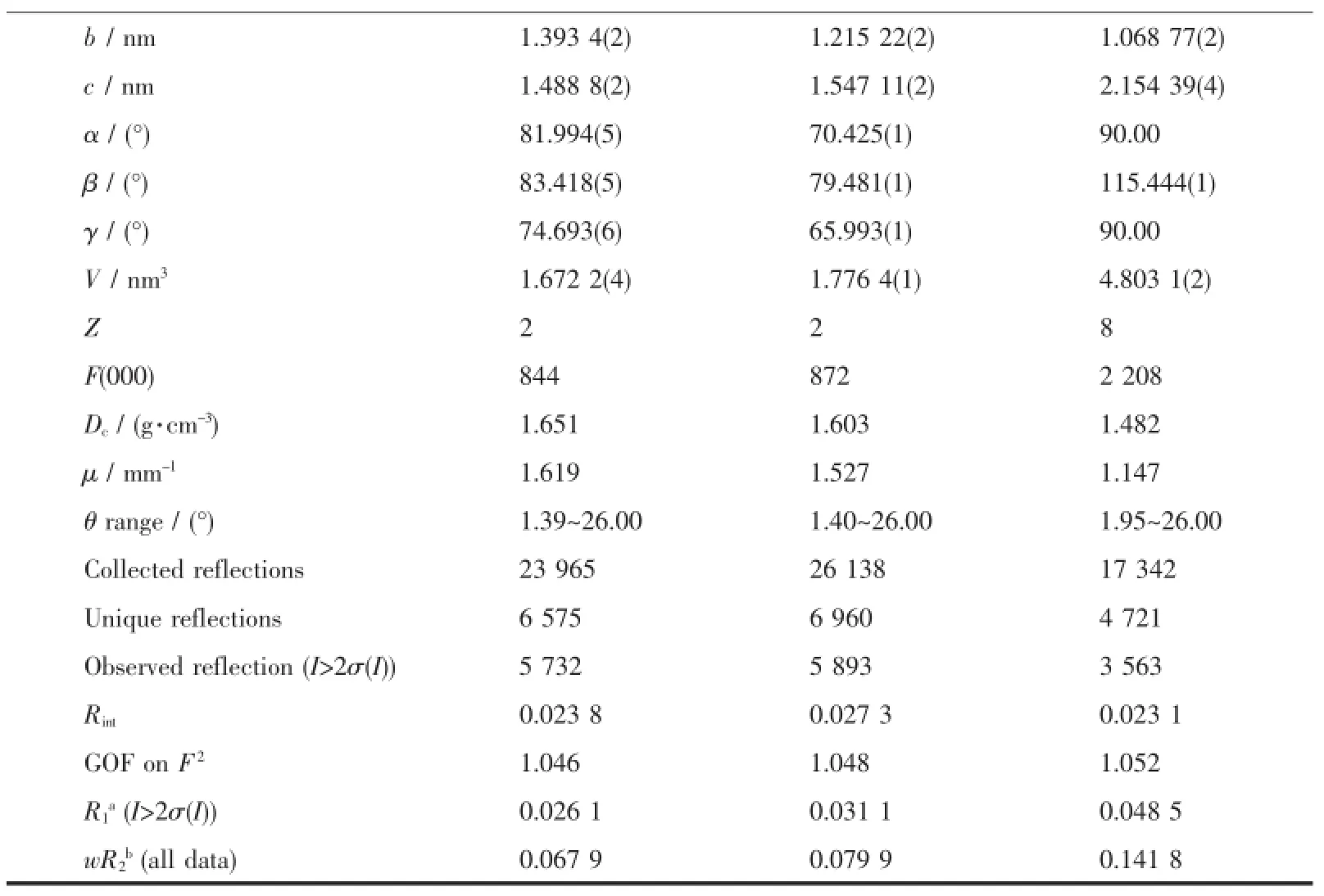
Continued Table 1
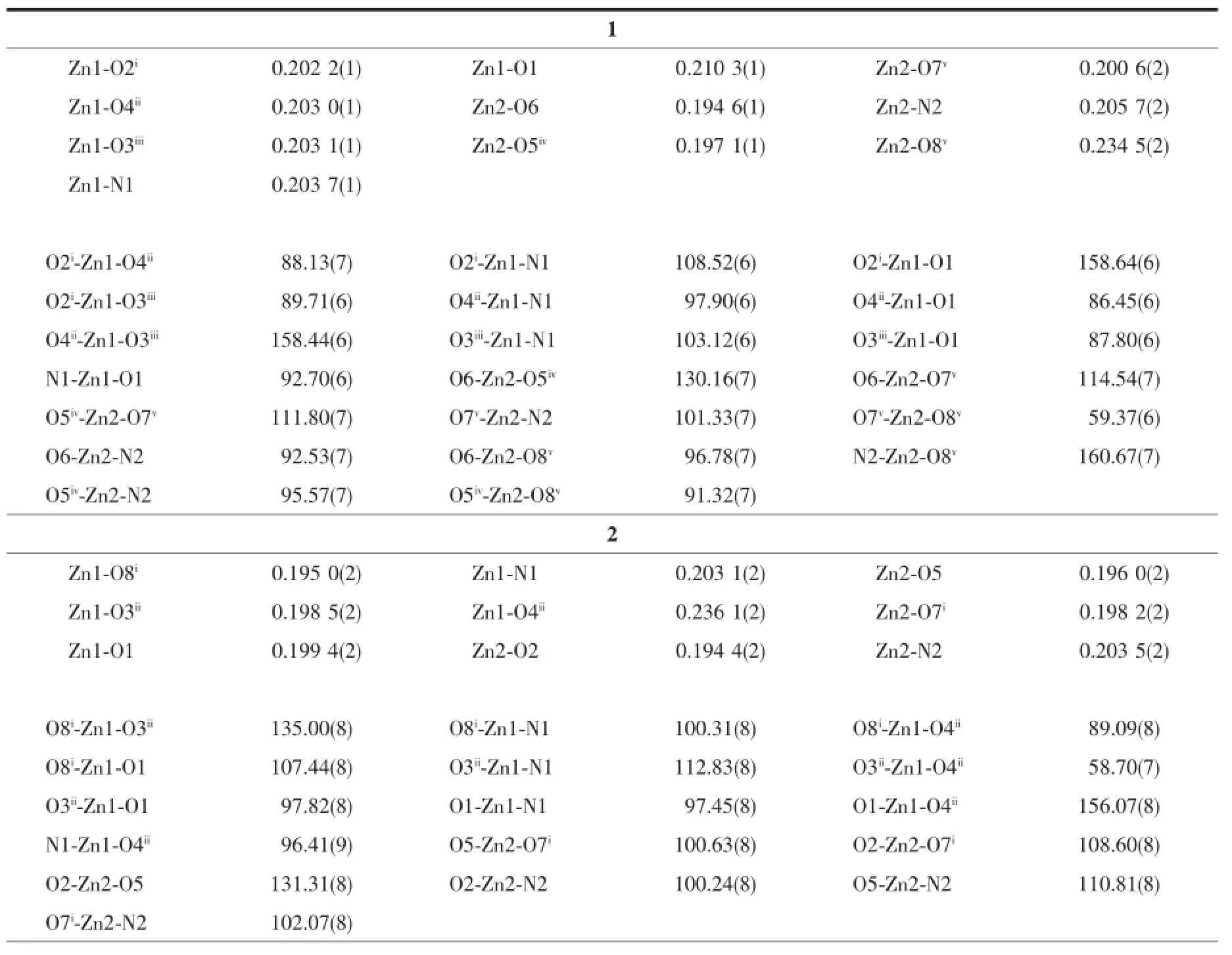
Table 2Selected bond lengths(nm)and bond angles(°)for 1~3
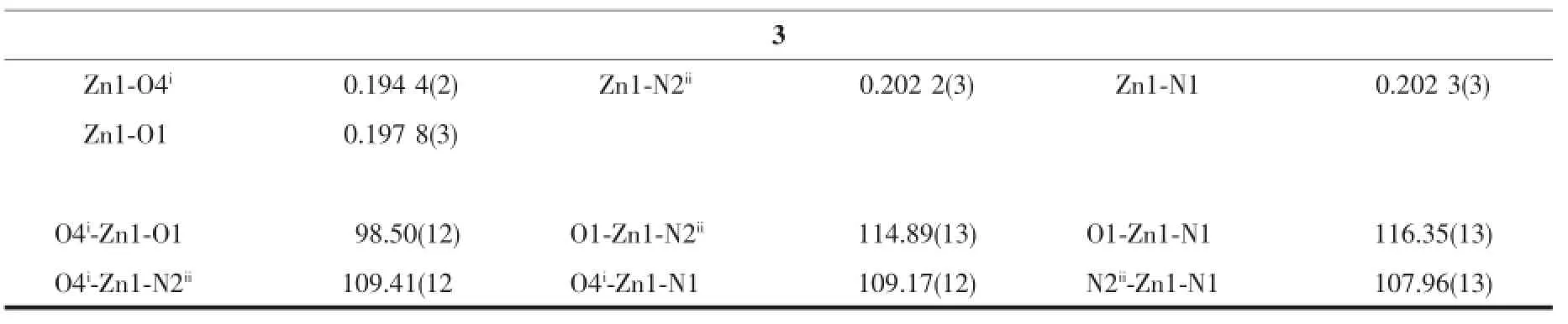
Continued Table 2
2 Results and discussion
2.1 Crystal structures of 1~3
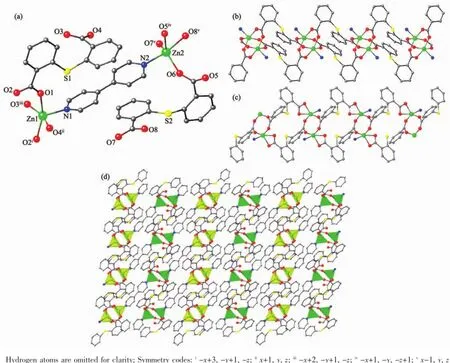
Fig.1(a)Coordination environment of Zn(Ⅱ)ions in 1;(b)Sub-chain with the dinuclear Zn(Ⅱ)units on the Zn1 site in 1; (c)Sub-chain with the dinuclear Zn(Ⅱ)units on the Zn2 site in 1;(d)2D(4,4)net in 1
Colourless block-like crystals of complex 1 were synthesized as a single phase by the hydrothermal reaction ofNaOH,2-(2-cyanophenylthio)benzoic acid and 4,4′-bipyridine.X-ray structural analysis shows that the asymmetric unit contains two crystallographically unique Zn(Ⅱ)ions,two 2,2′-thiobis (benzoic acid)ligands and one 4,4′-bipyridine ligand (Fig.1a).The 2,2′-thiobis(benzoic acid)ligand(tba) was generated via in situ hydrolyzation of 2-(2-cyanophenylthio)benzoic acid.The two Zn(Ⅱ)ions display differentcoordinationenvironments.Zn1isfivecoordinated by four O atoms from four different tba ligands(Zn-O 0.202 2(2)~0.210 3(2)nm)and one N atom from bpy ligands(Zn-N 0.203 7(2)nm)in an tetragonal pyramid geometry.Zn2 adopts a trigonalbipyramid coordination geometries with four O atoms from three different tba ligands(Zn-O 0.194 6(2)~0.234 5(2)nm)and one N atom from bpy ligand(Zn-N 0.205 7(2)nm).As shown in Fig.1,there are two types of tba units:One bridges pairs of Zn1 ions to construct paddle-wheel dinuclear units(Zn1…Zn1i0.298 5 nm)with two syn-syn bidentate carboxylate groups;another one links Zn2 ions to form the different dinuclear units(Zn2…Zn2iv0.372 4 nm)through its carboxylate groups as syn-syn bidentate bridge and chelate mode.Both carboxylate groups of the tba ligand are about perpendicular to each other(84.90° or 87.05°)to form an L-shaped coordination vector. The dinuclear Zn units are bridged by the tba ligands to form two types of one-dimension[Zn2(tba)2]nSubchains(Fig.1b and 1c).The bpy ligands extend the [Zn2(tba)2]nSub-chains into a two-dimensional network (Fig.1d).From the topological point of view,each dinuclear Zn units serves as an square-planar node and is linked to four nearest neighbours,thus resulting in a(4,4)topology.The adjacent layers are noninterpenetrated and parallel stacked with each other in an AA fashion.All phenyl ring groups of tba ligands point toward the space between adjacent layers and weak interlayer C-H…π interactions with the H…centroid distance of 0.282 nm have been observed between the phenyl ring groups of adjacent layers.
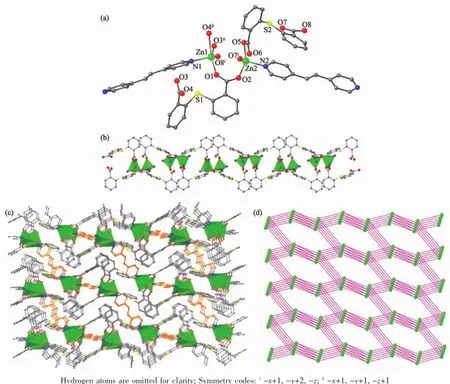
Fig.2(a)Coordination environment of Zn(Ⅱ)ions in 2;(b)Sub-chain with the dinuclear Zn(Ⅱ)units in 2; (c)3D net of 2 viewed along the b axis;(d)4-connected topological net in 2
As a comparative study for 1,2 was prepared by using1,2-bis(4-pyridyl)ethylene in place of 4,4′-bipyridine.However,complex 2 is a three-dimensional coordination network and is built from the different dinuclear Zn units.The asymmetric unit of 2 contains two crystallographically unique Zn(Ⅱ)ions,two 2,2′-thiobis(benzoic acid)ligands and two half-molecules of the 1,2-bis(4-pyridyl)ethylene ligand(Fig.2a).The two Zn(Ⅱ)ions display different coordination environments.The Zn1 is five-coordinated by four O atoms from three different tba ligands(Zn-O 0.195 0(2)~0.236 1(2)nm) and one N atom from bpe ligands(Zn-N 0.203 1(2) nm)in an tetragonal pyramid geometry.Zn2 adopts a tetrahedral coordination geometries with three O atoms from three different tba ligands(Zn-O 0.194 4(2)~0.198 2(2)nm)and one N atom from bpe ligand(Zn-N 0.203 5(2)nm).As shown in Fig.2b,each dinuclear Zn unit is surrounded by two syn-syn bidentate bridges,one chelate and one monodentate carboxylate donor group,which is then bridged by the tba ligands to give a one-dimensional sub-chain.The bpe ligands, as bridge modules,extend the sub-chains into a threedimensionalframework(Fig.2c).Apparently,the dinuclear Zn motifs can be viewed as the tetragonal′nodes′in the construction of this network,which is exactly a uninodal 4-connected diamond topology(Fig. 2d)with the short Schläfli symbol of 66.
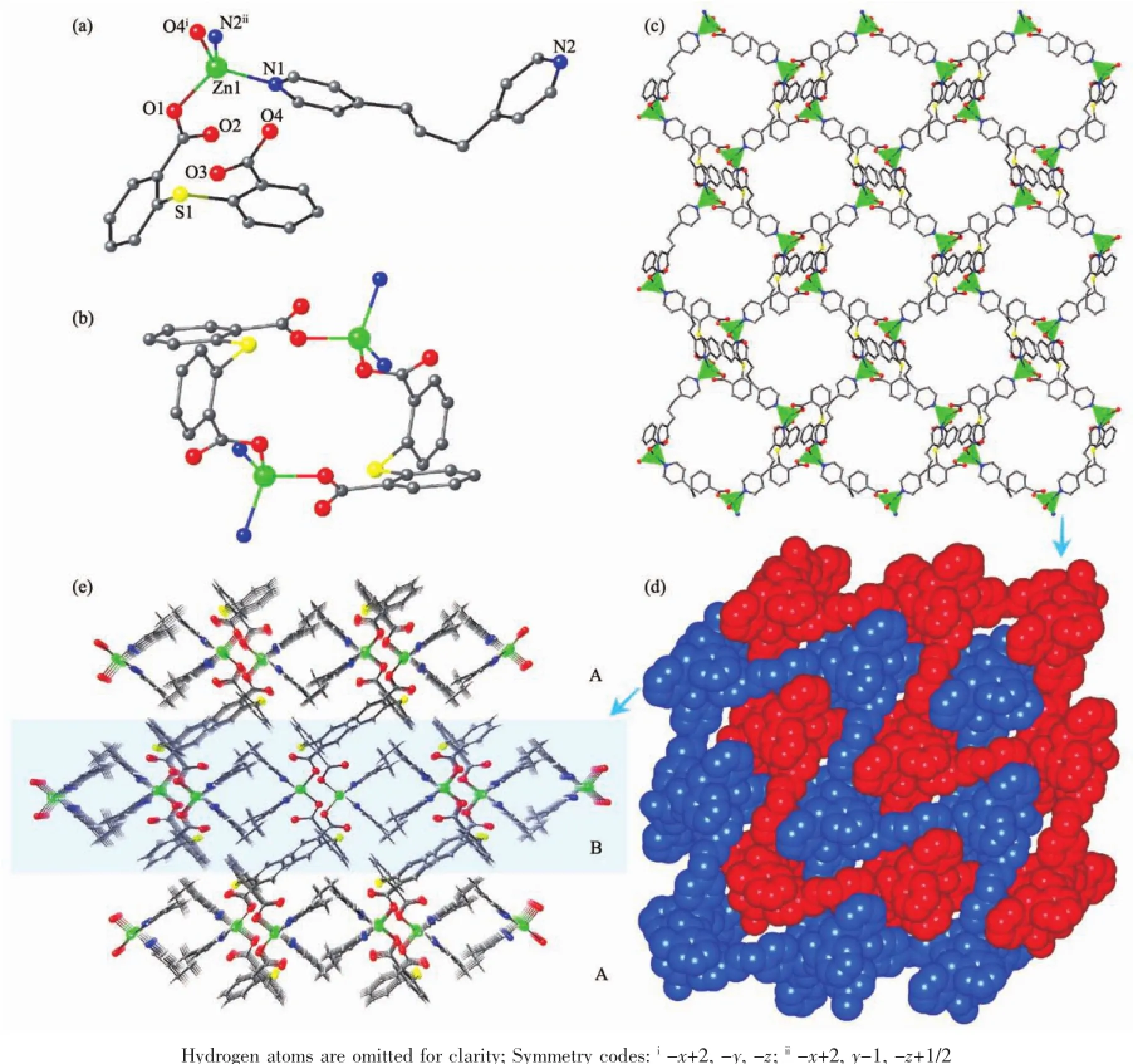
Fig.3(a)Coordination environment of Zn(Ⅱ)ions in 3;(b)[Zn2(tba)2]nunit in 3;(c)2D(4,4)net in 3; (d)2D polycatenated net in 3;(e)Molecular packing as repeating layers of type ABAB in 3
Another appropriate auxiliary ligand 1,3-bis(4-pyridyl)propane(bpp)[24]was selected for the assembled system and complex 3 was gained.In 3,the asymmetric unit contains one unique Zn(Ⅱ)ion,one 2,2′-thiobis (benzoic acid)ligand and one 1,3-bis(4-pyridyl)propane ligand(Fig.3a).The unique Zn(Ⅱ)ion is in a N2O2 tetrahedral coordination environment surrounded by two cis-related bpp ligands(Zn-N 0.202 2(3)~0.202 3(3) nm)and two oxygen atoms from two tba monodentate carboxylate group(Zn-O 0.194 4(2)~0.197 8(3)nm).Interestingly,two Zn(Ⅱ)ions are bridged by two tba ligands to form a quasi-planar Zn2(tba)2metallocycle as a second building unit(SBU)(Fig.3b).The intracycle opposite Zn…Zndistances are 0.571 4 nm.Each Zn2(tba)2macrocycle connects four neighbouring ones by four bpp bridges,which adopt the trans-gauche conformation to link the Zn2(tba)2dinuclear units into an overall(4,4)topological net.The net is parallel to the bc crystallographic plane and contains openings of a minimum size of 0.861 nm×0.861 nm as defined by the shortest trans-annular distance factoring van der Waals radii(Fig.3c).A particularly interesting structural feature of 3 is that each layer is interlocked with theneighbouringindependentlayerinparallel directions and generates a graceful 2D polycatenated network(Fig.3d)with(1/1)catenation.Owing to the prominent flexibility of the bpp ligands[25],the large openings of the layer accommodates another closed loop catenating with it.On the basis of the side view of these catenated layers,the window of the layer is a metallocycle composed of two Zn(Ⅱ)ions and two bpp linkers,and the corrugation of the layer results from the parallel alignment of the[Zn2(bpp)2]units(Fig.3e). The adjacent layers are stacked offset with respect to each other in an ABAB fashion by van der Waals interactions and hence maximize the intermolecular interactions for complementary shape,and interlayer C-H…O hydrogen bondings(C…O 0.316~0.318 nm) have been observed.
2.2 Photoluminescence properties
Coordination polymers consisting of transition metals with d10electronic configuration such as Zn(Ⅱ) and suitable organic linkers are found to exhibit excellent photoluminescence properties.Therefore,the complexes synthesized by using Zn metal ions might be employed as new luminescent materials.Consequently,the photoluminescence properties of complexes 1~3 have been studied in the solid state.The excitation and emission maxima(λex-maxand λem-max)of 1~3 at room temperature are depicted in Fig.4.Upon excitation at 409,439,and 399 nm,1~3 in the solid state exhibit photoluminescence at room temperature with emission maxima at ca.510,531 and 515 nm,respectively.To understand the nature of the emission bands,we also investigated the luminescence of tba in solid state at room temperature.The tba ligand is nearly nonfluorescent in the range of 400~800 nm for excitation wavelengths between 250 and 450 nm.However,in the complexes 1~3,the highest occupied molecular orbitals(HOMOs)are presumably associated with the π-bonding orbitals from the aromatic pyridyl rings, whereas the lowest unoccupied molecular orbitals (LUMOs)are associated mainly with the Zn-O(carboxy) σ*-antibonding orbital,localized more on the metal centres.Thus the origin of the emissions of 1~3 might be assigned to ligand-to-metal charge transfer(LMCT)[26]. The emission discrepancy of 1~3 is probably due to the differences of the coordination environment of centralmetalionsandtheconformationaland accumulation changes of the ligands.
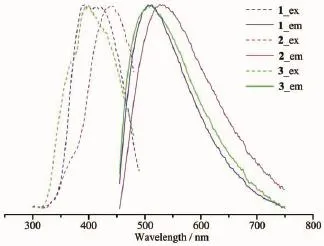
Fig.4Excitation(dashed line)and emission(solid line) spectra of 1~3 in the solid state at room temperature
3 Conclusions
In conclusion,we successfully synthesized and characterizedthreephotoluminescentcoordination polymers based on different dinuclear Zn units and a hydrolytic 2,2′-thiobis(benzoic acid)ligand.The tba ligand generated from in situ hydrolyzation of 2-(2-cyanophenylthio)benzoicaciddisplaysspecialL-shaped coordination vector in the construction of complex 1~3.It is reasonable that the thio group of the tba ligands shows special effect on the assembly progress,that is greatly different from the recognitionrole of other known dicarboxylate ligand.In 1~3,the two carboxylic groups of tba adopt different coordination configuration and make large dihedral angles with the basal plane defined by the sulfur group and benzene ring,whichisresponsibleforresultinginthe formation of different dinuclear Zn units.On the other hand,the introduction of different auxiliary ligands, 4,4′-bipyridine,1,2-bis(4-pyridyl)ethylene and 1,3-bis (4-pyridyl)propane,can also result in structural diversity.When using bpp instead bpy as auxiliary ligands, we obtained the complex 3 possessing interesting 2D→2D polycatenation,whichindicatethatthe flexible spacers have a significant effect on the entanglement of multiple motifs.The potential of the hydrolytic 2,2′-thiobis(benzoic acid)ligand to synthesize more coordination polymers with other second building units remain to be explored.
Supporting information is available at http://www.wjhxxb.cn
[1]Chughtai A H,Ahmad N,Younus H A,et al.Chem.Soc. Rev.,2015,44:6804-6849
[2]Lin Z J,Tong M L.Coord.Chem.Rev.,2011,255:421-450
[3]Pettinari C,Tbcaru A,Galli S.Coord.Chem.Rev.,2016,307: 1-31
[4]Zhang F,Wei Y Y,Wu X T,et al.J.Am.Chem.Soc.,2014, 136:13963-13966
[5]Hu S,Yu F Y,Zhang P,et al.Dalton Trans.,2013,42:7731-7740
[6]Steel P J.Acc.Chem.Res.,2005,38:243-250
[7]Du M,Li C P,Liu S C,et al.Coord.Chem.Rev.,2013,257: 1282-1305
[8]Chun H,Dybtsev D N,Kim H,et al.Chem.Eur.J.,2005,11: 3521-3529
[9]Park H J,Suh M P.Chem.Eur.J.,2008,14:8812-8821
[10]Roesky H W,Andruh M.Coord.Chem.Rev.,2003,236:91-119
[11]Rosi N L,Eckert J,Eddaoudi,M,et al.Science,2003,300: 1127-1129
[12]Higuchi M,Tanaka D,Korike S,et al.J.Am.Chem.Soc., 2009,131:10336-10337
[13]Kim Y K,Jung D Y.Chem.Commun.,2002:908-909
[14]Bi W H,Cao R,Sun D F,et al.Chem.Commun.,2004: 2104-2105
[15]Hu S,Zhang J P,Li H X,et al.Cryst.Growth Des.,2007,7: 2286-2289
[16]Wang R H,Hong M C,Luo J H,et al.Chem.Commun., 2003:1018-1019
[17]Moosun S B,Blair L H,Coles S J,et al.Transition Met. Chem.,2015,40:161-169
[18]Tong M L,Li L J,Mochizuki K,et al.Chem.Commun., 2003:428-429
[19]Chen B,Eddaoudi M,Hyde S T,et al.Science,2001,291: 1021-1023
[20]Kesanli B,Cui Y,Smith M R,et al.Angew.Chem.Int.Ed., 2005,44:72-75
[21]SAINT,Bruker AXS Inc.,Madison,WI,USA,2002.
[22]Sheldrick G M.SADABS Ver.2.05,University of Göttingen, Germany,1997.
[23]Sheldrick G M.Acta Crystallogr.,Sect.A,2008,A64:112-122
[24]Hu S,Tong M L.Dalton Trans.,2005:1165-1167
[25]Hu S,Zhou A J,Zhang Y H,et al.Cryst.Growth Des., 2006,6:2543-2550
[26]Zheng S L,Chen X M.Aust.J.Chem.,2004,57:703-712
2,2′-硫代-二(苯甲酸)和双吡啶配体构筑的三个双核锌配位聚合物
胡升*周常侠
(广东工业大学轻工化工学院,广州510006)
用水热法合成了3个配位聚合物并测定了其晶体结构,分子式分别为[Zn2(tba)2(bpy)]n(1),[Zn2(tba)2(bpe)]n(2)和[Zn(tba) (bpp)]n(3),(tba=2,2′-thiobis(benzoic acid),bpy=4,4′-bipyridine,bpe=1,2-bis(4-pyridyl)ethylene,bpp=1,3-bis(4-pyridyl)propane)。tba是由2-(2-cyanophenylthio)benzoic acid原位水解得到的。X射线单晶衍射分析表明配合物1含双核锌单元,由tba和bpy连成二维(4,4)网。配合物2是基于[Zn2(tba)2]n子链,由bpe连接而成的钻石拓扑三维结构。有趣的是,配合物3是一个由(4,4)网交缠形成的二维到二维的聚索烃。Tba配体构型和各双吡啶辅助配体引起了3个化合物的结构各异性。此外,研究了3个配位聚合物的荧光性质。
双核锌;荧光;晶体结构;配位聚合物;聚索烃
O614.24+1
A
1001-4861(2016)06-1111-09
2016-02-06。收修改稿日期:2016-04-13。
10.11862/CJIC.2016.126
广东省高等学校优秀青年教师培养计划(No.261532106)和广州市科技计划科学研究专项一般项目(No.201510010156)资助。
*通信联系人。E-mail:husheng@gdut.edu.cn

