PATHOLOGICAL CHANGES AND ETIOLOGICAL DIAGNOSIS IN FARMED ADULT RAINBOW TROUT (ONCORHYNCHUS MYKISS) ASSOCIATED WITH A LOW MORTALITY
FAN Wei, DUAN Ya-Jiao, HUANG Xiao-Li, DENG Yong-Qiang, DU Zong-Jun, GENG Yi, CHEN De-Fangand WANG Kai-Yu
(1. Department of Aquaculture, Sichuan Agricultural University, Chengdu 611130, China; 2. Sichuan Provincial Center for Animal Disease, Prevention and Control, Chengdu 611130, China; 3. College of Animal Science and Veterinary Medicine,Sichuan Agricultural University, Chengdu 611130, China)
PATHOLOGICAL CHANGES AND ETIOLOGICAL DIAGNOSIS IN FARMED ADULT RAINBOW TROUT (ONCORHYNCHUS MYKISS) ASSOCIATED WITH A LOW MORTALITY
FAN Wei1, DUAN Ya-Jiao1, HUANG Xiao-Li1, DENG Yong-Qiang2, DU Zong-Jun1, GENG Yi3, CHEN De-Fang1and WANG Kai-Yu3
(1. Department of Aquaculture, Sichuan Agricultural University, Chengdu 611130, China; 2. Sichuan Provincial Center for Animal Disease, Prevention and Control, Chengdu 611130, China; 3. College of Animal Science and Veterinary Medicine,Sichuan Agricultural University, Chengdu 611130, China)
Infectious haematopoietic necrosis (IHN) is a severe infectious disease in a variety of salmonids such as sockeye salmon (Oncorhynchus nerka) and rainbow trout (Oncorhynchus mykiss), and leads to necrosis in the haematopoietic organs and tissues including kidney and spleen and causes up to 100% mortality in larvae salmons. However, it can also infect adult trout and cause lower mortality. In September 2014, an outbreak emerged in a trout farm in Chongzhou, Sichuan Province, with about 30% cumulative mortality in 2-years rainbow trout (Oncorhynchus mykiss). Gross findings included dark skin color, hemorrhagic spots in muscle and abdominal wall. Histopathology showed severe necrosis in the hematopoietic tissues including spleen and interstitial kidney. A multiplex RT-PCR method was conducted to detect IHNV, VHSV and IPNV of fish tissues and cell culture. Only a 371 bp segment was amplified which was confirmed as an IHNV gene through sequencing and phylogenetic tree. Furthermore, the obvious cytopathic effect (CPE) was observed in epithelioma papulosum cyprini (EPC) cells inoculated with tissue filtrates. And 20 rainbow trouts (1.5 kg mean weight)4TCID50per mL, and the cumulative mortality reached 35% within 10 days. No bacteria and parasites were detected in the case. All the results demonstrated that it was due to IHNV infection in spite of the low mortality.
a 0.5 mL i.p. injection of the tissue filtrate of 10
Rainbow trout; Adult fish; IHNV; Dagnosis; Low mortality; Pathological change
Infectious haematopoietic necrosis (IHN) is a disease in fish that leads to necrosis in the haematopoietic organs and tissues including kidney and spleen, which was first reported and named by Amend[1]. It usually isolated from a variety of salmon such as sockeye salmon (Oncorhynchus nerka)and rainbow trout (Oncorhynchus mykiss), which is the most susceptible species to the virus[2-4].
The first outbreak of IHN was reported in the United States at Washington and Oregon fish hatcheriesin 1953[5]. Probably due to the commercial sale of eggs and fish which are infected, the virus was spread to Japan from Alaska in 1960s[6]. Subsequently, it was reported in France and Italy in 1987[7,8]. The infection of IHNV in China is due to the importing eggs from Japan and spreading to the northeast of China as early as 1985. It’s until 1990 that the virus was first isolated in Benxi, Liaoning Province[9]. IHNV was found in farmed halibut, rainbow trout and the imported paddlefish eggs in 2006[10]. Furthermore, it was also found by other researchers in China after 2010[11-13]. So far, 25-28 million eggs and 16000 tons of marketable fish which were 0.77% of the whole world production were produced by the main representative breeding area including Beijing,Jiangsu, Qinghai and Yunnan[14,15], but China has to import more than 10000 tons Atlantic salmon to meet the needs of the market every year[16]. And now, thefarming industry of Salmonidae is one of main cold water fish industry in China, the IHN pose a significant threat to the farming industry of salmonids in China.
Brief introduction of author: Fan Wei (1990—), E-mail: 379690242@qq.com
In this case report, we described the diagnostic evaluation of IHNV in adult rainbow trout that were submitted from a rainbow trout farm in Chongzhou,Sichuan Province in September 2014, with cumulative mortality about 30%. Analysis and diagnosis were performed following by necropsy, pathology,bacteriology and virology. Diagnostic investigation revealed that the rainbow trout were suffering from a systemic viral infection caused by IHNV. This suggested that IHN is an emmerging epizootic in farmed adult salmonids in China and our findings would provide a full consideration for the prevention of the disease in China.
1 Methods
1.1 Necropsy
Five adult rainbow trout, 4 live and 1 freshly died, from a Salmonid farm in Chongzhou, Sichuan Province in September, were submitted to our laboratory in SCAU for necropsy. The weight of the five fish was between 1.5 to 2 kilograms with a length of 50 to 60cm in size. The live fish were euthanized by using an overdose of MS-222. Firstly, all fish were examined the parasites of the gill and skin, subsequently, the examinations include gross external, internal lesions and content of the alimentary tract.
1.2 Histopathology
All the tissues for histopathology were collected and fixed immediately in 10% Neutral Buffered Formalin (1∶10 ratio of tissue to fixative): The tissues include gill, brain, eye, kidney, spleen, heart, liver, nares, gonad, esophagus, stomach, intestine, pyloric caeca, pancreas and body wall. Decalcified the bone and the cartilage (cranium, eye, body wall, and gill) by immersion for 24 hours in a commercially avai- lable decalcification fluid.
After the decalcification, these tissues were trimmed into cassettes, dehydrated in graded ethanol solutions, cleared in xylene, and embedded in paraffin wax. Tissues were cut to 5 μm and stained with hematoxylin and eosin (HE).
1.3 Bacteriology and Virology
A sterile swab of the kidney was provided at necropsy for routine bacteriology from each fish. Growing on blood agar at both 15℃ and 22℃. For virological examination, samples of these fish including heart, liver, spleen, kidney, pyloric caeca, intestine, gill and muscle were collected and stored at -80℃ immediately. Tissues including kidney and spleen were homogenized in a Stomacher with Hanks’ balanced salt solution (HBSS), and centrifuged at 3000 g for 20min. The supernatant was filtered through a 220 nm membrane filter, and 100 μL of the filtrate was used to inoculate to epithelioma papulosum cyprini (EPC) cells at 15℃. The cytopathic effects (CPE) was checked under microscopy daily.
Tissues were also tested for infectious pancreatic necrosis virus (IPNV), viral haemorrhagic septicaemia virus (VHSV) and IHNV by a multiplex reverse transcription-PCR (RT-PCR) method. The specific primers of these 3 virus had been developed previously[17]. Primers IHN-F and IHN-R recognize a 371 bp cDNA fragment within the N gene of IHNV. Primers VHSV-F and VHSV-R amplify a 625 bp cDNA fragment within the G gene of VHSV, Primers of IPNV recognize a 206 bp cDNA fragment within the VP2 gene of aquatic birnaviruses[18]. All these primers are put in table 1.
1.4 RNA extraction
Total RNA was extracted from tissues and infected cell culture by using the RNAiso Plus (TaKaRa,Dalian, China) according to the manufacturer’s protocols. Pulverizing the Mixing frozen tissues including kidney, head kidney, pyloric caeca, spleen, liver,heart, gill and muscle with liquid nitrogen, Samples were stored at -80℃ for RNA extraction subsequently.
1.5 RT-PCR amplification
Approximately 2 μg of total RNA was used for cDNA synthesis using the PrimerScript RT reagent Kit (TaKaRa, Dalian, China) with the manufacturer’s standard protocol. It performed with 10 μL of reverse transcribed cDNA in a 50 μL reaction mixture which consists of 25 μL of 10×PCR buffer (TaKaRa, Dalian,China), 3 μL ddH2O and 2 μL each primer, followed by cooling to 37℃ for 15min, 85℃ 15s. A multiplex RT-PCR assay was performed for detection of IPNV,VHSV and IHNV. The PCR protocol: initial denaturation at 94℃ for 4min, denaturation at 94℃ for 30s,annealing at 60℃ for 30s, extension at 72℃ for 90s,and final extension at 72℃ for 10min. The PCR products were confirmed by 1% Agarose gels, which contains 8 μL of ethidium bromide per 100 mL in 1× TAE electrophoresis buffer (40 mmol/L Tris, 20 mmol/L acetate, 2 mmol/L EDTA).

Tab. 1 Primers of IHNV, VHSV and IPNVs[19]
1.6 DNA Sequencing and Phylogenetic Analysis
The PCR amplifications were sent to a commercial sequencing facility (TaKaRa, Dalian, China). The generated sequences were compared with other IHNV sequences from NCBI’s GenBank sequence database using a Blast search algorithm. Genetic distances between each pair of sequences were calculated by Molecular Evolutionary Genetics Analysis ver. 5 (MEGA5), including all current available IHNV sequences of the N gene. Phylogenetic analysis was conducted using the neighbor-joining program with 1000 boot-strapped replicates. And the sequences were submitted to GenBank.
1.7 Animal infection assay
40 rainbow trout (1.5 kg mean weight) without IHNV were maintained in 2 tanks that received single-pass water at a temperature about 15℃. Fish were fed twice daily with a commercial rainbow trout diet. All fish were divided in two groups (group A and B) of 20 fishes each. Group A received 0.5 mL of saline (0.85%) intraperitoneal (i.p.) injection. Group B received a 0.5 mL i.p. injection of the tissue filtrate of 104TCID50per mL. Tissues (liver, kidney and spleen) from the dead fish were collected for virus isolation and RT-PCR detection.
2 Result
2.1 Necropsy Findings
Gross pathological changes of all five fish showed obvious black skin color. Wet mount evaluations of the gills from the four live fish were found to be negative for parasites. All five fish showed apparent internal changes as specified below. Three fish presented on slight muscle spotted hemorrhage and severe on abdominal wall. Two fish showed splenomegaly and unsmooth surface of spleen. What is more, it was filled with bile-stained mucus with no food in the intestinal tract. Only one fish showed obvious nephrorrhagia.
2.2 Histopathology Findings
All the five fish showed multi-tissues and systematic marked lesion especially in spleen and kidney and also some mild changes in liver and intestines. All the tissue sections of trunk kidney showed interstitial hemopoietic cells decreased with marked,focal or fusion necrosis (Fig. 1D). Tissue sections of spleen also showed marked necrosis, focal or bridging with infiltrated inflammatory cells (Fig. 1C). However, liver sections from fish showed mild necrosis with white blood cells around or in blood vessels (Fig. 1A and B). Mild inflammatory cells infiltrated in intestinal submucosa. No significant morphological changes were found in brain and heart. No obvious organisms were seen in any of tissues.
2.3 Examination of bacteriology and virology

Fig. 1 Pathological lesions in infected rainbow trout tissues
No parasites were found in the wet mounts and no colony was seen in any of these BA plates. All thefish were found to be free of significant bacterial and parasitic agents. However, tissue filtrates from kidney and spleen produced CPE in epithelioma papulosum cyprini (EPC) cells at 15℃. This demonstrated virus was considered to be the pathogen of the case(Fig. 2).
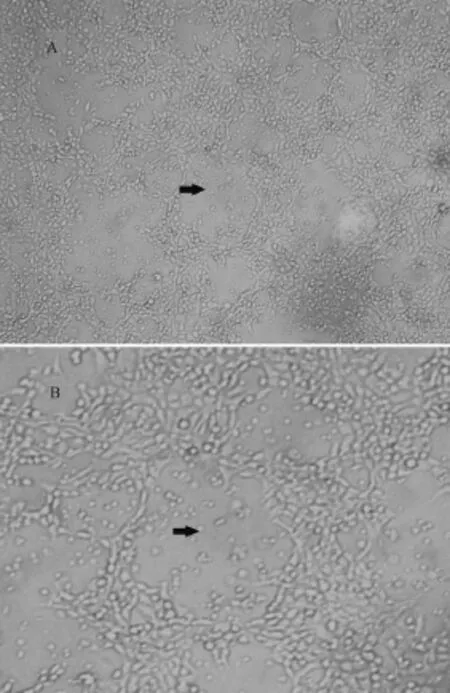
Fig. 2 Cytopathic effects (CPE) produced by inoculation of epithelioma papulosum cyprini (EPC) cells with tissue filtrate from diseased rainbow trout
2.4 Multiplex RT-PCR
Two 371 bp target brands were amplified from fish tissues and cell culture (Fig. 3). No specific brands of 206 bp or 625 bp for IPNV or VSHV were amplified. It referred to IHN once more according to the RT-PCR result.

Fig. 3 Typical agarose gels showing simultaneous RT-PCR identification of IHNV, VHSV and IPNV
2.5 DNA Sequencing and Phylogenetic Analysis
The RT-PCR product was sequenced and submitted to NCBI (accession number: KP117310). A GenBank BLAST search using the sequenced PCR products revealed a 93.8%—98.1% sequence identity to IHNV (Fig. 4). Phylogenetic tree showed KP117310 had the highest homology with the other two strains isolated from China earlier.
2.6 Animal infection assay
Clinical symptoms could be seen fish in group B in the second day, and the highest mortality occurred in the fourth day. The cumulative mortality of group B reached 35% within 10 days, while no mortality could be found in group A. Internal organs and muscle tissue from dead fish were collected for RTPCR detection. The results showed that 371 bp for IHNV purpose segment from individual dead fish of group B were amplified while samples from group A were negative. The results unequivocally demonstrated that the adult rainbow trout with low mobility were caused by IHNV.
3 Discussion
Infectious hematopoietic necrosis virus (IHNV)is a serious pathogen easily locating in cultured, wild,juvenile salmonids and producing an acute systemic disease. The main spread route of IHNV is water. IHNV could spreads between fry and juvenile.
It was hypothesized that the disease was most likely IHN through necropsy and pathology findings. For gross pathology found dotted hemorrhage in muscle, obvious splenomegaly and histopathology found marked necrosis in hematopoietic tissues including spleen and interstitial kidney, all those symptoms were the mainly characteristics of IHN. A multiplex RT-PCR assay was conducted to detect IHHV. The amplified fraction was demonstrated to be a IHNV gene through sequencing and analyzing through the phylogenetic tree. In addition, inoculation with tissue filtrates confirmed CPE which suggested a viral infection. No parasites or bacteria were found in all the five fish. Based on the results of cellulated detection, RT-PCR and pathology, it was fully supported the conclusion that the infection is caused by IHNV.
IHNV often cause up to 100% mortality in salmonid larve, fry and juveiles[19]. The fries of rainbow trout cause a mortality about 90% at a rainbow trout farm in Jilin Province[13]. However, some reports also demonstrated it could infect adult trout leading to lower mortality[20]. In this case, adult rainbow trout was infected and had a cumulative mortality about 30% at 15℃ which was the best temperature for IH-NV living, which confirmed that IHNV could infect 2-year rainbow trout in low mortality with no doubt.
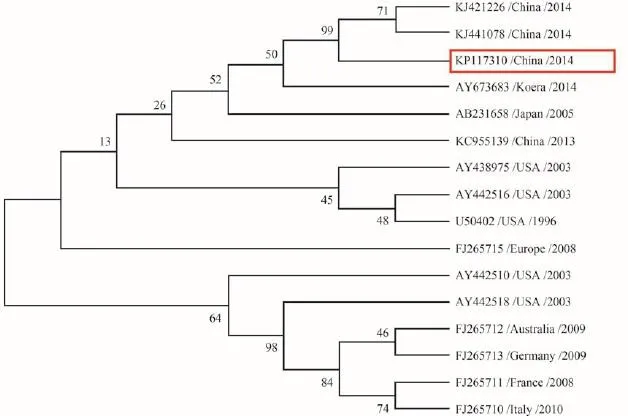
Fig. 4 Phylogenetic relationship of the partial nucleoprotein (N) sequences of 15 IHNV strains with KP117310
It’s known that IHNV may vary with the age or weitht of rainbow trout, and IHNV caused mortality in fish independent of their weight or age[21]. The smaller fish were more susceptible to IHNV[22]. Furthermore, the water temperature is one of the important factors that affect the morbidity and mortality of IHNV[23], the clinical symptoms were appeared obviously for 3 to 4 days following exposure to virus at water temperature of 8-15℃. In addition, IHNV is a RNA virus which is easy mutation, even though a mutational single gene in the genome can lead to a change of the virulence[24]. Comparative three strains of infectious hematopoietic necrosis virus in fry rainbow trout, the Idaho strain of IHNV was the most virulent and induced a 62% mortality over a 10 day period at water temperatures of 10℃. In contrast,strains of IHNV from Oregon and California caused only 4% and 6% mortality under the same conditions[25]. It was supposed that these were some relationship among water temperature, genotype and weight which we will focus next. Strikingly, the mortality of rainbow trout infected with IHNV was not always related to the weight.
IHNV has a serious impact on the Salmonidae industry, therefore, the detection and prevention of IHNV would be more and more important. The ef-
References:
[ 1 ]Amend D F, Mead W T Y R. A hematopoietic virus disease of rainbow trout and sockeye salmon [J]. Transactions of the American Fisheries Society, 1969, 98(4): 796—804
[ 2 ]Bootland L M, Leong J A C, Woo P T K, et al. Infectious haematopoietic necrosis virus [J]. Fish Diseases & Disorders, 2011, 3: 66—109 fective way of prevention IHN is to establish a good prevention system including strictly controlling diseases spread in the transportation process of commercial fish and fry, and selecting broodstock without carrying IHNV, and using vaccine to prevent. In 1996, the first report of genetic immunization of fish showed an IHNV DNA vaccine which provided a strong protective immunity to juvenile rainbow trout[26]. And then, several researchers also focused on vaccination study[27—32]. Although very effective in protecting fish against IHNV, only one DNA vaccine has been approved to date for use in Canada[33]. In Europe and in US, its commercialization is restricted due to safety concerns[34].
In the next work, we are going to do the surveillance about IHN infection and make a map of IHN distribution in China. We hope that would be helpful for IHN controlling in the process of international trade.
[ 3 ]Williams I V, Amend D F. A natural epizootic of infectious hematopoietic necrosis in fry of sockeye salmon (Oncorhynchus nerka) at Chilko Lake, British Columbia [J]. Journal of the Fisheries Research Board of Canada, 2011, 33(7): 1564—1567
[ 4 ]Breyta R, Jones A, Stewart B, et al. Emergence of MD type infectious hematopoietic necrosis virus in Washington State coastal steelhead trout [J]. Diseases of Aquatic Organisms,2013, 104(3): 179—195
[ 5 ]Rucker R R, Whipple W J, Parvin J R, et al. A Contagious Disease of Salmon Possibly of Virus Origin [M]. United State Government Printing Office, Washington D C. 1952,76
[ 6 ]Sano T, Nishimura T, Okamoto N, et al. Studies on viral diseases of Japanese fishes. VI. Infectious hematopoietic necrosis (IHN) of salmonids in the mainland of Japan [J]. Journal of the Tokyo University of Fisheries, 1977, 63(2): 81—85
[ 7 ]Laurencin F B. IHN (infectious haematopoetic necrosis) in France [J]. Bulletin of the European Association of Fish Pathologists, 1987, 7(4): 104
[ 8 ]Bovo G, Giorgetti G, Jørgensen P E V, et al. Infectious haematopoietic necrosis: first detection in Italy [J]. Bulletin of the European Association of Fish Pathologists, 1987, 7(5): 124
[ 9 ]Zhao Z Z, Niu L Q. Isolation and preliminary study of a rhabdovirus from rainbow trout (Oncorhynchus mykiss) in Benxi, China [J]. Acta Hydrobiologica Sinica, 1994, 18(4): 348—353 [赵志壮, 牛鲁祺. 中国本溪虹鳟一株弹状病毒的分离及初步研究. 水生生物学报, 1994, 18(4): 348—353]
[10]Liu H, Fan W H, Shi X J, et al. Detection and genetic analysis of infectious haematopoietic necrosis virus (IHNV) from cultured fish and imported fish larvae [J]. Journal of Huazhong Agricultural University, 2006, 25(5): 544—549[刘荭, 范万红, 史秀杰, 等. 国内养殖鱼类和进境鱼卵中传染性造血器官坏死病毒(IHNV)的检测及基因分析. 华中农业大学学报:自然科学版, 2006, 25(5): 544—549]
[11]Xu L M, Liu H B, Yin J S, et al. Prokaryotic expression and immunogenicity analysis of glycoprotein from infectious hematopoietic necrosis virus [J]. Chinese Journal of Virology, 2013, 29(5): 529—534 [徐黎明, 刘红柏, 尹家胜, 等.传染性造血器官坏死病毒糖蛋白原核表达及免疫原性分析. 病毒学报, 2013, 29(5): 529—534]
[12]Shen S S, Zheng X C, Liu H, et al. Genetic analysis of G gene of infectious hematopoietic necrosis virus isolated in China [J]. China Animal Health Inspection, 2012, 29(1): 37—41 [申斯思, 郑晓聪, 刘荭, 等. 两株传染性造血器官坏死病病毒的分离及其糖蛋白基因序列分析. 中国动物检疫, 2012, 29(1): 37—41]
[13]Luo X, Deng G C, Zhao C C, et al. Isolation and preliminary identification of rhabdovirus from Channa maculata cultured in pond [J]. Acta Hydrobiologica Sinica, 2013,37(4): 620—625 [罗霞, 邓国成, 赵长臣, 等. 池塘养殖斑鳢弹状病毒的分离与初步鉴定. 水生生物学报, 2013, 37(4): 620—625]
[14]Niu L Q, Zhao Z Z. The epidemiological IHN and IPN of rainbow trout in Northeast China [J]. Journal of Fisheries of China, 1988, 12(4): 327—332 [牛鲁祺, 赵志壮. 东北地区虹鳟IHN和IPN流行病学的初步研究. 水产学报, 1988,12(4): 327—332]
[15]Peng J, Zheng X C, Shi X J, et al. Determination of the complete genome sequence of infectious hematopoietic necrosis virus (IHNV) Ch20101008 and viral molecular evolution in China [J]. Infection Genetics & Evolution Journal of Molecular Epidemiology & Evolutionary Genetics in Infectious Diseases, 2014, 27: 418—431
[16]Sun D J, Wang B Q. Aquaculture of Salmonids in China [J]. Chinese Journal of Fisheries, 2010, 23(2): 56—63 [孙大江,王炳谦. 鲑科鱼类及其养殖状况. 水产学杂志, 2010, 23(2): 56—63]
[17]Sweeney A. Multiplex PCR assay for the simultaneous detection of IPNV and IHNV [D]. University of Maine, Maine. 1996
[18]Williams K, Blake S, Sweeney A, et al. Multiplex reverse transcriptase PCR assay for simultaneous detection of three fish viruses [J]. Journal of Clinical Microbiology, 1999,37(12): 4139—4141
[19]Woo P T, Leatherland J F, Bruno D W. Fish Diseases and Disorders [M]. CABI: Academic Press. 2011, 3
[20]Wolf K. Infectious hematopoietic necrosis [J]. Fish Viruses and Fish Viral Diseases, 1988: 83—114
[21]Lapatra S E. Factors Affecting Pathogenicity of Infectious Hematopoietic Necrosis Virus (IHNV) for Salmonid Fish [J]. Journal of Aquatic Animal Health, 1998, 10(2): 121—131
[22]Bergmann S M, Fichtner D, Skall H F, et al. Age and weight dependent susceptibility of rainbow trout Oncorhynchus mykiss to isolates of infectious haematopoietic necrosis virus(IHNV) of varying virulence [J]. Diseases of Aquatic Organisms, 2003, 55(3): 205—210
[23]Nishizawa T, Kinoshita S, Kim W S, et al. Nucleotide diversity of Japanese isolates of infectious hematopoietic necrosis virus (IHNV) based on the glycoprotein gene [J]. Diseases of Aquatic Organisms, 2006, 71(3): 267—272
[24]Lauring A S, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution [J]. Nature Reviews Microbiology, 2013, 11(5): 327—336
[25]Lapatra S E, Groff J M, Fryer J L, et al. Comparative pathogenesis of three strains of infectious hematopoietic necrosis virus in rainbow trout Oncorhynchus mykiss [J]. Diseases of Aquatic Organisms, 1990, 8(2): 105—112
[26]Anderson E D, Mourich D V, Fahrenkrug S C, et al. Genetic immunization of rainbow trout (Oncorhynchus mykiss)against infectious hematopoietic necrosis virus [J]. Molecular Marine Biology & Biotechnology, 1996, 5(2): 114—122
[27]Traxler G S, Anderson E, Lapatra S E, et al. Naked DNAvaccination of Atlantic salmon Salmo salar against IHNV[J]. Diseases of Aquatic Organisms, 1999, 38(3): 183—190
[28]Lorenzen N, Lorenzen E, Einer-Jensen K, et al. Immunity induced shortly after DNA vaccination of rainbow trout against rhabdoviruses protects against heterologous virus but not against bacterial pathogens [J]. Developmental & Comparative Immunology, 2002, 26(2): 173—179
[29]Einer-Jensen K, Delgado L, Lorenzen E, et al. Dual DNA vaccination of rainbow trout (Oncorhynchus mykiss) against two different rhabdoviruses, VHSV and IHNV, induces specific divalent protection [J]. Vaccine, 2009, 27(8): 1248—1253
[30]Kurath G, Garver K A, Corbeil S, et al. Protective immunity and lack of histopathological damage two years after DNA vaccination against infectious hematopoietic necrosis virus in trout [J]. Vaccine, 2006, 24(3): 345—354
[31]Corbeil S, Lapatra S E, Anderson E D, et al. Evaluation of the protective immunogenicity of the N, P, M, NV and G proteins of infectious hematopoietic necrosis virus in rainbow trout oncorhynchus mykiss using DNA vaccines [J]. Diseases of Aquatic Organisms, 1999, 39(1): 29—36
[32]Ballesteros N A, Alonso M, Saint-Jean S R, et al. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss) [J]. Fish & Shellfish Immunology, 2015, 45(2): 877—888
[33]Chico V, Ortega-Villaizan M A, Tafalla C, et al. The immunogenicity of viral haemorragic septicaemia rhabdovirus(VHSV) DNA vaccines can depend on plasmid regulatory sequences [J]. Vaccine, 2009, 27(13): 1938—1948
[34]Alonso M, Leong J A. Licensed DNA Vaccines against Infectious Hematopoietic Necrosis Virus (IHNV) [J]. Recent Patents on DNA & Gene Sequences, 2013, 7(1): 62—65
10.7541/2016.39
一例虹鳟低死亡率疾病的病原学及病理学研究
樊 威1段亚佼1黄小丽1邓永强2杜宗君1耿 毅3陈德芳1汪开毓3
(1. 四川农业大学水产系, 成都 611130; 2. 四川省动物疫病预防控制中心, 成都 611130; 3. 四川农业大学动物医学院, 动物疫病与人类健康四川省重点实验室, 成都 611130)
为确定病原类型和造成虹鳟低死亡率的原因, 研究对患病虹鳟进行了病理学观察、病毒的分离和鉴定以及动物感染实验。临床检查发现发病虹鳟体色变黑, 肌肉和腹壁点状出血。病理学观察发现虹鳟造血器官脾和肾间组织严重坏死。通过反转录PCR法检测坏死组织和病变细胞中传染性造血器官坏死病毒、出血性败血病毒和传染性胰腺坏死病毒, 并对得到的371 bp大小片段进行测序和构建进化树分析, 发现感染病原为传染性造血器官坏死病毒。同时, 给体重为1.5 kg健康虹鳟腹腔注射104TCID50的组织滤液, 累计死亡率达到35%。除此之外, 将组织滤液接种到鲤鱼上皮瘤细胞系后出现了特征性病变。在实验过程中未发现细菌或寄生虫感染。结果证实引起虹鳟低死亡率的病原为传染性造血器官坏死病毒。
虹鳟; 成鱼; 传染性造血器官坏死病毒; 诊断; 低死亡率; 病理变化
S941.41 Document code: A Article ID: 1000-3207(2016)02-0287-07
date: 2015-09-15; Accepted date: 2015-12-07
Supported by the Sichuan Science and Technology Program (2014NZ0003)
Huang Xiao-Li (1979—), E-mail: hxldyq@126.com

