十二指肠降部黏膜免疫荧光试验辅助诊断过敏性紫癜的价值及临床局限性
宋 瑛 石杰如 唐子斐 冯佳燕 陈 莲 黄 瑛
·论著·
十二指肠降部黏膜免疫荧光试验辅助诊断过敏性紫癜的价值及临床局限性
宋 瑛1,4石杰如2,4唐子斐2冯佳燕3陈 莲3黄 瑛2
目的 探讨十二指肠降部免疫荧光试验与黏膜病变程度在过敏性紫癜(HSP)辅助诊断中的价值和临床局限性。方法 收集2014年4月至2015年12月复旦大学附属儿科医院消化科以腹痛为主要症状初诊的HSP或怀疑HSP,且辅助诊断至少有胃镜检查并行十二指肠降部黏膜免疫荧光试验的患儿,从病史中查阅紫癜的记录情况,采集HSP患儿行胃镜检查时十二指肠黏膜病变程度及其组织荧光试验结果;本文以胃镜直视下充血、水肿为轻度病变,糜烂和溃疡为中重度病变;本文以典型皮肤紫癜出现在胃镜检查前为早出紫癜,之后为晚出紫癜;根据免疫荧光试验结果分为阴性和阳性。结果 符合本文纳入和排除标准的54例HSP患儿进入本文分析,男31例,女23例;平均(8.1±2.7)岁;门诊病例14例,住院病例40例;早出紫癜36例(76.7%),晚出紫癜18例。十二指肠降部黏膜均受累,免疫荧光阳性31例(57.4%),阴性23例(其中弱阳性6例)。十二指肠降部黏膜轻度病变14例(25.3%),重度病变40例(其中糜烂19例,溃疡21例)。免疫荧光阴性的HSP患儿中黏膜中重度病变的比例明显多于阳性,差异有统计学意义[91.3%(21/23)vs61.3%(19/31),P=0.013];早出紫癜的HSP患儿黏膜轻度病变免疫荧光阳性比例(10/31,32.2%)多于阴性(2/23,8.7%),差异有统计学意义(P=0.039);不论免疫荧光结果阳性与否:早或晚出紫癜HSP患儿黏膜病变轻度与中重度比例差异均无统计学意义、晚出紫癜的HSP患儿黏膜轻度病变的比例差异无统计学意义、早出或晚出紫癜的HSP患儿黏膜中重病变的比例差异亦均无统计学意义。结论 十二指肠降部黏膜较皮肤组织免疫荧光试验阳性率低,可能与取活检处黏膜的病变严重程度有关,不排除与取活检的部位、数量、深度及器械等多种因素有关。
过敏性紫癜; 胃镜; 儿童; 免疫荧光
2010年欧洲抗风湿病联盟/欧洲儿科风湿病学会制定了过敏性紫癜(HSP)最新的诊断及分类标准[1]。明显皮肤紫癜作为诊断HSP的首要条件,其他4项作必要的辅助条件之一,弥漫性腹痛、任何部位活检提示IgA沉积、急性关节炎/关节痛、血尿或蛋白尿等肾脏受损表现[1]。基于皮肤的免疫荧光试验诊断的敏感度和特异度分别为81%~86%和83%~84%[2,3],但皮肤活检需要全身麻醉下进行,肾脏穿刺也只有在考虑肾脏受累时才进行,两者都存在创伤大及出现严重并发症的风险。儿童HSP累及关节病变不多,累及消化道占51%~74%[4- 7],其中以腹痛为主要临床症状占42%[8],所以常在伴有腹痛疑似HPS或HSP患者中行胃镜检查,虽然大部分研究肯定了胃镜在HSP诊断中潜在价值[9,10],但目前并没有把胃镜检查结果纳入到HSP诊断依据中,可能与十二指肠黏膜的类似病变也存在于克罗恩病、卓- 艾综合征、小肠结肠耶尔森菌感染、嗜酸细胞性胃肠炎、白塞氏综合征和辐射或药物诱发的损伤等,临床上行胃镜检查时常从十二指肠降部黏膜取组织标本行免疫荧光试验[11],然而很少有关注胃镜下十二指肠降部黏膜免疫荧光试验及其病变程度在HSP辅助诊断中的价值研究。
1 方法
1.1 研究设计 本文为病例系列报告。基于复旦大学附属儿科医院(我院)胃镜室操作流程,胃镜下常规钳取胃窦、胃体和病变部位的黏膜送检病理。本文仅收集HSP患儿胃镜十二指肠降部黏膜病变程度评价结果及其组织荧光试验结果,考察作为HSP辅助诊断的价值和临床的局限性。
1.2 HSP的诊断标准 明显皮肤紫癜伴有如下任何一条:①弥漫性腹痛;②任何部位活检提示IgA沉积;③急性关节炎/关节痛;④血尿或蛋白尿等肾脏受损表现[1]。
1.3 我院HSP诊断流程 以腹痛为主要症状就诊伴典型皮肤紫癜的可直接确诊HSP,同时行胃镜检查了解黏膜病变程度并排除其他疾病,取十二指肠降部黏膜组织行免疫荧光检查;以腹痛就诊虽无皮肤紫癜,但临床高度疑似诊断者,亦行胃镜检查并取十二指肠降部黏膜组织行免疫荧光检查,对症治疗并随访至皮肤紫癜出现。若胃镜下高度怀疑HSP,对症治疗效果不好,即使没有皮肤紫癜,也可考虑诊断性治疗,并随访到紫癜出现。
1.4 十二指肠降部黏膜病变程度判断 回顾性收集纳入分析病例的胃镜影像学资料,出于研究的目的在不知晓临床资料的情况下由2名2年以上胃镜操作经验的胃镜医生共同判断黏膜正常、充血、水肿、糜烂和溃疡。本文以胃镜直视下充血、水肿判断为轻度病变,糜烂和溃疡判断为中重度病变。
1.5 纳入标准 2014年4月至2015年12月于我院消化科以腹痛为主要症状初诊的HSP或怀疑HSP的患儿;②辅助诊断至少有胃镜检查并行十二指肠降部黏膜免疫荧光试验;③怀疑HSP的患儿随访至紫癜出现确诊HSP。
1.6 排除标准 诊断HSP同时,伴以下任意疾病诊断,如细菌性肠炎、血液系统肿瘤、炎症性肠病、川崎病、白塞氏病及系统性红斑狼疮。
1.7 截取资料及其原则 从纳入分析的病历中截取来我院初诊时年龄、性别、主诉时的腹痛症状,从病史中查阅紫癜的记录情况,本文以典型皮肤紫癜出现在胃镜检查前为早出紫癜,之后为晚出紫癜。根据十二指肠降部黏膜免疫荧光试验报告的结果分为阴性、弱阳性及阳性,其中弱阳性归为阴性。
1.8 统计学方法 采用SPSS16.0软件进行统计学分析。研究对象一般特征资料采用描述性统计学方法进行。分类变量资料两组间比较采用Pearson卡方检验,单格子频数小于5者采用Fisher精确概率法检验。P<0.05为差异有统计学意义。
2 结果
2.1 一般情况 符合本文纳入排除标准的54例HSP患儿进入本文分析,男31例,女23例;年龄3岁5个月至15岁3个月,平均(8.1±2.7)岁;门诊病例14例,住院病例40例;早出紫癜36例(76.7%),晚出紫癜18例(自胃镜检查后1~23 d,中位数9.5 d)。
2.2 胃镜与病理检查结果 54例HSP患儿胃镜下黏膜病变累及胃底2例、胃体12例、胃窦27例、十二指肠球部23例,54例均有累及十二指肠降部。十二指肠降部黏膜轻度病变14例,重度病变40例(其中糜烂19例,溃疡21例)。胃镜下黏膜表现为黏膜的广泛充血水肿红斑(图1A)、糜烂(图1B),散在出血点、出血斑及浅表不规则溃疡(图1C)。54例HSP患儿均行十二指肠降部黏膜免疫荧光试验,阳性31例(57.4%),阴性23例(其中弱阳性6例);病理切片均未发现典型的血管炎表现,图2A~C显示,免疫荧光阳性者可见小血管壁及周围绿色荧光带。
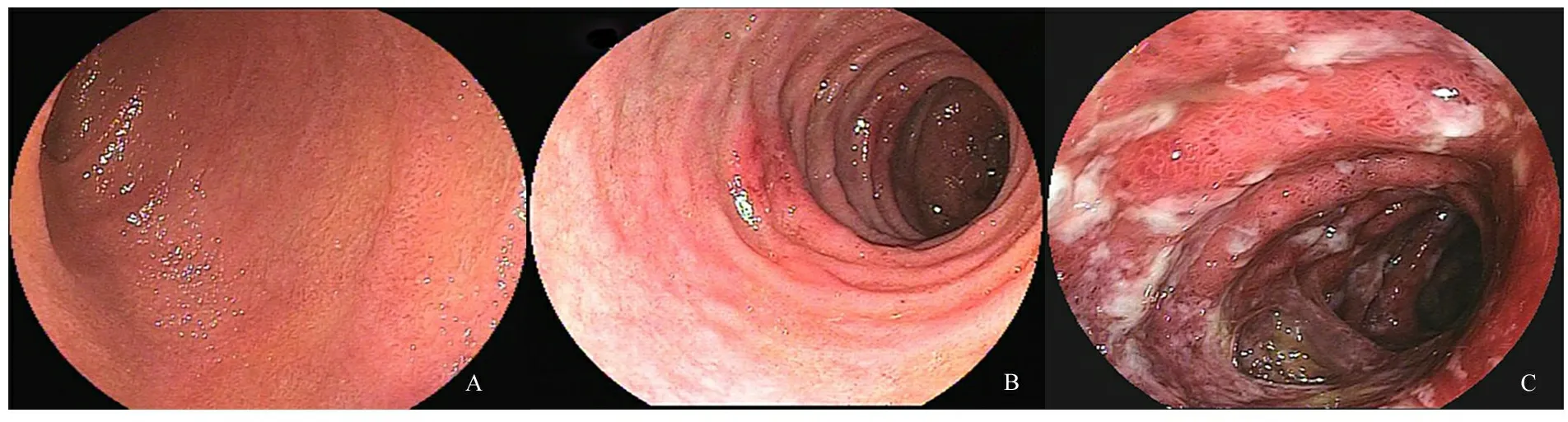
图1 胃镜下HSP十二指肠降部黏膜特征性改变
注 A:十二指肠黏膜充血水肿,B:十二指肠黏膜糜烂,C:十二指肠黏膜溃疡
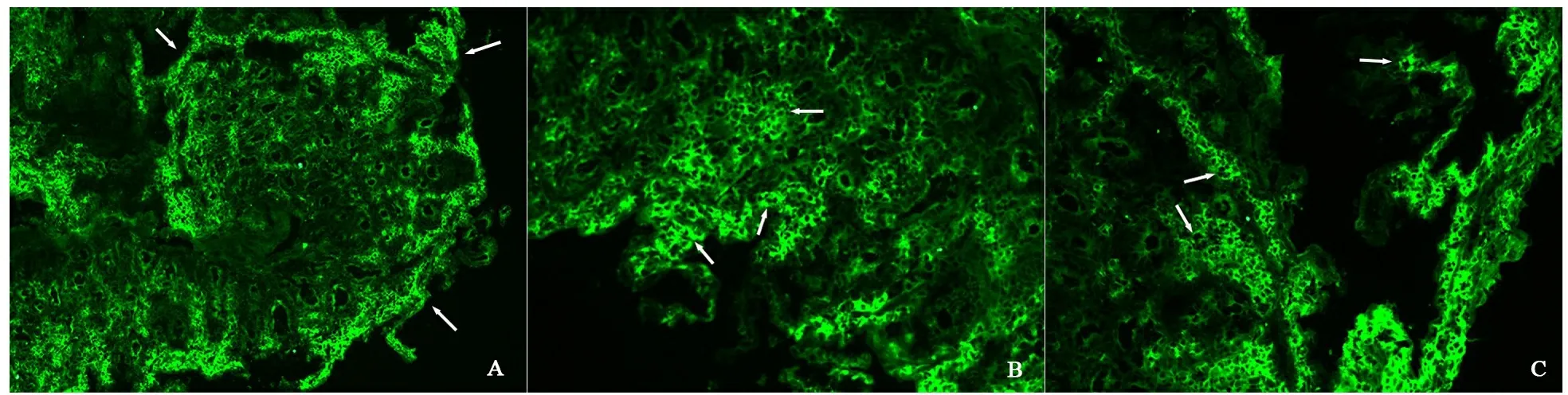
图2 十二指肠黏膜免疫荧光阳性区域主要分布图
注 A:100倍光镜,十二指肠黏膜降部免疫荧光阳性区域主要分布在黏膜浅层小血管处;B、C:200倍光镜,十二指肠降部黏膜免疫荧光分布在小血管及其周围形成绿色荧光带
2.3 HSP患儿十二指肠降部黏膜免疫荧光试验及其病变程度 十二指肠黏膜免疫荧光阴性的HSP患儿23例中黏膜中重度病变21例(91.3%),十二指肠黏膜免疫荧光阳性的HSP患儿31例中黏膜中重度病变19例(61.3%),差异有统计学意义(P=0.013)。表1显示,早或晚出紫癜HSP患儿,不论十二指肠黏膜免疫荧光结果阳性还是阴性,黏膜病变轻度与中重度比例差异均无统计学意义;早出紫癜的HSP患儿黏膜轻度病变十二指肠黏膜免疫荧光阳性比例(10/31,32.2%)多于阴性(2/23,8.7%),差异有统计学意义(P=0.039);不论十二指肠黏膜免疫荧光结果阳性与否:早或晚出紫癜HSP患儿黏膜病变轻度与中重度比例差异均无统计学意义、晚出紫癜的HSP患儿黏膜轻度病变的比例差异无统计学意义、早出或晚出紫癜的HSP患儿黏膜中重病变的比例差异亦均无统计学意义。
表1 HSP患儿十二指肠降部黏膜免疫荧光试验及其 黏膜病变程度(n)
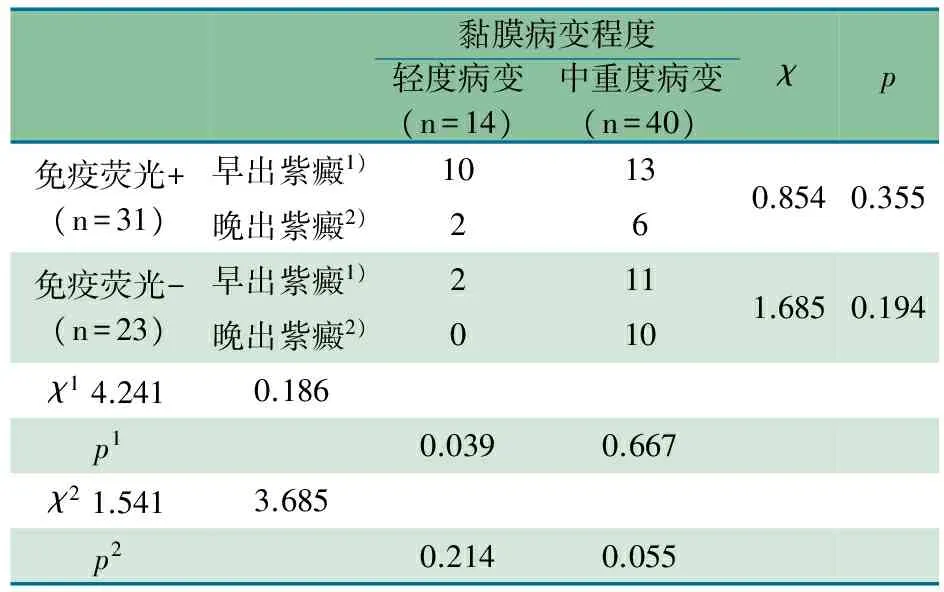
黏膜病变程度轻度病变(n=14)中重度病变(n=40)χp免疫荧光+(n=31)早出紫癜1)1013晚出紫癜2)260.8540.355免疫荧光-(n=23)早出紫癜1)211晚出紫癜2)0101.6850.194χ14.2410.186p10.0390.667χ21.5413.685p20.2140.055
注χ1和P1为早出紫癜患儿中黏膜轻度病变在免疫荧光试验阴性中的比例高于免疫荧光试验阳性;χ2和P2为晚出紫癜患儿中黏膜中重度病变在免疫荧光试验阳性和阴性中的比例无明显差异
3 讨论
目前临床上对HSP的诊断所遵循的诊断标准中皮肤紫癜是核心症状[1],但是有12%~19%的患儿在病初甚至在整个疾病过程中始终不出现紫癜[4,12,13],在没有关节及肾脏病变等的辅助诊断条件的情况下,临床上有腹痛症状的患 儿常规行胃镜检查。HSP患儿上消化道主要累及十二指肠降部,存在典型皮肤紫癜但同时伴有腹痛的患儿胃镜检查一方面可以直观地观察到十二指肠降部黏膜病变的特点,另一方面对仅有腹痛症状,但又高度怀疑HSP的患儿,通过胃镜下观察消化道黏膜病变的特点,对HSP的诊断提供帮助并与其他疾病进行鉴别。
HSP的发病机制是由IgA为主的免疫复合物沉积于小血管壁继而激活补体系统而引起的自身免疫性小血管炎[1],沉积在组织血管壁的免疫复合物是本病发病的关键因素,可以通过组织免疫荧光手段进行检测[2],较早的研究报道皮肤组织免疫荧光阳性率高达93%~100%[14- 16],且与HSP有强烈的相关性[17- 19]。
本研究十二指肠降部黏膜组织免疫荧光阳性率仅为57.4%,远远低于皮肤组织的免疫荧光阳性率。本研究发现十二指肠降部黏膜免疫荧光阴性的患儿,其胃镜下黏膜为中重度病变的比例越高,同样也在早发紫癜中十二指肠降部黏膜免疫荧光阴性的患儿,其胃镜下黏膜为中重度病变的比例也越高。可能与以下因素有关:①HSP患者体内产生的循环免疫复合物主要沉积在具有环形或攀形结构的小血管处,而消化道黏膜微循环冗长且仅在绒毛的小血管处形成攀状结构,故决定了免疫复合物主要沉积在小肠绒毛的小血管中,而较少沉积在黏膜下层的小静脉和小动脉内。这一特点也使病变常局限在黏膜表层,胃镜下黏膜轻度病变者免疫荧光试验阳性率高,黏膜中重度表现为糜烂和浅表性溃疡[2,11,20]反而阳性率低。②对于黏膜中重度病变的患儿来说,能够体现免疫复合物沉积的所需要血管壁结构遭到破坏且沉积的免疫复合物很大程度上也已被降解清除[21- 24];③内镜医生在胃镜钳取黏膜组织时一般会选取有病理意义的典型病变部位,典型病变部位由于黏膜病变较重可能钳取不到有病理意义的组织;④限于内镜下活检钳的尺寸很难取到黏膜下层,不能观察到小动脉、小静脉的病理变化[21,22]。本研究的结果提示,黏膜免疫荧光阳性率低应谨慎看待,黏膜免疫荧光结果的阴性可能为假象,临床工作中需要结合患儿的临床特点、胃镜下十二指肠黏膜的病变严重程度等多方面因素考虑。
由于消化道黏膜血管解剖结构方面的特点加之取材方面的主观客观因素,可能导致了十二指肠黏膜免疫荧光的总阳性率低于皮肤,黏膜病变程度越重免疫荧光阳性率越低。有文献报道,HSP患者非紫癜部位的皮肤组织免疫荧光也可呈阳性[25],由此提示高度怀疑HSP时,需要消化道黏膜取活检行免疫荧光检测时,可考虑在病变部位或相对正常部位多点取活检,并尽可能取得深达黏膜下层的组织。
关于胃镜下黏膜病变程度的划分未见文献报道,本研究以轻度(充血、水肿)和中重度(糜烂、溃疡)划分胃镜下十二指肠黏膜病变程度,结合黏膜免疫荧光检查可在一定程度上提高对HSP累及消化道黏膜的识别力。由于胃镜检查方便、快捷、安全性高,可在发病早期通过胃镜直观的观察并评估黏膜病变的范围及严重程度,而且早于免疫荧光试验,而且可以省去行免疫荧光试验的费用。鉴于此,亟需建立内镜下黏膜病变评估方法。
[1] Ruperto N, Ozen S, Pistorio A, et al. EULAR/PRINTO/PRES criteria for Henoch- Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part I: Overall methodology and clinical characterisation. Ann Rheum Dis, 2010, 69(5): 798- 806
[2] Linskey KR, Kroshinsky D, Jr MM, et al. Immunoglobulin- A- associated small- vessel vasculitis:a 10- year experience at the Massachusetts General Hospital. J Am Acad Dermatol, 2012, 66(5): 813- 822
[3] Larson AR, Granter SR. Utility of Immunofluorescence Testing for Vascular IgA in Adult Patients With Leukocytoclastic Vasculitis. Am J Clin Pathol, 2014, 142(3): 370- 374
[4] Trapani S, Micheli A, Grisolia F, et al. Henoch Schönlein Purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5- year period and review of literature. Semin Arthritis Rheum, 2005, 35(3): 143- 153
[5] Chang WL, Yang YH, Lin YT, et al. Gastrointestinal manifestations in Henoch- Schönlein purpura: a review of 261 patients. Acta Paediatr, 2004, 93(11): 1427- 1431
[7] Lindenauer SM, Tank ES. Surgical aspects of Henoch- Schonlein's purpura. Surgery, 1966, 59(6): 982- 987
[8] Schwab J, Benya E, Lin R, et al. Contrast enema in children with Henoch-Schönlein purpura. J Pediatr Surg, 2005, 40(8): 1221- 1223
[9] Esaki M, Matsumoto T, Nakamura S, et al. GI involvement in Henoch-Schönlein purpura. Gastrointest Endosc, 2002, 56(6): 920- 923
[10] Zhang Y, Huang X. Gastrointestinal involvement in Henoch- Schönlein purpura. Scand J Gastroenterol, 2008, 43(9): 1038- 1043
[11] Gunasekaran TS, Berman J, Gonzalez M. Duodenojejunitis: Is It Idiopathic or Is It Henoch- Schönlein Purpura Without the Purpura?. J Pediatr Gastroenterol Nutr, 2000, 30(1): 22- 28
[12] Saulsbury FT. Henoch- Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore), 1999, 78(6): 395- 409
[13] Kato S, Ozawa KN, Naganuma H, et al. Immunoglobulin A enteropathy: a possible variant of Henoch- Schönlein purpura. Dig Dis Sci, 2004, 49(11- 12): 1777- 1781
[14] Giangiacomo J, Tsai CC. Dermal and glomerular deposition of IgA in anaphylactoid purpura. Am J Dis Child, 1977, 131(9): 981- 983
[15] Asamer H, Wohlfarth B, Schabel F, et al. Dittrich P.Clinical and immunological aspects of the Schoenlein- Henoch syndrome. Schweiz Med Wochenschr, 1974, 104(34): 1188- 1192
[16] Tsai CC, Giangiacomo J, Zuckner J. Letter: dermal IgA deposits in Henoch- Schönlein purpura and Berger's nephritis. Lancet, 1975, 1(7902): 342- 343
[17] Jennette JC, Falk RJ. Small- vessel vasculitis. Current Rheumatology Reports, 2007, 338(4): 1512- 1523
[18] Piette WW, Stone MS. A cutaneous sign of IgA- associated small dermal vessel leukocytoclastic vasculitis in adults (Henoch- Schönlein purpura). Arch Dermatol, 1989, 125(1): 53- 56
[19] Egan CA, Taylor TB, Meyer LJ, et al. IgA1 is the major IgA subclass in cutaneous blood vessels in Henoch- Schönlein purpura. Br J Dermatol, 1999, 141(5): 859- 862
[20] Cheungpasitporn W, Jirajariyavej T, Howarth C B, et al. Henoch- Schönlein purpura in an older man presenting as rectal bleeding and IgA mesangioproliferative glomerulonephritis: a case report. J Med Case Rep, 2011, 5(1):1- 5
[21] Pillebout E, Thervet E, Hill G, et al. Henoch- Schönlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol, 2002, 13(5): 1271- 1278
[22] Choong CK, Beasley SW. Intra- abdominal manifestations of Henoch- Schönlein purpura. J Paediatr Child Health, 1998, 34(5): 405- 409
[23] Tizard EJ. Henoch- Schönlein purpura. Current Paediatrics, 1977, 1(6055): 190- 191
[24] Davin JC, Weening J. Henoch- Schönlein purpura nephritis: an update. Eur J Pediatr, 2001, 160(12): 689- 695
[25] Murali NS, George R, John GT, et al. Problems of classification of Henoch Schönlein purpura: an Indian perspective. Clin Exp Dermatol, 2002, 27(4): 260- 263
(本文编辑:张崇凡,孙晋枫)
ThevalueandlimitationofmucosalimmunofluorescenceofduodenaldescendingtoassistdiagnosisofHenoch-Schönleinpurpura
SONGYing1,4,SHIJie-ru2,4,TANGZi-fei2,FENGJia-yan3,CHENLian3,HUANGYing2
(1DepartmentofPediatrics,FirstPeople'sHospitalofKunshan,Kunshan215300,China; 2DepartmentofDigestive,ChildrenHospitalofFudanUniversity,Shanghai201102,China; 3DepartmentofPathology,ChildrenHospitalofFudanUniversity,Shanghai201102,China; 4hasequalcontributiontothestudy)
Corresponding Author:HUANG Ying,E- mail:yhuang815@163.com
ObjectiveTo study the value of immunofluorescence test and the severity of mucosal lesions of the duodenum descending portion in the diagnosis of Henoch-Schönlein purpura.MethodsTo collect the situation of purpura from the medical history and the degree of mucosal lesions and the immunofluorescence results of duodenum under endoscopic examination. The hyperemia and edema of mucosal were defined as mild lesions, the erosion and ulcer were defined as moderate to severe lesions observed under the endoscopy. The typical skin purpura appeared before the examination of gastroscopy was named as early onset purpura, and after that was named as later onset purpura. According to the results of immunofluorescence test was divided into negative and positive.ResultsThe newly diagnosed Henoch- Schönlein purpura or suspected Henoch- Schönlein purpura patients with the main symptom of abdominal pain were collected who were treated in Department of Gastroenterology of Children's Hospital of Fudan University from April 2014 to December 2015. Fifity- four cases who finished the gastroscopic examination and immunofluorescence test of duodenum portion mucosal were enrolled in the study. Thirty- one cases were males and 23 cases were females; the average age was 8.1±2.7 years; 14 cases were outpatient patients, 40 cases were hospitalized patients; 36 patients (76.7%) were with early onset purpura, 18 cases were with later onset purpura. Duodenal mucosal lesions manifested as congestion and edema in 14 cases, manifested as erosion and ulcers in 40 cases (including erosion in 19 cases and ulcers in 21 cases). Thirty- one cases (57.4%) were immunofluorescence positive of duodenal mucosa and negative in 23 cases. The frequency of moderate to severe mucosal lesions was significantly higher in patients with duodenal mucosal immunofluorescence-negative Henoch- Schönlein purpura than that in positive ones (91.3%vs61.3%,P=0.013). The frequency of mild mucosal lesions in patients with immunofluorescence positive of duodenal mucosa was higher than that with negative on the basis of all the patients with early onset purpura, the difference was statistically significant (χ2=4.241,P=0.039). There was no significant difference of the degree of mucosal lesions of duodenal portion between early or later onset purpura regardless of the result of immunofluorescence positive or negative of duodenal descending portion mucosal. There was no significant difference in the proportion of mucosal mild lesions with later onset purpura between immunofluorescence positive and negative of duodenal descending portion mucosal. There was no statistically significant difference in the proportion of moderate to severe lesions between patients with early onset purpura and patients with later onset purpura regardless of the result of immunofluorescence positive or negative of duodenal descending portion mucosal.ConclusionClinical diagnosis of Henoch- Schönlein purpura with negative results of mucosal immunofluorescence should be cautious. It may be mainly associated with the severity of the lesion and can't rule out the biopsy factors, such as location, quantity, depth and equipment.
Henoch-Schönlein Purpura; Gastroscopy; Children; Immunofluorescence
1江苏省昆山市第一人民医院儿内科 昆山,215300;2复旦大学附属儿科医院消化科 上海,201102;3复旦大学附属儿科医院病理科 上海,201102;4 共同第一作者
黄瑛,E-mail: yhuang815@163.com
10.3969/j.issn.1673-5501.2016.06.010
2016-12-02
2016-12-20)

