Consequences of early adverse rearing experience(EARE) on development: insights from non-human primate studies
Bo Zhang
1Yunnan Key Laboratory of Primate Biomedical Research, Kunming Yunnan 650500, China
2Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming Yunnan 650500, China
3National Institute of Health, Bethesda, Maryland, USA
Consequences of early adverse rearing experience(EARE) on development: insights from non-human primate studies
Bo Zhang1,2,3,*
1Yunnan Key Laboratory of Primate Biomedical Research, Kunming Yunnan 650500, China
2Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming Yunnan 650500, China
3National Institute of Health, Bethesda, Maryland, USA
Early rearing experiences are important in one’s whole life, whereas early adverse rearing experience (EARE) is usually related to various physical and mental disorders in later life. Although there were many studies on human and animals, regarding the effect of EARE on brain development, neuroendocrine systems, as well as the consequential mental disorders and behavioral abnormalities, the underlying mechanisms remain unclear. Due to the close genetic relationship and similarity in social organizations with humans, non-human primate (NHP) studies were performed for over 60 years. Various EARE models were developed to disrupt the early normal interactions between infants and mothers or peers. Those studies provided important insights of EARE induced effects on the physiological and behavioral systems of NHPs across life span, such as social behaviors (including disturbance behavior, social deficiency, sexual behavior, etc), learning and memory ability, brain structural and functional developments (including influences on neurons and glia cells, neuroendocrine systems, e.g., hypothalamic-pituitary-adrenal (HPA) axis, etc). In this review, the effects of EARE and the underlying epigenetic mechanisms were comprehensively summarized and the possibility of rehabilitation was discussed.
Early adverse rearing experience; Nonhuman primates
INTRODUCTION
One of factors affecting life-long health of humans is the stability of early childhood, especially children’s relationship with their mothers. John Bowlby's attachment theory suggests that individual's social relationship throughout life is influenced by the initial attachment with the mother (Bowlby, 1969). Attachment theory is a psychological, evolutionary and ethological theory concerning relationships among humans. Within the theory, attachment means an affectional bond or tie between an individual and an attachment figure (usually a caregiver). The core is that a child needs to build relationship with at least one primary caregiver to develop normal social and emotional behaviors. In many orphans, the lack of normal attachment to parents would cause behavioral and physical problems in childhood and possibly continuing throughout adult life (McEwen, 2003). Adults with adverse experience were more vulnerable to physical, psychosocial and mental disorders (Maughan & McCarthy, 1997;1Pirkola et al., 2005). In human, early adverse rearing experience (EARE) usually refers to child abuse, which is a worldwide problem and is defined as neglect or physical, sexual or emotional mistreatment or abuse of children (Newton & Vandeven, 2009, 2010). Although human based studies revealed compelling associations between EARE and psychological outcomes, both retrospective and prospective studies showed their limits, e.g., inaccurate selfreport due to biased or even false memory, failure in controlling accompanying environmental and genetic factors. Therefore, the long-term effects of EARE on subjects were usually not the direct consequences, but were inevitably intervened or masked by uncontrollable factors. However, experimental animals can be raised in laboratory environments, therefore allow researchers to carry out randomized prospective longitudinal studies, e.g., rigorously control or systematically manipulate early experiences throughout the entire period of investigation.
Rodents are easy to manipulate genetically, and the related studies indicate EARE as a developmental risk factor with profound, long-term effects on later life (Meaney, 2001; Pryce et al., 2005b; Sánchez et al., 2001). Whereas the high similarities of NHPs with humans make it irreplaceable in investigating the effects of EARE on physiological and behavioral development, e.g., NHPs and chimpanzees in specific, share over 90% and 98.8% genomes with human beings, respectively (Lovejoy, 1981). High similarities were found in both biological (Azmitia & Gannon, 1986; Uylings & van Eden, 1991) and socioecological aspects, e.g., social organizations and clear dominance hierarchies (Bailey & Aunger, 1990; DeVore, 1990; Wright, 1990). The phenomenon that in NHPs, 2%-10% of infants were physically abused or neglected by their mothers in group-living conditions, allow the possible screening of natural child abuse models (Maestripieri & Carroll, 1998; Maestripieri et al., 1997). Moreover, like humans NHPs has prolonged postnatal period of maturation during which mother–infant relationship and neural system development can be influenced by environment and early life experience (Levine & Wiener, 1988; Suomi, 2005).
Harlow (Harlow & Harlow, 1965) introduced the concept of affectional systems to characterize the relationships in the social groups of primates, and five distinct affectional systems were described, including the infant-mother affectional system, the maternal affectional system, the age-mate/peer affectional system, the heterosexual affectional system and the paternal affectional system. The infant-mother and the maternal affectional systems in Harlow’s affectional systems are similar to Bowlby’s concept of mother-infant attachment theory in humans. In normal living group, most monkey infants virtually spend all of their initial days or weeks of life clinging with their biological mothers, ventral to ventral, during which, specific and strong attachment bonds are built. When about 2-month old, infants begin to explore the physical and social environment, spending increasing amount of time participating social interactions, especially playing with peers. From 6-month of age until puberty, playing with peers becomes the major social activity (Hinde & Spencer-Booth, 1967; Suomi, 1997, 2005). In fact, the infant and juvenile monkeys always maintain a close social relationship with their mothers, while the mother plays the role of protector especially under stressful situations, and mentor in teaching developing appropriate social behaviors. Accordingly, the studies regarding EARE usually involve disruption of the normal infant-mother relationships, by maternal deprivation of newborns, maternal separation or induced stress on older infants and juvenile monkeys. Although some epidemiological studies in humans suggest possible direct relationships between EARE and abnormal behaviors in later life, no solid evidence was raised to prove the precise impact of childhood adversities on psychiatric disorders (Benjet, 2010; Bick & Nelson, 2016; Gershon et al., 2013; Kessler et al., 1997; Kessler & Wang, 2008; Klein et al., 2013; Sheridan et al., 2010). The over 60 years NHP studies shed lights on the understanding of the influences of EARE on physiological and behavioral development, including social behaviors (e.g., disturbance behavior, social deficiency, sexual behavior, etc), learning and memory ability, brain structural and functional development (e.g., development of neurons and glia cells, neuroendocrine dysregulation, etc). In this review, the previous findings on EARE were systematically summarized, and the underlying epigenetic mechanisms and the potential methods of rehabilitation were thoroughly discussed.
EARE MODELS IN NON-HUMAN PRIMATES
Controlled rearing conditions in standard laboratory settings are designed to simulate natural environments. The infants are reared by their mothers and live in a group consisted of other infants, juveniles and adults, allowing infants to be exposed to complex social interactions. In abnormal rearing conditions, the mother deprivation method is applied. The newborn is taken away from their mothers at birth and is reared in incubators with regular medical attention and laboratory nursery. A period of time (usually 1-month) later, when able to feed themselves, infants are moved to other rearing conditions depending on aims of research, e.g., be reared alone in social isolation condition, with nursery/peers of the same age in nursery/peer rearing condition, with a surrogate in surrogate mother/foster rearing condition, etc. They could also be separated from mothers at later time for once (temporary maternal separations) or several times (repetitive maternal separations); or even though staying with their mother all the time, but still suffer from EARE (maternal neglect).
Social isolation
Social isolation (including total and partial social isolation) is initially described in early 1960s by Harlow and his colleagues, and has been used ever since to raise monkeys in simulating social behavior deficits in humans (Table 1). In total isolation, the infant is reared in a cage alone without any auditory, visual, olfactory and tactile contact with conspecifics, including mothers, peers and other monkeys (Baysinger et al., 1972; Harlow & Harlow, 1962; Harlow et al., 1964, 1965). In partial social isolation, although infants are separately caged from their mothers, peers, and social groups, they have auditory, visual, and olfactory but not tactile contact with their conspecifics (Cross & Harlow, 1965; Mason & Sponholz, 1963; Struble & Riesen, 1978; Suomi et al., 1971). These early studies by Harlow and his colleagues, especially their extreme manipulations, including total isolation, "pit of despair" and "rape rack" devices, were controversial and were most likely forbidden to perform due to ethical issues. In 1950s, many researchers assumed that the only necessity of mother was supplying food to infants, whereas excessive intimacy between mother and infant would hinder the growth of infant, or even induce over dependence in adulthood. Harlow disagreed with the viewpoints; performed a series of isolation studies on primates to prove that to acquire necessary social skills, to obtain both physically and psychologically healthy development, infants need mothers’affection, as well as normal social interaction and emotional relationship with peers. However, their intention was to prove the essential role of mother's love to infants, in theform of her availability all the time, her physical touching, caring and protection, which was an obvious fact to us today without any necessity to prove.
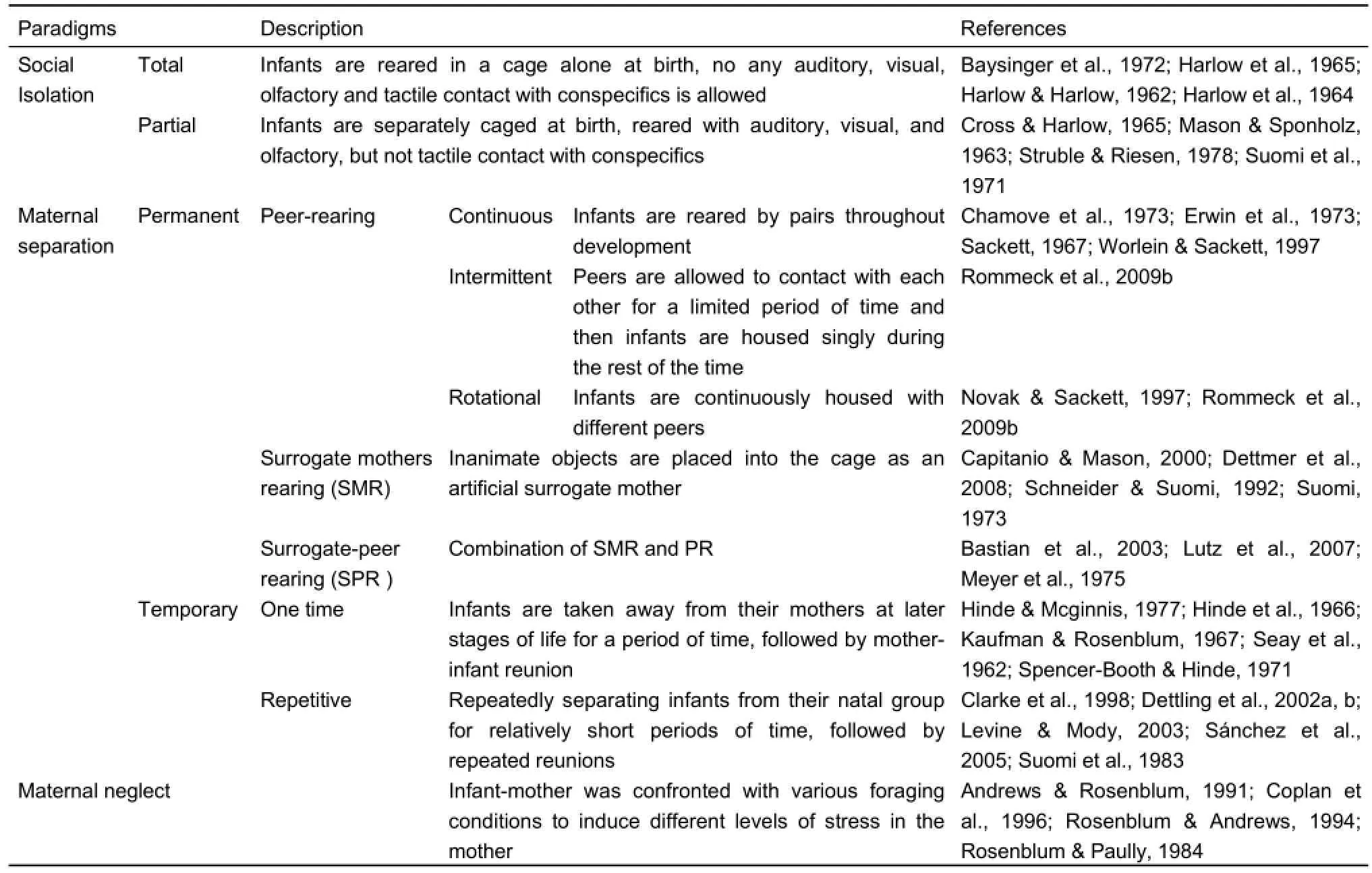
Table 1 Early adverse rearing experience (EARE) methods
However, although isolation models are important in highlighting the devastating consequences of maternal deprivation, the extreme manipulations could induce severe cognitive and emotional deficits, or even self-injurious behaviors, which are very difficult to remediate in primates. Therefore, less severe rearing conditions were developed afterwards at least partially due to ethical considerations.
Maternal separations
Peer-rearing (PR) (or nursery rearing, NR) (including continuous pair rearing, intermittent and rotational peer rearing) is another widely used rearing condition, in which infants were reared together with peers of the same age (Chamove et al., 1973; Erwin et al., 1973; Sackett, 1967; Worlein & Sackett, 1997) (Table 1). In continuous pair rearing condition, infants are usually reared by pairs throughout development (Chamove et al., 1973; Fekete et al., 2000; Hotchkiss & Paule, 2003; Novak & Sackett, 1997). Intermittent peer rearing allow peers to contact with each other for a limited period of time, and then infants are housed singly during the rest of the time (Rommeck et al., 2009b). Within the rotational peer rearing condition, infants are continuously peer housed with different infant partners (Novak & Sackett, 1997; Rommeck et al., 2009b). Previous study showed that continuous rotational pairing induces a behavioral profile quite similar with that of mother rearing in socially complex environment (Rommeck et al., 2011). Compared with social isolation, PR is less severe and thus more widely used in recent NHP EARE studies. Surrogate mothers rearing (SMR) is another early rearing method, in which inanimate object is placed into the cage as an artificial surrogate mother (Capitanio & Mason, 2000; Dettmer et al., 2008; Eastman & Mason, 1975; Harlow, 1958; Harlow & Zimmermann, 1959; Hennessy & Kaplan, 1982; Kaplan, 1974; Mason & Berkson, 1975; Roy et al., 1978; Schneider & Suomi, 1992; Suomi, 1973). Infants could quickly develop attachment with surrogate mothers, and some studies indicated that the infants usually preferred cloth surrogate mothers than wired ones (Harlow, 1958; Harlow & Zimmermann, 1959). Previous study reported that surrogate mothers could affect the behaviors of infants, and different characters of surrogate mothers such as mobility and orientation had different influences (Dettmer et al., 2008). Surrogate-peer rearing (SPR) method is a combination of SMR and PR, in which the infants are reared with inanimate surrogate mothers (SMR condition) during the initial several months of life, and then are allowed to have peer interactions for a limited period of time (PR condition) (Bastian et al., 2003; Lutz et al., 2007; Meyer et al., 1975). Comparing with permanent removal of the mother, infants are not separated from their mothers right away at born intemporary maternal separations, but after a period of time usually several hours, days or weeks, following by mother-infant reunion (Hinde et al., 1966; Hinde & McGinnis, 1977; Kaufman & Rosenblum, 1967; Seay et al., 1962; Spencer-Booth & Hinde, 1971). Temporary maternal separation usually contains a onetime separation although different time delay could be adopted. A modified version of one-time separation is repetitive motherinfant separation, in which infants are separated from and reunited with their natal group repeatedly for relatively short periods of time (Clarke et al., 1998; Dettling et al., 2002b; Levine & Mody, 2003; Sánchez et al., 2005; Suomi et al., 1983). The impact of these procedures appeared to be further intensified if the separations were unpredictable (Levine, 2000; Sánchez et al., 2005). Unlike social isolation, maternal separation adopted relatively mild manipulations, the presence of surrogate mothers and the opportunity of direct contact with mothers and peers added social complexity to the infants’ living environment, therefore could avoid severe social and emotional deficits associated with mothers’ absence.
Maternal neglect
Compared with isolation and maternal separation methods described above, maternal abuse and neglect during early life are more common in humans, therefore are more widely used on NHPs to study adult mood and anxiety disorders. In NHP maternal neglect models, in order to induce stress in the mother, infant mothers are confronted with various foraging conditions, such as variable/unpredictable foraging demand (VFD), consistently low foraging demand (LFD) and consistently high (but predictable) foraging demand (HFD). Mothers in LFD condition have easy access to food while those in high foraging demand have to work hard to get food (Andrews & Rosenblum, 1991; Coplan et al., 1996; Rosenblum & Andrews, 1994; Rosenblum & Paully, 1984). The advantage of this model is that even though infants are still in adverse situation, the severe adverse experience of mother and peer deprivation can be avoided. In addition, other rearing strategies are applied in this model, i.e., infants were reared by a female which was not their biological mother (Maestripieri, 2005; Novak & Suomi, 1991); infants were housed with non-reproductive female adults (Champoux et al., 1989b).
EARE EFFECTS
Although partial social isolation tends to induce less severe defects than total social isolation, the expression of behavior defects is similar. Isolated monkeys reared without exposure to companions during early life, especially the first 6 months, develop a pervasive pattern of abnormalities referred to as the isolation syndrome. Mason (Mason, 1968) summarized the syndrome under four headings: (1) abnormal posturing and movements, such as rocking; (2) motivational disturbances, such as excessive fearfulness or arousal; (3) poor integration of motor patterns, such as inadequate sexual behavior; (4) deficiencies in social communication, such as failure to withdraw after being threatened by an aggressing animal. In this section, the effects of EARE on social behaviors, learning and memory ability, brain structural and functional developments, including influences on neurons and glia cells, neuroendocrine dysregulation, especially stress related HPA axis will be reviewed.
Social behavior
Effects of EARE on social behaviors are detailed in Table 2.
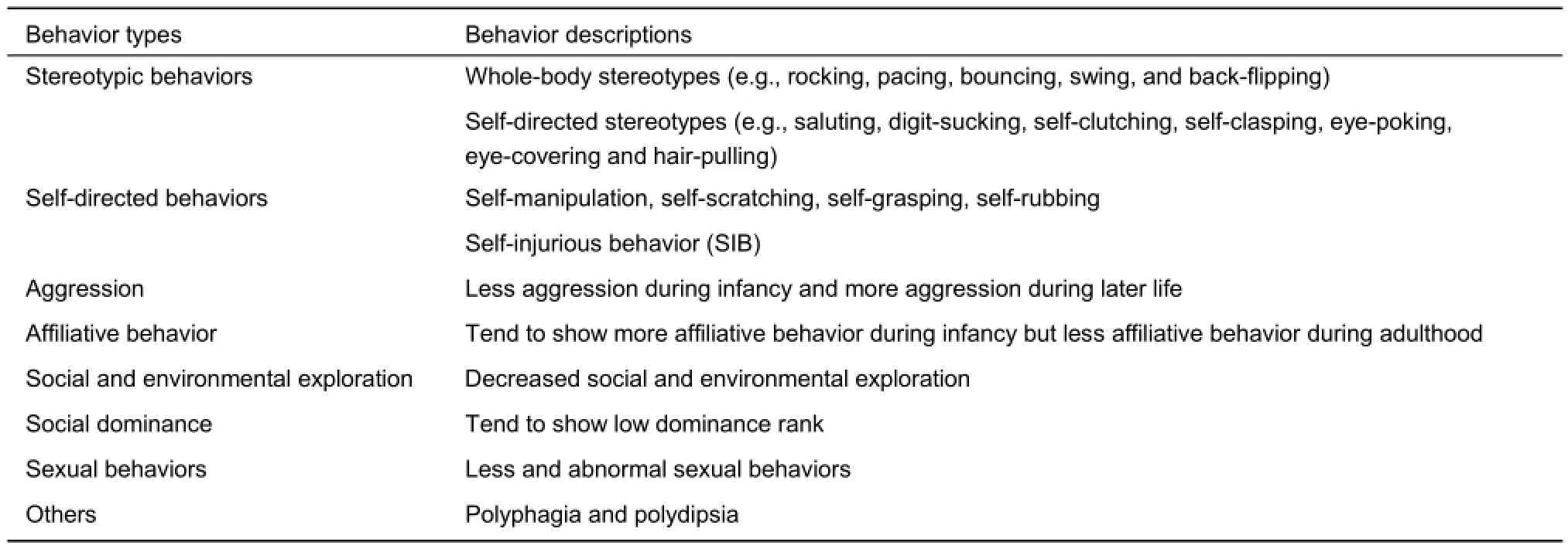
Table 2 Effects of EARE on social behaviors
Disturbance behavior
Monkeys exposed to adverse early experience tended to show more disturbance behaviors, such as stereotypic and selfdirected behaviors, motivational disturbances and social deficiency. The isolated monkeys appeared to show more disturbance behaviors, including crouching, clutching, rocking, pacing, flipping, hugging, clasping, thumb-sucking (Harlow & Harlow, 1962; Harlow & Suomi, 1971a; Mason & Sponholz, 1963; Mitchell, 1968; Suomi et al., 1971). Among these monkeys, some abnormal movements, such as rocking and self-grasping, could present very early in their lives, even at the first month (Baysinger et al., 1972). Additionally, some of these behaviors could turn into stereotypic behaviors, including repetitive movements or postures, as well as ritualized movements, and could be divided into whole-body stereotypes(e.g., rocking, pacing, bouncing, swing, and back-flipping), selfdirected stereotypes (e.g., saluting, digit-sucking, self-clutching, self-clasping, eye-poking, eye-covering and hair-pulling) and other idiosyncratic behaviors (e.g., teeth grinding, head tossing, or making noise by blowing air into the cheeks). It was reported that whole-body stereotypes were much more common than self-directed stereotypes (Lutz et al., 2003). Previous studies indicated that isolated monkeys showed more repetitive whole-body stereotypes (Mitchell, 1968), while PR monkeys showed more selfdirected stereotypes (Lutz et al., 2003; Suomi et al., 1971).
EARE exposed monkeys tended to show more self-directed behaviors. Isolated monkeys showed self-manipulation, selfscratching, self-grasping, self-rubbing, and autoeroticism while in isolation (Baysinger et al., 1972), or showed remarkable increases in self-clasping soon after removal from isolation (Harlow et al., 1965; Suomi et al., 1974), or self-clutching after surrogate mother removing (Harlow & Zimmermann, 1959). PR reared infants and juvenile monkeys showed increased selfstimulation behaviors, including self-sucking, self-clinging, selfclasping and other self-directed behaviors (Champoux et al., 1991; Lutz et al., 2003; Suomi et al., 1971). Moreover, shortterm stress by temporary physical restrictions could also induce significant increases in self-clasping and huddling behaviors when the infants returned to their home cages (Harlow & Suomi, 1971a). Those self-directed behaviors often turned into selfinjurious behavior (SIB), with males showing a much higher level of vulnerability than females (Cross & Harlow, 1965; Gluck & Sackett, 1974; Lutz et al., 2003; Suomi et al., 1971), and PR monkeys usually much more vulnerable than MR monkeys (Rommeck et al., 2009a). Surrogate mothers appeared to provide a certain degree of contact acceptability, security and trust sufficient for isolated monkeys to suppress existing self-directed disturbance activity, and to initiate crude social interactions with other isolated monkeys (Harlow & Suomi, 1971b). However, Lutz et al. (Lutz et al., 2007) reported that SPR monkeys showed significantly more self-biting comparing to PR and MR reared animals, and it was suggested that surrogate rearing in combination with lower levels of social contact during play may be risk factors for the later development of self-biting behavior. Actually, self-directed behaviors were hypothesized to result from the redirection of normal social behaviors toward one's own body and were suggested to be symptoms of some mental diseases (Goosen, 1981; Mason & Berkson, 1975). These findings indicate that EARE exposed monkeys could be used as an ideal model of related human mental disorders from behavioral perspective.
Social deficiency
In natural environments, infants and juvenile monkeys are supposed to be more active in joining the social play with peers, but monkeys exposed to EARE show decreased social playing. Isolated monkeys showed less (Harlow et al., 1965; Mitchell, 1968), or even no contact playing at all (Harlow et al., 1965). Pair and peer reared infants (Chamove et al., 1973), VFD reared infants (Andrews & Rosenblum, 1991; Rosenblum & Paully, 1984), repeated parental deprivation infants (Dettling et al., 2002b; Levine & Mody, 2003) all showed less social playing compared with MR infants. Lack of sufficient social interaction led to the fact that EARE exposed monkeys could not successfully adapt to living in a large social group (Griffin & Harlow, 1966; Harlow & Harlow, 1962; Mason & Sponholz, 1963; Ruppenthal et al., 1991). Not only social interaction, studies also showed decreased environmental exploration in isolated monkeys (Griffin & Harlow, 1966; Mason & Sponholz, 1963; Mitchell, 1968), VFD and PR monkeys (Rosenblum & Paully, 1984; Ruppenthal et al., 1991). Another major index of exploratory behavior is locomotor activity, while some NHP studies showed less locomotion in isolated adults (Harlow & Suomi, 1971a; Mason & Sponholz, 1963; Mitchell, 1968) and PR infants (Feng et al., 2011), others found no differences in PR adults (Winslow et al., 2003), or even higher activity levels in PR infants during the first month after isolation (Champoux et al., 1991). Therefore, there was no agreed tendency of EARE influence on locomotor activity in monkeys, making it an invalid measure of exploratory behavior if used alone (Wright, 1983).
Another domain of EARE induced social deficiency is social dominance. In monkey society, social dominance is a complex phenomenon mediated by different mechanisms and various factors such as kinship, age, sex, and physical factors like body weight, appearance and health (Bernstein & Cooper, 1999; Bernstein & Mason, 1963; Morgan et al., 2000; Sprague, 1998; Takahashi, 2002). Kinship seemed to be the major factor in determining dominant rank at least until puberty (Koford, 1963; Koyama, 1967), but became weaker during the development (Bernstein & Williams, 1983). Both dominance formation and maintenance among males in a living group are usually achieved by aggressive behavior such as fighting, with the stronger and more aggressive subjects winning and thus becoming dominant. However, appropriate use of aggression is critical for both acquiring and maintaining social status, as overly aggressive monkeys may risk social ostracism from their conspecifics. Moreover, aggressive behavior was not indispensable to obtain and keep dominance status and dominance sustained without aggression was more stable than that formed on the basis of aggression (Fonberg, 1988).
Monkeys exposed to EARE tended to show less aggression during infancy (Chamove et al., 1973; Harlow et al., 1965), and more aggression during later life (Chamove et al., 1973; Mitchell, 1968; Suomi et al., 1974; Winslow et al., 2003). The aggressive monkeys exposed to EARE may repeatedly attack a helpless infant or attempt to attack a dominant male, while infant-directed aggression is abnormal adult-directed aggression is both abnormal and suicidal (Chamove et al., 1973; Mitchell, 1968; Suomi et al., 1974; Winslow et al., 2003). On the other hand, studies showed EARE exposed monkeys showed heightened fear in all age stages (Champoux et al., 1991; Dettling et al., 2002b; Levine & Mody, 2003; Mitchell, 1968). It seems that EARE makes monkeys more emotional in two opposite directions, both aggression and fear. In addition to aggression, affiliative behavior, such as grooming and proximity, is also important in establishing and maintaining alliances and reinforcing the dominance hierarchy. Affiliative behavior was suggested to be more positively related to dominance rank than kinship in Japanese monkeys (Singh et al., 1992). On thecontrary to aggression, EARE exposed monkeys showed more affiliative behavior during infancy (Chamove et al., 1973; Rosenblum & Paully, 1984; Ruppenthal et al., 1991), but less affiliative behavior during adulthood (Kraemer & McKinney, 1979; Rosenblum & Paully, 1984; Winslow et al., 2003). With more aggressive and less affiliative behavior which both contribute to acquiring and reinforcing social dominance, EARE exposed adult monkeys are supposed to have low social dominant rank in a living group, and studies indeed indicated that both isolated and PR adult monkeys showed low social dominance (Kraemer & McKinney, 1979; Mitchell, 1968; Ruppenthal et al., 1991).
Sexual behavior
Monkeys exposed to EARE demonstrated less or abnormal sexual behaviors (Chamove et al., 1973; Harlow et al., 1966; Harlow, 1962; Harlow et al., 1965; Mitchell, 1968). Abnormal sexual behaviors (abortive mount) is defined as any improperly oriented mount, accompanied by pelvic thrusting including standing-to-head, standing-to-side and ventral lie-on (Wallen et al., 1981). Males usually were not mount properly as they engaged in varied but misplaced heterosexual efforts, while females were not maintain the sexual present (stood quadripedally with the perineal area directed towards the recipient) or turned their bodies when mounted. Mount behavior includes no-foot-clasp mount and foot-clasp mount, which could be differentially affect by different EARE. Males with short access periods with peers (0.5 h) rarely or never foot-claspmounted peers, while those given 24 h access regularly footclasp-mounted peers (Wallen et al., 1981). Isosexually reared males showed less foot-clasp mounting and more presenting than heterosexual males, while conversely, isosexually reared females showed statistically more mounting and less presenting than heterosexual females (Goldfoot et al., 1984). Moreover, females exposed to EARE also showed abnormal maternal behaviors, in a way that those never experienced mother caring not only were unable to exhibit caring to their own offspring, but also far more likely to display inadequate, abusive or neglectful behavior toward their offspring (Bridges et al., 2008; Champoux et al., 1992; Harlow & Suomi, 1971b; Seay et al., 1964; Suomi, 1978; Suomi et al., 1974; Suomi & Ripp, 1983), consistent with human findings showing abusive behavior appeared to be transmitted across generations (Roustit et al., 2009).
Primate studies also showed other EARE induced behavioral effects besides listed above, including polyphagia and polydipsia in isolated adults (Miller et al., 1969), more vulnerable to excessive alcohol consumption (Fahlke et al., 2000; Higley et al., 1991) and elevated response to both aversive and rewarding stimuli (Nelson et al., 2009) in PR monkeys and abnormal sleep rhythmicity (Barrett et al., 2009; Boccia et al., 1989; Kaemingk & Reite, 1987; Reite et al., 1974; Reite & Short, 1978). An interesting research showed EARE significantly influenced the development of lateralisation, as PR monkeys demonstrated greater left-hand bias compared to MR reared monkeys (Bennett et al., 2008). Despite of EARE effects described above, recent research suggested that modern PR practices might not result in inevitable perturbations in aggressive, rank-related, sexual, and emotional behavior in rhesus monkeys (Bauer & Baker, 2016).
Learning and memory
Early primate studies showed EARE exposed adults performed adequately on simple discriminations or delayed-response (Gluck et al., 1973), but showed impairments in certain complex tasks such as those requiring engaging working memory with dynamic rules or delays or response inhibition (Beauchamp & Gluck, 1988; Beauchamp et al., 1991; Gluck et al., 1973; Gluck & Sackett, 1976; Sánchez et al., 1998). These results were obtained mostly from adult monkeys separated from their mothers at birth and reared in total isolation for 9-12 months. PR reared juvenile monkeys also showed cognitive deficits, they had more difficulty acquiring the delayed non-matching to sample (DNMS) task and were also impaired in object but not spatial reversal learning (Sánchez et al., 1998). Moreover, even brief social isolation impaired performance in a multiple videotask assessment in adult rhesus monkeys (Washburn & Rumbaugh, 1991) and impaired reversal learning and behavioral inhibition in adult marmosets (Pryce et al., 2004a, b). These results were consistent with the results of human studies, which showed the post institutionalized children (Bauer et al., 2009) and childhood exposed to neglect and abuse (Majer et al., 2010) were associated with impaired learning and memory during adulthood. Although those studies revealed EARE induced impairment of learning and memory ability in a task dependent way in adult monkeys, other primate studies indicate exposure to mild early life stress improves prefrontal dependent response inhibition in primates, suggesting its beneficial effect on cognitive control (Parker et al., 2005, 2012).
Brain structure and function
The first documentation of the effects of negative early experiences on monkey brain was provided by Martin et al. (1991), which showed significant alterations in the chemo architecture of the striatum 19-24 years after social deprivation. Additionally, Siegel et al. (1993) demonstrated that early social deprivation resulted in an increase in the amount of nonphosphorylated neurofilament protein in hippocampal dentate gyrus granule cells in rhesus monkeys. Further studies showed structure and function changes in many brain regions including amygdala, hippocampus, prefrontal cortex (PFC), anterior cingulate cortex (ACC), corpus callosum and cerebellum etc, both in humans and animals exposed to EARE (Andersen, 2015; Bick & Nelson, 2016; Gilmer & McKinney, 2003; Gorman et al., 2002; Hart & Rubia, 2012; Korosi et al., 2012; McEwen, 2003; Worlein, 2014)(Table 3).
Amygdala
Amygdala is a group of almond-shaped nuclei located deep within the medial temporal lobes of the brain in complex vertebrates. It was considered as the emotion center and responsible for emotion reactions like reward, fear and anxiety (Davis, 1992; Gallagher & Chiba, 1996; Ledoux, 2003; Phelps, 2006). Rodent studies showed acceleration of amygdala development in early weaning rodents (Kikusui & Mori, 2009;Ono et al., 2008). The limited amount of primate studies found no significant amygdala volume changes (Howell et al., 2014), but functional changes including decreased SERT binding potential (Ichise et al., 2006) and differential expression of one gene GUCY1A3 (Sabatini et al., 2007) in amygdala of EARE exposed monkeys. However, human studies in maltreated children showed contrary results, with some studies found no volume changes (De Bellis et al., 2001; De Brito et al., 2013; Hanson et al., 2010; Woon & Hedges, 2008), while others revealed decreased volume (Edmiston et al., 2011; Hanson et al., 2015; Luby et al., 2013) or greater volume and elevated response (Lupien et al., 2011; Mehta et al., 2009; Tottenham et al., 2010). Furthermore, those studies found greater volume and elevated response of amygdala (Mehta et al., 2009; Tottenham et al., 2010) were performed several years after the institutionalized children adopted by high socio-economic status families. These data suggested that EARE modified amygdala changes was resistant to recovery, and it was consistent with primate research that suggested abnormal behaviors was resistant to environmental enrichment treatments (Lutz et al., 2004; Lutz & Novak, 2005; Novak et al., 1998; Rommeck et al., 2009a). Similarly, in adults exposed to EARE some studies found no significant changes of amygdala volume (Bremner et al., 1997; Cohen et al., 2006), while others found larger volume (Evans et al., 2016; Lyons-Ruth et al., 2016), interrupted regulation of negative emotion (Kim et al., 2013), increased response to potential rewards (Casement et al., 2014), elevated amygdala responses to threat but not happy faces (Javanbakht et al., 2015). In addition to amygdala structure and activity changes, its connectivity with other brain regions was also affected (Barch et al., 2016; Jedd et al., 2015). Despite those controversial results, the influence of EARE on emotion such as the elevated response to emotion stimuli both in human and primates (Casement et al., 2014; Javanbakht et al., 2015; Nelson et al., 2009) should be mainly achieved through its influence on amygdala.
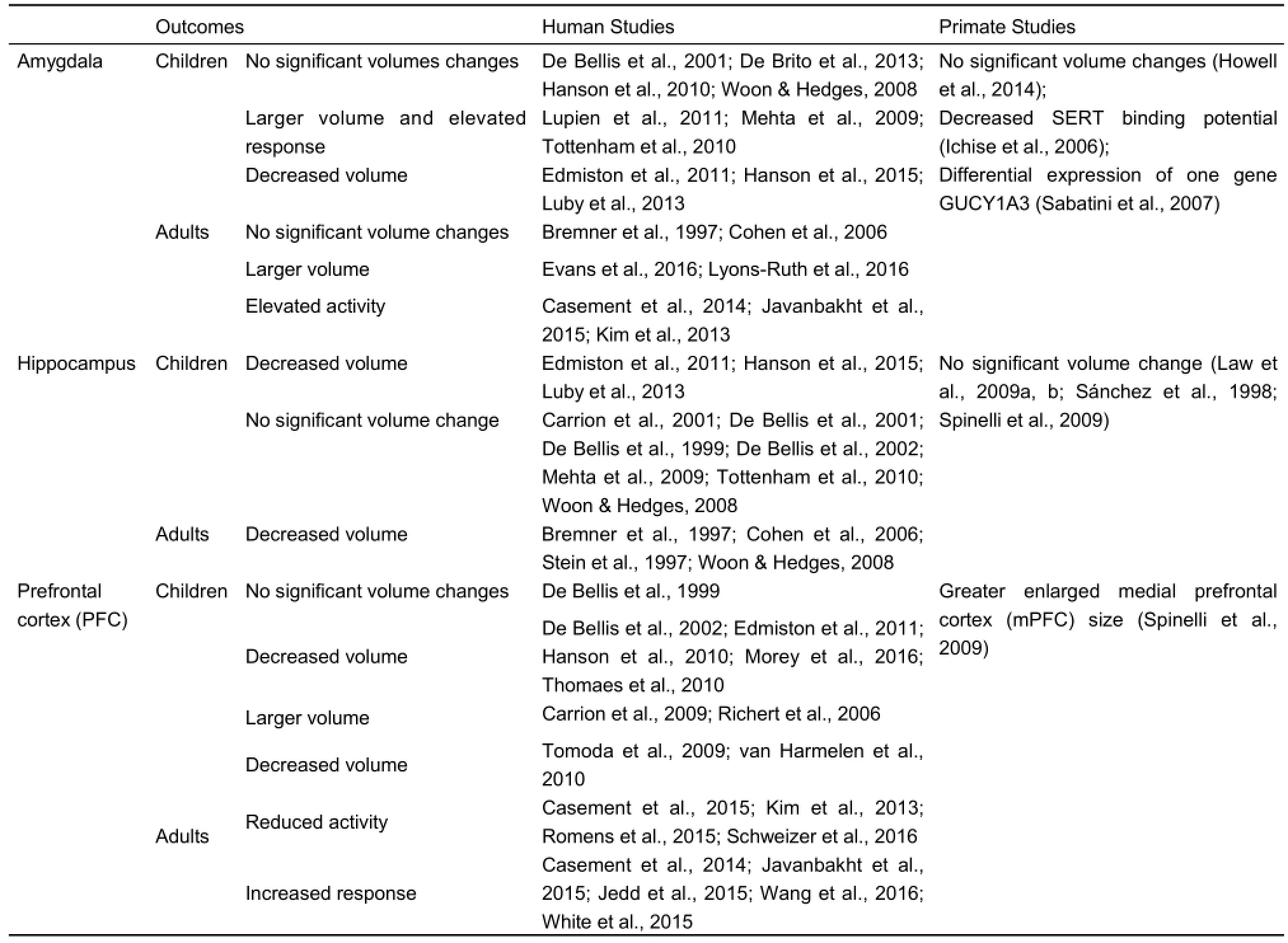
Table 3 Effects of EARE on brain structure and function
Hippocampus
Hippocampus, a major component of the brains located inside the medial temporal lobe and beneath the cortical surface, is involved in episodic, declarative, contextual, and spatial learning and memory, as well as being a component in the control of autonomic and vegetative functions (Buckley, 2005; Eichenbaum, 2001; Eichenbaum et al., 1992, 1996; Manns &Eichenbaum, 2006; Opitz, 2014; Shohamy & Turk-Browne, 2013). In human studies, EARE induced significant reduction of hippocampal volume was an consistent finding in adults (Bremner et al., 1997; Cohen et al., 2006; Hart & Rubia, 2012; McCrory et al., 2011; Stein et al., 1997; Woon & Hedges, 2008). However, children and adolescents studies showed inconsistent results, with few found decreased volume (Edmiston et al., 2011; Hanson et al., 2015; Luby et al., 2013), while most found no significant change (Carrion et al., 2001; De Bellis et al., 2001, 1999, 2002; Mehta et al., 2009; Tottenham et al., 2010; Woon & Hedges, 2008). Primate studies also found no significant hippocampal volume change in PR (Sánchez et al., 1998; Spinelli et al., 2009) and repeated mother deprived (Law et al., 2009b) juvenile monkeys, suggesting changes of hippocampus seemed to happen later in life compared to early life amygdala changes. Two possible explanations could account for the discrepancy of children and adult findings. Firstly, that might due to the fact that the hippocampus develops mainly in the first years of life, therefore less affected by exposure to adversity in childhood and adolescence (Houston et al., 2014; Lenroot & Giedd, 2006; Richards & Xie, 2015). Another possibility is that EARE might not have an immediate effect on the hippocampus but induced changes over time, and long-term effects of EARE exposure may be delayed and became manifest only in later phases of development when the vulnerable brain reaches maturation (Andersen & Teicher, 2004; Brunson et al., 2005; Gluckman & Hanson, 2004; Gluckman et al., 2007; Sapolsky et al., 1985). Moreover, human studies found interesting results concerned with influence of EARE exposure on structure and activity of hippocampus and amygdala, with decreased hippocampal volume and activity in humans exposed to adulthood stress (Bremner et al., 2007; Lupien et al., 2007; Rauch et al., 2000) and adults experiencing EARE (Bremner et al., 1997; Cohen et al., 2006; Stein et al., 1997; Woon & Hedges, 2008), while increased amygdala volume and activity in humans exposed to adulthood stress (Bremner et al., 2007; Lupien et al., 2007; Rauch et al., 2000) and adults experiencing EARE (Mehta et al., 2009; Tottenham et al., 2010). Although the biological mechanism and meaning of this phenomenon remains unclear, that might contribute to or even be the direct reason for the impaired learning and memory ability (decreased hippocampal volume and activity related) and elevated response to emotional stimuli (increased amygdala volume and activity related) described above.
Prefrontal cortex
The prefrontal cortex (PFC) is the anterior part of the frontal lobes of the brain and implicated in planning complex cognitive behaviors, personality expression, decision making and moderating correct social behavior. Children and adolescents studies showed inconsistent results of EARE induced PFC structural changes, with findings of either no significant differences (De Bellis et al., 1999), or significantly smaller volume (De Bellis et al., 2002; Edmiston et al., 2011; Hanson et al., 2010; Morey et al., 2016; Thomaes et al., 2010) or significantly larger volume (Carrion et al., 2009; Richert et al., 2006). In contrast, decreased PFC volume in adults exposed to childhood maltreatment was a consistent finding (Tomoda et al., 2009; van Harmelen et al., 2010). That might due to the fact that PFC continues to develop during adolescence (Houston et al., 2014; Lenroot & Giedd, 2006; Richards & Xie, 2015), therefore might be particularly vulnerable to the effects of stress during adolescence. In addition to the structural changes, EARE could also induce PFC functional changes, with some human adults exposed to EARE showing reduced prefrontal cortex activity during monetary reward anticipation and emotion regulation (Casement et al., 2015; Kim et al., 2013; Romens et al., 2015; Schweizer et al., 2016), while others showing increased response to potential rewards and threatening faces and in passive viewing conditions (Casement et al., 2014; Javanbakht et al., 2015; Jedd et al., 2015; Wang et al., 2016; White et al., 2015). One primate report indicated PR juvenile monkeys showed greater enlarged medial prefrontal cortex (mPFC) size (Spinelli et al., 2009). Moreover, both rodent and primate studies revealed the direct underlying epigenetic mechanisms of EARE on PFC through influencing differential gene expression, histone acetylation and DNA methylation (Blaze et al., 2015a; Provençal et al., 2012; Wall et al., 2012). Studies regarding EARE effects on PFC in primates are rare, and further investigations are necessary.
Other brain regions
The anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex, and appears to play a role in a wide variety of rational cognitive functions, such as reward anticipation, decision-making, empathy and emotion (Devinsky et al., 1995; Drevets et al., 2008). It can be divided anatomically into dorsal and ventral components, with dorsal part connected with PFC making its involvement in cognition possible, and the ventral part connected with amygdala making its involvement in emotion possible (Bush et al., 2000; Morecraft et al., 2007). Human studies showed reduced volume of adult ACC in people with mood disorders (Botteron et al., 2002; Drevets et al., 1997; Yamasue et al., 2003), adults exposed to early life stress (ELS) (Cohen et al., 2006) and abuse-related Posttraumatic stress disorder ( PTSD ) (Kitayama et al., 2006; Thomaes et al., 2010) and major depressive disorder (Treadway et al., 2009). On the contrary, a primate study found enlarged ACC in PR juvenile monkeys (Spinelli et al., 2009). Moreover, an epigenetic study showed parental separations in infant marmoset affected expression of genes in the ACC of adolescent monkeys (Law et al., 2009a). Additionally, both human and primate studies revealed EARE affected cerebellum, with human studies showing decreased cerebellum (Bauer et al., 2009; Edmiston et al., 2011), while a primate study revealing larger cerebellar vermis area in PR juvenile monkeys (Spinelli et al., 2009). EARE effect on primate cerebellum might due to the fact that macaque cerebellum has high density of glucocorticoid receptors (GRs) (Sánchez et al., 2000), which put it particularly vulnerable to stress hormones related over stimulation. Striatum was another brain region affected by EARE, with increased response to potential rewards (Casement et al., 2014) and elevated dopamine responses to amphetamine (Oswald et al.,2014), and a potential neurobiological mechanism linking earlylife adversity and altered ventral striatal development was indicated (Goff & Tottenham, 2015). In addition to those specific regional changes, PR chimpanzees showed less global whiteto-grey matter volume and cortical folding (Bogart et al., 2014). Structural connectivity between different brain regions was also affected by EARE, as studies showed affected corpus callosum, a wide and flat bundle of axons beneath the cortex connecting left and right cerebral hemispheres and facilitating interhemispheric communication, in a inconsistent way that most human studies showing EARE reduced corpus callosum (De Bellis et al., 1999; Rinne-Albers et al., 2016; Teicher et al., 2004, 1997), while few showing no significant changes (Mehta et al., 2009). Primate studies also found either decreased corpus callosum size (Sánchez et al., 1998) or no significant changes (Spinelli et al., 2009).
Neurons and glia cells
Neurons are the basic unit of brain. Neuronal network is responsible for the daily cognitive and emotional behaviors. Glia cell is a group of non-neuronal cells that support and protect the neurons in the brain. Rodent studies showed that maternal separation could induce morphological alteration of the apical dendrites of CA3 pyramidal neurons (Kwak et al., 2008); could increase corticotropin releasing factor (CRF)-containing neurons in amygdala (Becker et al., 2007); and could decrease in vivo firing activity of amygdala neurons (Adams & Rosenkranz, 2016) and sex related neurogenesis (Oomen et al., 2009). Chronic stress could induce atrophy of dendrites in hippocampus of rats (Brunson et al., 2005; McEwen, 1999) and tree shrews (Magariños et al., 1996), and could induce hippocampal neuroplasticity changes (Fenoglio et al., 2006). Bartesaghi and colleagues used guinea-pig as animal model to investigate the effects of early isolation on neurons, and they found that early isolation could induce morphologic changes of neurons in entorhinal cortex and hippocampus (Bartesaghi et al., 2003a, b; Bartesaghi & Serrai, 2001, 2004). Although primate studies found neuronal morphological changes in EARE exposed monkeys (Bryan & Riesen, 1989; Floeter & Greenough, 1979; Stell & Riesen, 1987; Struble & Riesen, 1978), these early findings were limited to cerebellum, somatosensory and motor cortex, with limited information on other important brain regions, such as hippocampus, amygdala and PFC. Recent studies showed that different environments could induce neuron plasticity changes in the key brain regions involved in learning and memory. Complex environment could enhance complexity of the dendritic tree and density of dendritic spine in hippocampus and PFC in monkeys (Kozorovitskiy et al., 2005). Early parental deprivation in the marmoset monkey could produce long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder (Law et al., 2009b). So these neuron morphological and plasticity changes might explain and account for how EARE take effects on cell level, and then further more leading to behavioral changes.
As EARE effects on glia cells, rodent studies revealed that EARE could induce long-term changes of astrocyte density and numbers in many brain regions, including PFC, mPFC, hippocampus, cingulate cortex and amygdala (Leventopoulos et al., 2007), and could alter behavioral, autonomic and endocrine responses to environmental challenge (Musholt et al., 2009; Rüedi-Bettschen et al., 2006). Although there was no direct evidence pointing out that glia cell changes were responsible for those altered responses in rats, those studies at least suggested the involvement of glia cell in EARE induced effects. Moreover, human studies showed that glial cell depletion in many brain regions was related to mood disorders, as the number of glia cell was reduced in PFC of both major depressive disorder (MDD) and bipolar disorder (BD) patients (Öngür et al., 1998), in the amygdala of major depressive disorder patients (Bowley et al., 2002) and in anterior cingulate cortex of major depressive disorder and schizophrenia patients (Cotter et al., 2001). Considering the important trophic influence of glia on neurons, glia cell deficits induced by EARE could possibly be responsible for EARE effects on neurons and furthermore to abnormal behavioral function. If that is true, how does it happen? Rodent Studies showed that stress related hormone glucocorticoid receptors (GRs) were also expressed in glia cells (Bohn et al., 1991; Jung-Testas & Baulieu, 1998; Vielkind et al., 1990). Glucocorticoid is the product of the HPA axis, so EARE might take effects through its influence on stress related hormones, like glucocorticoid, and then exert influence on glia cells leading to various effects (Jauregui-Huerta et al., 2010). Indeed, in vitro and in vivo studies showed that glucocorticoids could influence gene expression in glia cells (Bohn et al., 1994; Kumar et al., 1985) and could regulate the concentration of glial fibrillary acidic proteins (O'Callaghan et al., 1989). By playing central roles in learning and memory, hippocampal astrocyte number was dose-dependently increased by corticosterone treatment (Bridges et al., 2008), and glial responses in hippocampus was also regulated by glucocorticoid through influencing gene expression (Nichols et al., 2005). However, few studies were performed to investigate this issue and EARE affected glia cell changes were link directly to behavioral outcomes without solid evidence. In primate studies, there are lack of evidence to support that EARE affects glia cell structural and functional changes, and furthermore, induces behavioral outcomes.
Lateralisation
Some studies suggested that the influence of EARE on different brain hemisphere might be different, and different type of EARE might take effects differentially on the same brain structure. A human study found that the institutionalized children had greater right amygdala volume, while the left amygdala volume was smaller in the children experienced longer periods of deprivation (Mehta et al., 2009). Another human study showed that patients with child abuse-related complex PTSD showed reduced gray matter concentration in right hippocampus and right dorsal ACC, but not in the left areas (Thomaes et al., 2010). In primate studies, maternal separation was associated with activation in the right dorsolateral PFC and decreased activity in the left dorsolateral PFC of juvenile rhesus monkeys (Rilling et al., 2001). Not only brain structure and functionshowed lateralisation affection by early experiences, behavioral research also found lateralisation in primates, as an interesting research showed that PR monkeys demonstrated greater lefthand bias compared to MR reared monkeys (Bennett et al., 2008). The number of lateralisation related studies is limited and the underlying mechanism remains unknown, which certainly adds complexity to the understanding of the influence EARE on brain structure and functional changes and the related abnormal behavioral outcomes.
Other EARE effects
Young animals are particularly vulnerable to EARE effects
Adverse experience has its influence over all life stages, including early, middle and later life, in which infants are especially vulnerable to EARE and the consequences could persistent into later life. That might due to the fact that the most sensitive period of the whole life is the early stage, during which the body is undergoing profound physiological development, such as HPA axis, and brain is also undergoing profound neural development, such as neurogenesis. The amygdala developes rapidly during the early postnatal period in animals, e.g., in rats, cats and primates (Kikusui & Mori, 2009; Lupien et al., 2009; Payne et al., 2010; Wakefield & Levine, 1985). Stress related hormones and receptors were also maximally expressed in the brain early in development (Avishai-Eliner et al., 1996; Baram & Hatalski, 1998; Meaney & Szyf, 2005; Pryce et al., 2005a; Vazquez et al., 2006). These early physiological development heighten the vulnerability of the brain to environmental exposures. On the other hand, the proper development needs proper environmental stimuli, and the natural and best stimuli during early life is the attachment between caregivers, especially mothers, and infants, as mothers could supply tactile contact, physical warmth, nourishment, and psychological comforts. As stated in attachment theory and affectional system, infants need to develop a stable relationship with the mother for social and emotional development to occur normally, while various EARE intervene the forming of the bonds of this relation, therefore both short-term and long-term devastating influence are inevitable.
Sexual differences in EARE effects
Humans studies showed that affectability of various mental disorders were sex-related during development, with boys showing higher tendencies to develop aggression and novelty seeking behaviors (Farrington & Loeber, 2000) while girls more susceptible to anxiety and depression (Kessler, 2003). Additionally, EARE influence might also be sex related, e.g., corpus callosum volume reduction was only found in EARE exposed males (De Bellis et al., 1999). Similarly, animal studies also revealed the vulnerability of males to EARE in rodents (Galea et al., 1997; Kikusui & Mori, 2009) and primates (Clarke, 1993; Cross & Harlow, 1965; Mitchell, 1968; Rommeck et al., 2009a; Suomi et al., 1971). On the contrary, other studies showed preference of EARE on females in humans (Heim & Nemeroff, 2001; Klimes-Dougan et al., 2001), rodents (Hoyer et al., 2013; Ziabreva et al., 2003b) and primates (Sánchez et al., 2005). Previous studies showed that stress could induce decreasing in number and length of apical dendritic branch of medial prefrontal cortex in male rats, whereas increasing in apical dendritic length in female rats (Garrett & Wellman, 2009). Isolated males showed less dendritic branches, shorter dendritic length and smaller dendritic spine density than control males, while isolated females had more dendritic branches than control females in guinea pig (Bartesaghi et al., 2003a). Neurogenesis was significantly increased in male but decreased in female offspring after maternal deprivation in rats (Oomen et al., 2009). The mechanism of those sexual differences remains unclear, but one possible explanation is the gender related physiological differences, such as neuroendocrine system and brain structure and function, which may induce different behavioral and physiological responses in male and female subjects. .
Time effects of EARE
Early life is a time of heightened susceptibility to EARE and expression of adverse experiences induced effects would be different across life time, therefore the time of administration of adverse experiences and subjects age of measurement might partially explain the discrepant findings across studies (Tottenham & Sheridan, 2009).
The time of adverse experiences administration is important, as different brain regions might have unique windows of vulnerability to stress, e.g., human studies indicate that the time window of hippocampus, corpus callosum and frontal cortex is at ages of 3-5, 9-10 and 14-16 years, respectively (Andersen et al., 2008). Rodent studies revealed the critical importance of specific time windows early in life for the outcome of maternal separation (Bock et al., 2005; Gos et al., 2008; Pryce et al., 2005b). Early primate studies by Harlow et al. showed the importance of administration time of adverse experiences (isolation), in a way that isolation beginning at birth generated most severe effects and persisting abnormalities (Harlow et al., 1965; Mitchell, 1968), while the isolation starting until later in life would produce less severe effects and persistent abnormalities (Harlow et al., 1965; Mitchell, 1968). Moreover, different lasting period of EARE also produced different effects even was all initiated at birth, i.e., 3 months isolation only induced reversible debilitating behavioral deficits, while at least six months isolation generated most severe effects and persisting abnormalities; 3 months isolation induced least, 6 months isolation induced moderate and 12 months isolation induced most severe defects (Griffin & Harlow, 1966; Harlow et al., 1965; Mitchell, 1968). These studies suggested that both the time point of administration of EARE and the lasting period have different influences on behavioral and biological outcomes.
Human studies showed different, or even contrary effects of EARE in children and adults, suggesting EARE might induce differential outcomes across lifespan. For example, childhood abuse induced significant reduction of hippocampal volume in adults (Bremner et al., 1997; Cohen et al., 2006; Stein et al., 1997; Woon & Hedges, 2008) but not in children (Carrion et al., 2001; De Bellis et al., 2001, 1999; Woon & Hedges, 2008); EARE induced hypercortisolism in children (Essex et al., 2002;Fernald & Gunnar, 2009; Flinn & England, 1997; Kaufman et al., 1997) but hypocortisolism in adults (Carpenter et al., 2009; Elzinga et al., 2008); adults with abuse related PTSD showed ACC volume reductions (Kitayama et al., 2006; Thomaes et al., 2010), whereas pediatric PTSD showed increased ACC (Richert et al., 2006). Primate studies also found similar results, e.g., monkeys exposed to EARE showed less aggression during infancy (Chamove et al., 1973; Harlow et al., 1965) but more aggression during latter life (Chamove et al., 1973; Mitchell, 1968), whereas showed more affiliative behavior during infancy (Chamove et al., 1973; Rosenblum & Paully, 1984; Ruppenthal et al., 1991) but less during adulthood (Kraemer & Mckinney, 1979; Rosenblum & Paully, 1984; Winslow et al., 2003). Monkeys exposed to EARE showed more activity during infants (Champoux et al., 1991) but less activity during adulthood (Harlow & Suomi, 1971a; Mason & Sponholz, 1963; Mitchell, 1968); The number and style of stereotypies exhibited in monkeys also varied by age, e.g., the number of whole-body stereotypies were negatively correlated with age, whereas self-directed stereotypies were positively correlated; moreover younger monkeys exhibited more pacing, body-flipping, and swinging, while older ones exhibited more hair-pulling and saluting (Lutz et al., 2003). These studies showed different, or even opposite effects of EARE on behavioral and biological outcomes between infants and adults, indicating EARE induce different outcomes across lifespan.
MECHANISMS UNDERLYING EARE INDUCED EFFECTS
Neuroendocrinological mechanisms
Some recent study linked behavioral outcomes with EARE affected neuroendocrine systems, and suggested that EARE might modulate subsequent social behaviors through regulating both the production and body’s sensitivity to neurotransmitters and hormones (Cushing & Kramer, 2005). Moreover, studies indicate that the involved neurotransmitters and hormones were mainly monoamine neurotransmitter serotonergic systems, including serotonergic system and catecholamine system (both noradrenergic system and dopaminergic system), and glucocorticoid hormones (cortisol in non-human primates and humans), oxytocin and growth hormone (GH)(Table 4).
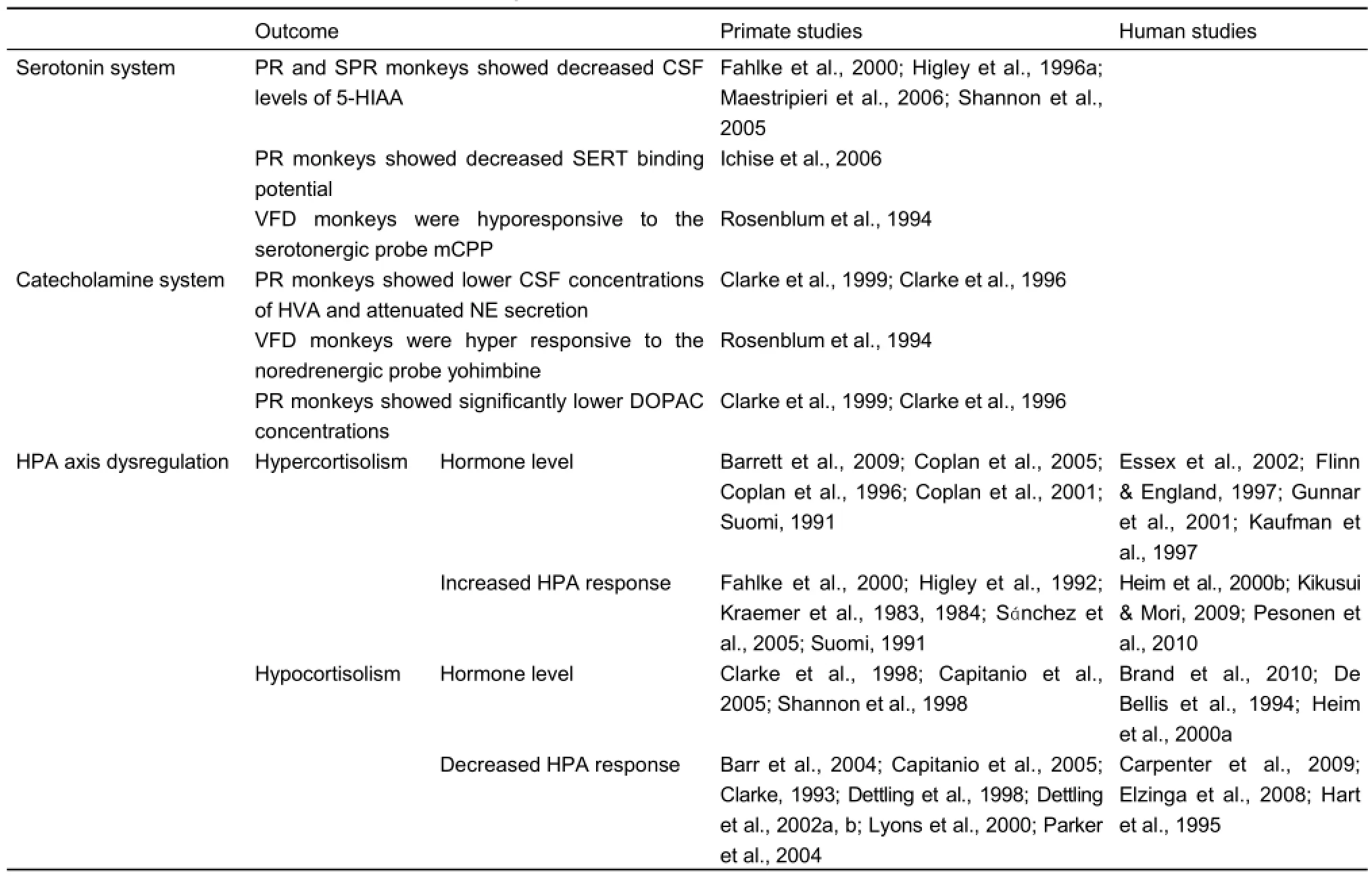
Table 4 EARE induced effects on neuroendocrine systems
Monoamine and hormone systems
The serotonergic system was shown to moderate the effects of EARE on the risk of depression in humans (Eley et al., 2004; Kaufman et al., 2004), and primate studies also indicate the role of serotonin system in regulating the effects of EARE. Maternal rejected, PR and SPR reared infant monkeys exhibited lower CSF 5-HIAA concentrations (Fahlke et al., 2000; Higley et al., 1996a; Maestripieri et al., 2006; Shannon et al., 2005); PR monkeys showed decreased SERT binding potential across arange of brain areas (Ichise et al., 2006); VFD reared monkeys were hyporesponsive to the serotonergic probe meta-Chlorophenylpiperazine (mCPP) (Rosenblum et al., 1994). Moreover, epigenetic studies also indicate the role of serotonin system plays in EARE induced HPA axis dysfunction (Barr et al., 2004; Rosenblum et al., 1994; Shannon et al., 2005; Spinelli et al., 2007) and subsequent abnormal behavioral outcomes (Barr et al., 2003, 2004; Law et al., 2009b; Maestripieri et al., 2006; Vicentic et al., 2006; Ziabreva et al., 2003a). Many studies showed catecholamine system is another candidate through which EARE takes its effect. PR monkeys showed attenuated Norepinephrine (NE) secretion (Clarke et al., 1999, 1996) and reduced CSF concentrations of catecholamine metabolite (Clarke et al., 1999, 1996), while VFD reared monkeys were hyper responsive to the noredrenergic probe yohimbine (Rosenblum et al., 1994). It was further suggested that EARE might influence the differentiation of noradrenergic neurons and thus alter HPA responses stress during adulthood (Liu et al., 2000). Dopamine system (another catecholamine system) might also be involved in EARE effects, as significantly lower concentrations of dopamine metabolite were revealed in PR infant monkeys (Clarke et al., 1999, 1996) and history of childhood adversity was positively associated with striatal dopamine responses to amphetamine (Oswald et al., 2014).
Additionally, primate studies indicate potential hormonal pathways through which EARE takes effects, including oxytocin, growth hormone (GH), and most importantly, cortisol. Monkeys exposed to EARE showed abnormal aggressive and affiliative behaviors, and oxytocin was suggested to be a neuropeptide for affiliation and involved in the regulation of social bonding behaviors (Insel, 1992; Lim & Young, 2006). Therefore, oxytocin is a possible pathway for EARE to take effects, which indeed was probed by Winslow et al. (Winslow et al., 2003), showing that the decrease in affiliative behavior in PR rhesus monkeys was significantly and positively correlated with cerebrospinal oxytocin. Another hormone, GH, was also related to early adversity, as PR and social separation experiences in infant monkeys showed abnormal GH levels (Champoux et al., 1989a; Laudenslager et al., 1995). Most importantly, the main target of EARE under investigation is HPA axis. While some studies showed blunt HPA response, and thus decreased cortisol and ACTH levels (Barr et al., 2004; Capitanio et al., 2005; Clarke, 1993; Dettling et al., 1998, 2002a, b; Lyons et al., 2000; Parker et al., 2004), others showed the opposite (Barrett et al., 2009; Coplan et al., 2005, 1996, 2001; Suomi, 1991). Although consistent results were not achieved, the importance of HPA dysregulation in EARE induced effects was suggested.
Hypothalamic-pituitary-adrenal (HPA) axis dysregulationEARE is associated with elevated levels of stress and fear. The adverse impact of stress on brain development was suggested to be largely through hypothalamic-pituitaryadrenal (HPA) axis both in humans (Loman & Gunnar, 2010) and primates (Sanchez, 2006). The effects of EARE on HPA circadian rhythmicity and the function of HPA axis were reviewed in this section.
Circadian rhythmicity
TheHPA axis is a complex set of direct influences and feedback interactions among three endocrine glands, i.e., hypothalamus, pituitary gland, and adrenal glands. Under basal conditions, HPA axis exhibits a circadian rhythmicity with a peak around the time of waking and a trough during the quiescent time of the activity cycle (Dickmeis et al., 2013; Leliavski et al., 2015; Tsang et al., 2016, 2014). So cortisol levels typically follow the circadian rhythm with levels highest occurring about 20 minutes after awakening in the morning (cortisol awakening response, CAR) and declining throughout the day. Alterations in the normal pattern of HPA rhythmicity, including CAR response and diurnal decrease of cortisol, were found in human studies. Most studies found higher morning cortisol level than controls in maltreated children (Cicchetti & Rogosch, 2001; Cutuli et al., 2010) and EARE exposed adults (Gonzalez et al., 2009; Gustafsson et al., 2010), while some found lower morning cortisol level (Carlson & Earls, 1997). Moreover, different kind of EARE might have differential influence on morning cortisol values, as studies indicate more emotionally and sexually abused children showed higher morning cortisol values, whereas more severe physically neglected and abused children showed lower levels (Bruce et al., 2009; Cicchetti & Rogosch, 2001). Additionally, EARE exposed children also showed higher incidences of atypical diurnal rhythmicity patterns, such as a peaking in the afternoon or evening (Cicchetti et al., 2010; Dozier et al., 2006). Similarly, abnormal HPA circadian rhythmicity were also found in limited amount of primate studies on rhesus monkeys, with morning peak occurring late in PR infants (Thomas et al., 1995) and flattened diurnal rhythm in repetitive maternal separation exposed infants (Sánchez et al., 2005). However, a recent study found no shift in diurnal patterns of cortisol in PR reared juvenile rhesus monkeys (Barrett et al., 2009). Although how EARE induces those abnormal HPA axis circadian rhythmicity, and its different or even contrary effects remains unknown, these HPA axis circadian rhythmicity abnormalities certainly contribute to various abnormal behavioral outcomes.
HPA axis dysregulation
In humans, the HPA axis develops over the initial several years of life and is highly sensitive to EARE (De Weerth et al., 2003; Watamura et al., 2004). The key elements of the HPA axis are as following: the hypothalamus synthesizes and secretes corticotropin-releasing hormone (CRH); CRH stimulates the secretion of adrenocorticotropic hormone (ACTH) in pituitary gland; ACTH acts on the adrenal cortices, which then produces glucocorticoid hormones (mainly cortisol in NHPs and humans); glucocorticoids in turn act back on the hypothalamus and pituitary to suppress CRH and ACTH production in a negative feedback cycle. When activated in response to a stressor, the HPA axis participates in a cascade of neuroendocrine responses, and a typical HPA stress response involves a period of increased glucocorticoids in circulation induced by stimulation of elevated levels of CRH and ACTH, followed by a return to baseline levels induced by negative feedback of glucocorticoids(Herman & Cullinan, 1997). Thus CRH, ACTH and glucocorticoids levels could indicate the reactivity levels of HPA axis, with CRH an important neurotransmitter in HPA axis to initiate the autonomic and behavioral changes in response to stress (Heinrichs et al., 1995; Krohg et al., 2008; Ohmura & Yoshioka, 2009; Smagin & Dunn, 2000). Studies showed EARE induced elevated cerebrospinal fluid (CSF) concentrations of CRH levels in mother deprived rats (Ladd et al., 1996) and VFD reared infant monkeys (Coplan et al., 1996). Not only the CRH levels was increased, a study showed that EARE increased the density of CRH binding sites in many brain regions, including PFC cortex, amygdala and hippocampus (Anisman et al., 1998). As analysis of CRH requires sampling of CSF, it was hard to perform the experiment on healthy humans. On the other hand, analysis of ACTH and glucocorticoids (cortisol) only requires blood or urine sampling, so they are more widely investigated in humans.
Glucocorticoids was revealed to be released from the adrenal cortex during neuroendocrine responses to stress (Herman et al., 2003, 1996), and then regulate HPA axis via negative feedback by binding to two types of receptors, mineralocorticoid receptors (MRs) with high affinity (important in proactive maintenance of HPA basal activity), and GRs with low affinity (primarily responsible for negative feedback). Glucocorticoids could pass through the blood-brain barrier to influence brain function (Zarrow et al., 1970), and MRs’ expression was significantly greater in monkey infants than other ages (Pryce et al., 2005a). Therefore, HPA axis was one of the major pathways through which EARE induces stress and shapes brain development, particular in infants. Glucocorticoids could facilitate HPA axis activation by occupying its receptors in amygdala, leading CRH increase within amygdala (Kolber et al., 2008), whereas it could also suppress HPA axis by occupying its hippocampal receptors (van Haarst et al., 1997). Amygdala and hippocampus are important brain regions for socio-emotional functioning and learning and memory throughout development, and they have a high density of receptors for unbound glucocorticoids, therefore are major targets of EARE affected HPA axis (Johnson et al., 2005; Sánchez et al., 2000). Additionally, EARE could affect HPA axis function bidirectionally, with some studies showing attenuated basal and challenge induced levels of cortisol (hypocortisolism), while others showing elevated levels in both conditions (hypercortisolism).
Hypercortisolism
Human studies showed that EARE could induce hypercortisolism of basal cortisol level in children, reflected by elevated levels of cortisol (Essex et al., 2002; Flinn & England, 1997; Gunnar et al., 2001) and ACTH (Kaufman et al., 1997) in EARE exposed children. Primate studies also showed EARE induced hypercortisolism, reflected by increased plasma coritsol and ACTH in PR infants and juvenile monkeys (Barrett et al., 2009; Suomi, 1991). The elevated cortisol levels in hairs of PR infants indicate the long time accumulation of EARE outcomes (Dettmer et al., 2012). Increased basal cortisol levels were found to be induced by prenatal stress (Pryce et al., 2011). Persistently elevated CSF concentrations of CRF in both infants and mothers under VFD conditions were reported (Coplan et al., 2005, 1996). Not only EARE could affect infants and children, it was suggested that childhood abuse was associated with a persistent sensitization of the HPA axis to stress in human adults (Elzinga et al., 2008), e.g., adults exposed to EARE had higher HPA reactivity during the Trier Social Stress Test (TSST) (Heim et al., 2000b; Pesonen et al., 2010). Animal studies also showed hyper-response of HPA axis activity when facing stress in both infants and adults exposed to EARE. Rodent studies revealed higher HPA response to novelty stress in earlyweaned mice (Kikusui & Mori, 2009). Primate studies showed hyper-responsiveness in EARE reared monkeys, reflected by increased cortisol response to stress in monkeys exposed to PR rearing (Fahlke et al., 2000; Suomi, 1991), VFD rearing (Coplan et al., 2001), repetitive maternal separation (Sánchez et al., 2005) and parental deprivation (Higley et al., 1992; Sánchez et al., 2005). Amphetamine challenge test also revealed neurochemical and behavioral hyper-responseness in isolated monkekys (Kraemer et al., 1983, 1984). All those studies suggested EARE induced hypercortisolism, reflected by elevated basal and stress or challenge facing levels of cortisol, ACTH or CRF.
Hypocortisolism
EARE induced hypocortisolism was also a common finding (Gunnar & Vazquez, 2001), e.g., maltreated children showed decreased basal levels of cortisol (Brand et al., 2010; Heim et al., 2000a) and ACTH (De Bellis et al., 1994). Primate studies revealed attenuated basal levels of cortisol and ACTH in PR monkeys (Capitanio et al., 2005; Clarke et al., 1998; Shannon et al., 1998). When facing stress or challenge, EARE exposed children and adults both showed blunt cortisol response and thus reduced cortisol level (Carpenter et al., 2009; Elzinga et al., 2008; Hart et al., 1995). Similarly, primate studies showed blunt HPA responses during stress and thus decreased coritsol and ACTH levels in PR reared infants (Barr et al., 2004; Capitanio et al., 2005; Clarke, 1993), in young adults exposed to maternal deprivation and intermittent separation (Capitanio et al., 2005; Lyons et al., 2000) and in the hairs of PR infants (Feng et al., 2011) of rhesus monkeys. EARE induced hypocortisolism was also found in other monkey species, including maternal neglect exposed juvenile Goeldi's monkeys (Dettling et al., 1998), intermittent stress exposed squirrel monkeys (Parker et al., 2004) and parental deprivation exposed marmosets (Dettling et al., 2002a, b). All those studies indicate hypocortisolism reflected by decreased basal and stress or challenge facing levels of cortisol or ACTH.
As described above, it is controversial as to the effects of EARE on HPA axis, with some studies showing hypercortisolism while other showing hypocortisolism. There are several possible reasons. Firstly, different types of EARE vary between different research, and even for a same type of EARE, the procedures, manipulations, tests and measuring indexes could be different in different experiments. Secondly, different genotype among human races or animal species could contribute to the divergence as well, in a way that individuals with certain
genotype may be more sensitive to a particular type of EARE than others. In addition, subjects’ personality or temperaments could also partially contribute to the divergence, e.g., children with inhibited temperaments tended to have higher cortisol levels than extroverted children (Gunnar et al., 1995; Kagan et al., 1988), indicating that long-term consequences of EARE may not uniform across subject populations..
A sample of EARE induced neurotransmitter and hormonal changes related behavioral outcomes - social status of primates
Studies suggest that EARE could induce abnormal changes of neurotransmitters and hormones and then influence social status of primates. Serotonergic system was the most widely studied neurotransmitter involved. Primate studies showed that different levels of CSF serotonin (5-HT) or its main metabolite 5-Hydroxyindoleacetic acid (5HIAA) were related to different social status, with higher levels related to more dominant status (Higley et al., 1996b; Raleigh et al., 1983). Additionally, serotonergic drugs were found to be able to influence dominance status, in a way that serotonergic enhancing drugs increase social dominance while serotonergic reducing drugs decrease dominance (Raleigh et al., 1991). 5-HT seems to be positively related to social dominance status, and studies suggested that might due to its influence on affiliative and aggressive behaviors which are important factors in dominance formation and maintenance. Primate studies showed positive correlation between CSF 5-HIAA and affiliative approaching and grooming behavior (Mehlman et al., 1995; Raleigh et al., 1985) and negative correlation between CSF 5-HIAA and aggressive behavior (Higley et al., 1996a), which were consistent with the previous presumption that affliliative behavior was much more effective in acquiring and reinforcing social dominance than aggressive behaviors. Supporting evidence also came from another genetic primate study that suggested certain serotonin transporter (5-HTT/SERT) diplotypes might modulate acquisition of dominance status (Miller-Butterworth et al., 2007). Beside serotonergic system, dopaminergic systems might also affect social dominance status, as dopamine transporter (DAT) gene variants were suggested to be associated with social rank in cynomolgus monkeys (Miller-Butterworth et al., 2008). As to hormones, although a study showed that cortisol concentration was significantly higher in dominant monkeys (Czoty et al., 2009), most studies failed to find the relationship between cortisol level and social rank (Czoty et al., 2009; Goo & Sassenrath, 1980; Morgan et al., 2000; Stavisky et al., 2001). Those studies suggested that EARE could influence social status of primates through its influence on neurotransmitters and hormones. The effects of EARE should not be limited on social status but also might on some other abnormal behaviors.
Genetic and epigenetic influences of EARE effects
Developing is a dynamic process involving constant and reciprocal interactions between organisms and the environments. Emerging evidence suggests that epigenetic modifications may serve as a critical mechanism through which experiences occurring during the lifespan can have sustained
effects in developmental outcomes (Daskalakis et al., 2013). Epigenetics refers to the study of inherited changes in phenotype (appearance) or gene expression caused by mechanisms other than changes in the underlying DNA sequence, such as modifications of transcription of the genome by chemical markers regulation, and variation in gene expression rather than gene sequence is the key concept. Moreover, epigenetics is used to describe the dynamic interactions between genome and the environment (Jablonka & Lamb, 2002). Research suggested that environmental events can modify the epigenetic status of the genome by activating intracellular pathways to regulate interaction between transcription factors and their DNA binding sites, leading to changes in gene expression and eventually different levels of proteins (Bagot & Meaney, 2010; Zhang & Meaney, 2010). This is the biological basis for the interplay between environmental factors and the genome in the regulation of individual differences in behaviors and cognition. Both animal and human studies suggest that EARE can lead to lasting changes in neurotransmitter systems and brain function, and then induce cognitive and behavioral changes. However, there was remarked inter-individual variations in responses to adversity (Collishaw et al., 2007; Rutter, 2007), and these variations might be due to different genotype, different living environment and interaction between the genome and environment.
Genetic influences
Different genotype could induce different behavioral outcomes. Allelic variation of the monoamine oxidase A (MAOA) gene was implicated in aggressive behaviors (Volavka et al., 2004). Both human (Caspi et al., 2002; Craig, 2005; Kim-Cohen et al., 2006) and primate (Karere et al., 2009) studies showed that genotype conferring low MAOA activity was related to mental health problems. These findings may partially explain the variability in developmental outcomes associated with maltreatment, e.g., why not all victims of maltreatment grow up to with abnormal behaviors like antisocial problems, and they provide epidemiological evidence that genotypes can moderate children's sensitivity to environmental insults. Similar results was revealed in 5-HTT genotype, as short promoter region of the serotonin transporter (5-HTTLPR) allele was related to increased anxious behavior in primates (McCormack et al., 2009) and highest emotional problem scores in human (Kumsta et al., 2010). Those evidences suggest the importance of genotype in behavioral outcomes.
Epigenetic influences of EARE on gene expression
Environmental and life experience could exert influences on gene expression and time course analysis indicate that maternally induced epigenetics might emerge during the postnatal period and could sustain into adulthood (Weaver et al., 2004). Epigenetic regulation of gene expression is particularly important during the early stages of development, and it is one of the main mechanisms mediating the long-term effects of maternal care on development (Champagne, 2008; Champagne & Curley, 2009; Diorio & Meaney, 2007; Meaney, 2001; Zhang et al., 2006). For example, rodent studies showed that postnatalmaternal licking/grooming (LG) behavior could induce increased hippocampal GR expression (Caldji et al., 1998; Francis et al., 1999; Liu et al., 1997; Weaver, 2007), while low levels of LG during neonates led to reduced expression of estrogen receptor in hypothalamus and reduced response to estrogen (Champagne et al., 2001, 2006, 2003). As to the effects of EARE on gene expression, isolation attenuated social interaction induced gene expression in rodents (Ahern et al., 2016; Lukkes et al., 2012, 2013; Shishkina et al., 2015; Wall et al., 2012). A human study also showed EARE related down regulation of genes containing GR response elements (Miller et al., 2009). In primate studies, early maternal separation could lead to gene expression changes in many brain regions, including differential expression of gene GUCY1A3 in amygdala, decreases in hippocampal growth associated protein 43 (GAP-43) mRNA and 5-HT receptor mRNA (Law et al., 2009b) and a selective long-term effect on expression of genes in ACC (Law et al., 2009a). Moreover, epigenetics is not a binary response across the whole brain. Different genes in different brain regions can be affected in different ways, e.g., early maternal deprivation could either induce reduction of gene expression (Liu et al., 1997; Roceri et al., 2002) or up-regulation of gene expressions (Plotsky et al., 2005; Ziabreva et al., 2000).
Epigenetic influences of EARE on neurobiological and behavioral outcomes
Epigenetic influences on gene expression may lead to different expression patterns of proteins, thus different levels of hormones and neurotransmitters, ultimately lead to different behavioral outcomes. Studies suggested that EARE might exert its effects on behavioral outcomes independent of genotype. Primate studies revealed the importance of environment and life experience independent of genotype. MR monkeys showed significantly up-regulated level of 5-HTT during maternal separation, while NR monkeys did not (Kinnally et al., 2008). With the same low-activity Monoamine oxidase A (MAOA) genotype, MR reared monkeys were more aggressive than the PR monkeys (Newman et al., 2005). Higher 5-HTT cytosinephosphate-guanosine (CpG) methylation, but not rh5-HTTLPR genotype, exacerbated the effects of early life stress on behavioral stress reactivity in infant monkeys (Kinnally et al., 2010). Additionally, from behavioral perspective alone, infant monkeys exposed to mother abuse showed the same tendency to their offspring, regardless of whether they were reared by their biological mothers or by foster mothers (Maestripieri, 2005). Rodent studies also supported the important role of environment and life experience on behavioral outcomes. Rodent studies suggested that maternal care behaviors especially postnatal maternal LG could be transmitted from the mother to her female offspring, so female offspring who received low levels of LG also provided low levels of this form of maternal care to their own offspring (Fleming et al., 2002). Cross-fostering studies in rodents indicate that this intergenerational transmission of behaviors was the result of early experience rather than genetic inheritance (Champagne & Meaney, 2001). For example, the biological offspring of low-LG mothers reared by high-LG dams resembled the normal offspring of high-LG mothers (Francis et al., 1999). All those studies indicate the importance of environment and life experience, especially maternal interactions on the subsequent expression of behaviors, rather than the genetic contributions. Moreover, primate studies showed the importance of the interaction between genetic factors and environmental experience on neurobiological outcomes, as CSF 5-HIAA concentrations were significantly influenced by genotype in the PR but not MR reared monkeys (Bennett et al., 2002), and 5-HTT gene variation affected HPA axis activity in response to stress in a way that cortisol levels increased during separation in MR but decreased in PR monkeys (Barr et al., 2004).
Transgenerational epigenetic programming
Among the epigenetic mechanisms of EARE, such as DNA methylation, histone modifications, and micro-RNA expression, DNA methylation was the most intensively studied epigenetic phenomenon (Babenko et al., 2015; Blaze et al., 2015b; Jawahar et al., 2015; Provençal et al., 2015; Vaiserman, 2015a, b; Zheng et al., 2014). Actually, not only EARE exposure of the offspring themselves could lead to long time biological and behavioral outcomes, EARE exposure of parents could also influence their offspring, which was defined as transgenerational epigenetic programming phenomenon and has drawn much attention recently. Related studies suggested the underlying epigenetic mechanisms of maternal transgenerational influence to be DNA methylation, histone modifications, and micro-RNA expression (Babenko et al., 2015; Bale, 2014; Blaze & Roth, 2015; Gröger et al., 2016; Miska & Ferguson-Smith, 2016; Nagy & Turecki, 2015).
In addition to maternal influences, fathers can exert influences on offspring development either through direct care in living social environment, or indirectly through interacting with maternal influences. Some recent studies showed the important influence of paternal early experiences on infant development through non-social mechanisms, even in the absence of direct contact with offspring. This emerging field focuses on how environmental influences can epigenetically alter paternal sperm DNA methylation, histone modification and micro-RNA expression, and ultimately change the phenotype and behavior of offspring (Braun & Champagne, 2014; Curley et al., 2011; Day et al., 2016; Kinnally & Capitanio, 2015; Rodgers et al., 2013; Yuan et al., 2016).
DISCUSSION
Rehabilitation
Human studies indicate the possibility of rehabilitation of EARE induced deficits, e.g., Fisher et al. (2000, 2006, 2007) suggested that the improvements of caring following EARE had the potential to prevent or reverse EARE induced HPA axis dysfunction, such as normalizing perturbed diurnal cortisol patterns and reducing basal salivary cortisol level (Fernald & Gunnar, 2009). In primate studies, total social isolation was once considered to induce permanent defect, which was described as learning deficit in some studies, because isolated monkeys were lack of physical interactions and had noopportunity for social learning with conspecifics, or to gradually develop sophisticated social behaviors (Mitchell, 1968; Sackett, 1969; Suomi et al., 1974). These abnormities might be rehabilitated by socializing the isolate monkeys with conspecifics. Some studies reported that after the isolated monkeys was paired with “therapist" monkeys, less selfdirected disturbance activities, or stereotypic behaviors, but more social contact and exploratory behaviors were observed (Harlow & Suomi, 1971b; Suomi, 1973). Less severe selfinjurious behaviors were found when isolated monkeys were reared with surrogates (Brunelli et al., 2014; Harlow & Suomi, 1971b) or social housing (Lutz & Novak, 2005). Additionally, environmental enrichment treatments were used to eliminate abnormal behaviors and to normalize the behavioral repertoire of EARE exposed monkeys (Lutz & Novak, 2005; Novak et al., 1998; Rommeck et al., 2009a). It was suggested that environmental enrichment devices could only ameliorate less severe forms of abnormal behavior but not more severe forms of self-injurious or non-injurious self-abuse behaviors (Rommeck et al., 2009a). Those studies indicate rehabilitation is possible, at least partially, but it requires combination of multiple rehabilitation methods, such as socializing, environmental enrichment, and considerable time and effort.
Establishing NHP mental disorder models with EARE methods
EARE exposed NHPs showed signs of various mental disorders, including anxiety, autism and depression etc., making it a potential animal model to study human mental disorders, among which depression model was the mostly investigated one (Gilmer & McKinney, 2003; Pryce et al., 2005b; Worlein, 2014). The influence of EARE on NHPs is through daily life and accumulates over a period of time, making it a more natural model of mental disorders than those induced by drugs or invasive surgeries. The diagnosis of mental disorders in humans usually depends on questionnaire investigation and verbal communication between patients and doctors, which are not doable in NHPs. Therefore NHPs studies usually include daily group living or single subject observation and behavioral analysis, biochemical index analysis (e.g., hormones), brain structural and functional changes analysis by using modern imaging methods, However, due to the complexity of mental disorders, it is very difficult to diagnose mental disorders in NHPs. Different disorders might show very similar behavioral symptoms and biochemical abnormalities, therefore additional indexes are necessary in diagnosing. For example, in NHPs, anxiety and depression share symptoms of stereotypic behaviors and elevated cortisol level in response to stressor, so, more depression specific symptoms, such as lacking of responses to stimulus are needed for diagnosing. It is difficult to differentiate if a monkey was depressed or autistic as in both cases, preference of staying away from social activity and being alone in a corner would be shown. Certain metal disorders could be divided into many subtypes, e.g., depression includes unipolar, bipolar and atypical depression, etc, which certainly adds more complexity in establishing NHP animal models. These might explain the fact that despite being an ideal and irreplaceable animal model, studies on EARE induced NHP mental disorder models are limited.
CONCLUDING REMARKS
In summary, as an irreplaceable animal model, NHP EARE experiments were performed for over 60 years and revealed important insights into understanding the effects of EARE on development and underlying mechanisms of related physiological and psychological diseases. Although much has been learned to date, there is much more to understand about EARE impact on developmental trajectory. Now, with the help of emerging cutting edge technologies, such as new brain imaging method, gene modification, optogenetics, etc, future EARE studies will further clarify these issues and help to cure the diseases.
ACKNOWLEDGEMENTS
The author wishes to thank Prof. Yuan-Ye Ma and the anonymous reviewers and editors of Zoological Research for their valuable opinions and suggestions.
REFERENCES
Adams T, Rosenkranz JA. 2016. Social isolation during postweaning development causes hypoactivity of neurons in the medial nucleus of the male rat amygdala. Neuropsychopharmacology, 41(7): 1929-1940.
Ahern M, Goodell DJ, Adams J, Bland ST. 2016. Brain regional differences in social encounter-induced Fos expression in male and female rats after post-weaning social isolation. Brain Research, 1630: 120-133.
Andersen SL, Teicher MH. 2004. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology, 29(11): 1988-1993. Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. 2008. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3): 292-301.
Andersen SL. 2015. Exposure to early adversity: points of cross-species translation that can lead to improved understanding of depression. Development and Psychopathology, 27(2): 477-491.
Andrews MW, Rosenblum LA. 1991. Attachment in monkey infants raised in variable- and low-demand environments. Child Development, 62(4): 686-693.
Anisman H, Zaharia MD, Meaney MJ, Merali Z. 1998. Do early-life events permanently alter behavioral and hormonal responses to stressors? International Journal of Developmental Neuroscience, 16(3-4): 149-164.
Avishai-Eliner S, Yi SJ, Baram TZ. 1996. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research, 91(2): 159-163.
Azmitia EC, Gannon PJ. 1986. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Advances in Neurology, 43: 407-468.
Babenko O, Kovalchuk I, Metz GAS. 2015. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience & Biobehavioral Reviews, 48: 70-91.
Bagot RC, Meaney MJ. 2010. Epigenetics and the biological basis of gene × environment interactions. Journal of the American Academy of Child & Adolescent Psychiatry, 49(8): 752-771.
Bailey RC, Aunger R. 1990. Humans as primates: the social relationships of Efe pygmy men in comparative perspective. International Journal of Primatology, 11(2): 127-146.
Bale TL. 2014. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues in Clinical Neuroscience, 16(3): 297-305.
Baram TZ, Hatalski CG. 1998. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends in Neurosciences, 21(11): 471-476.
Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, Tillman R, Luby J. 2016. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. American Journal of Psychiatry, 173(6): 625-634.
Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. 2003. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior, 2(6): 336-340.
Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. 2004. Rearing condition and rh5-HTTLPR interact to influence limbichypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry, 55(7): 733-738.
Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. 2009. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology, 34(7): 1029-1040.
Bartesaghi R, Serrai A. 2001. Effects of early environment on granule cell morphology in the dentate gyrus of the guinea-pig. Neuroscience, 102(1): 87-100.
Bartesaghi R, Raffi M, Severi S. 2003a. Effects of early isolation on layer ii neurons in the entorhinal cortex of the guinea pig. Neuroscience, 120(3): 721-732.
Bartesaghi R, Severi S, Guidi S. 2003b. > effects of early environment on pyramidal neuron morphology in field CA1 of the guinea-pig. Neuroscience, 116(3): 715-732.
Bartesaghi R, Severi S. 2004. effects of early environment on field CA2 pyramidal neurons in the guinea-pig. Neuroscience, 123(3): 703-714.
Bastian ML, Sponberg AC, Sponberg AC, Suomi SJ, Higley JD. 2003. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Developmental Psychobiology, 42(1): 44-51.
Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. 2009. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry, 66(12): 1100-1106.
Bauer SA, Baker KC. 2016. Persistent effects of peer rearing on abnormal and species-appropriate activities but not social behavior in group-housed rhesus macaques (Macaca mulatta). Comparative Medicine, 66(2): 129-136.
Baysinger CM, Brandt EM, Mitchell G. 1972. Development of infant social isolate monkeys (Macaca mulatta) in their isolation environments. Primates, 13(3): 257-270.
Beauchamp AJ, Gluck JP. 1988. Associative processes in differentially reared monkeys (Macaca mulatta): sensory preconditioning. Developmental Psychobiology, 21(4): 355-364.
Beauchamp AJ, Gluck JP, Fouty HE, Lewis MH. 1991. Associative processes in differentially reared rhesus monkeys (Macaca mulatta): blocking. Developmental Psychobiology, 24(3): 175-189.
Becker K, Abraham A, Kindler J, Helmeke C, Braun K. 2007. Exposure to neonatal separation stress alters exploratory behavior and corticotropin releasing factor expression in neurons in the amygdala and hippocampus. Developmental Neurobiology, 67(5): 617-629.
Benjet C. 2010. Childhood adversities of populations living in low-income countries: prevalence, characteristics, and mental health consequences. Current Opinion in Psychiatry, 23(4): 356-362.
Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. 2002. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry, 7(1): 118-122.
Bennett AJ, Suomi SJ, Hopkins WD. 2008. Effects of early adverse experiences on behavioural lateralisation in rhesus monkeys (Macaca mulatta). Laterality: Asymmetries of Body, Brain and Cognition, 13(3): 282-292.
Bernstein IS, Mason WA. 1963. Group formation by rhesus monkeys. Animal Behaviour, 11(1): 28-31.
Bernstein IS, Williams LE. 1983. Ontogenetic changes and the stability of rhesus monkey dominance relationships. Behavioural Processes, 8(4): 379-392.
Bernstein IS, Cooper MA. 1999. Dominance in assamese macaques (Macaca assamensis). American Journal of Primatology, 48(4): 283-289.
Bick J, Nelson CA. 2016. Early adverse experiences and the developing brain. Neuropsychopharmacology, 41(1): 177-196.
Blaze J, Roth TL. 2015. Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Seminars in Cell & Developmental Biology, 43: 76-84.
Blaze J, Asok A, Roth TL. 2015a. Long-term effects of early-life caregiving experiences on brain-derived neurotrophic factor histone acetylation in the adult rat mPFC. Stress, 18(6): 607-615.
Blaze J, Asok A, Roth TL. 2015b. The long-term impact of adverse caregiving environments on epigenetic modifications and telomeres. Frontiers in Behavioral Neuroscience, 9: 79.
Boccia ML, Reite M, Kaemingk K, Held P, Laudenslager M. 1989. Behavioral and autonomic responses to peer separation in pigtail macaque monkey infants. Developmental Psychobiology, 22(5): 447-461.
Bock J, Gruss M, Becker S, Braun K. 2005. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cerebral Cortex, 15(6): 802-808.
Bogart SL, Bennett AJ, Schapiro SJ, Reamer LA, Hopkins WD. 2014. Different early rearing experiences have long-term effects on cortical organization in captive chimpanzees (Pan troglodytes). Developmental Science, 17(2): 161-174.
Bohn MC, Howard E, Vielkind U, Krozowski Z. 1991. Glial cells express both mineralocorticoid and glucocorticoid receptors. The Journal of Steroid Biochemistry and Molecular Biology, 40(1-3): 105-111.
Bohn MC, O'banion MK, Young DA, Giuliano R, Hussain S, Dean DO, Cunningham LA. 1994. In vitro studies of glucocorticoid effects on neurons and astrocytes. Annals of the New York Academy of Sciences, 746: 243-258.
Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. 2002.Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry, 51(4): 342-344.
Bowlby J. 1969. Attachment and Loss: Volume I: Attachment. London: The Hogarth Press and the Institute of Psycho-Analysis.(请核对出版信息)
Bowley MP, Drevets WC, Öngür D, Price JL. 2002. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry, 52(5): 404-412.
Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. 2010. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology, 35(5): 686-693.
Braun K, Champagne FA. 2014. Paternal influences on offspring development: behavioural and epigenetic pathways. Journal of Neuroendocrinology, 26(10): 697-706.
Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, Mccarthy G, Innis RB, Charney DS. 1997. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biological Psychiatry, 41(1): 23-32.
Bremner JD, Elzinga B, Schmahl C, Vermetten E. 2007. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research, 167: 171-186.
Bridges N, Slais K, Syková E. 2008. The effects of chronic corticosterone on hippocampal astrocyte numbers: a comparison of male and female Wistar rats. Acta Neurobiologiae Experimentalis, 68(2): 131-138.
Bruce J, Fisher PA, Pears KC, Levine S. 2009. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Developmental Psychobiology, 51(1): 14-23.
Brunelli RL, Blake J, Willits N, Rommeck I, Mccowan B. 2014. Effects of a mechanical response-contingent surrogate on the development of behaviors in nursery-reared rhesus macaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 53(5): 464-471.
Brunson KL, Kramár E, Lin B, Chen YC, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. 2005. Mechanisms of late-onset cognitive decline after earlylife stress. Journal of Neuroscience, 25(41): 9328-9338.
Bryan GK, Riesen AH. 1989. Deprived somatosensory-motor experience in stumptailed monkey neocortex: dendritic spine density and dendritic branching of layer IIIB pyramidal cells. The Journal of Comparative Neurology, 286(2): 208-217.
Buckley MJ. 2005. The role of the perirhinal cortex and hippocampus in learning, memory, and perception. The Quarterly Journal of Experimental Psychology Section B: Comparative and Physiological Psychology, 58(3-4): 246-268.
Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6): 215-222.
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. 1998. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America, 95(9): 5335-5340.
Capitanio JP, Mason WA. 2000. Cognitive style: problem solving by rhesus macaques (Macaca mulatta) reared with living or inanimate substitute mothers. Journal of Comparative Psychology, 114(2): 115-125.
Capitanio JP, Mendoza SP, Mason WA, Maninger N. 2005. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta). Developmental Psychobiology, 46(4): 318-330. Carlson M, Earls F. 1997. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences, 807: 419-428.
Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. 2009. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biological Psychiatry, 66(1): 69-75.
Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. 2001. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry, 50(12): 943-951.
Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL. 2009. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Research: Neuroimaging, 172(3): 226-234.
Casement MD, Guyer AE, Hipwell AE, Mcaloon RL, Hoffmann AM, Keenan KE, Forbes EE. 2014. Girls' challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience, 8: 18-27.
Casement MD, Shaw DS, Sitnick SL, Musselman SC, Forbes EE. 2015. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Social Cognitive and Affective Neuroscience, 10(3): 416-423.
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. 2002. Role of genotype in the cycle of violence in maltreated children. Science, 297(5582): 851-854.
Chamove AS, Rosenblum LA, Harlow HF. 1973. Monkeys (Macaca mulatta) raised only with peers. A pilot study. Animal Behaviour, 21(2): 316-325.
Champagne F, Diorio J, Sharma S, Meaney MJ. 2001. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences of the United States of America, 98(22): 12736-12741.
Champagne F, Meaney MJ. 2001. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research, 133: 287-302.
Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ. 2003. Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the medial preoptic area. Endocrinology, 144(11): 4720-4724.
Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ. 2006. Maternal care associated with methylation of the estrogen receptorα1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology, 147(6): 2909-2915.
Champagne FA. 2008. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology, 29(3): 386-397. Champagne FA, Curley JP. 2009. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience & Biobehavioral Reviews, 33(4): 593-600.
Champoux M, Coe CL, Schanberg SM, Kuhn CM, Suomi SJ. 1989a. Hormonal effects of early rearing conditions in the infant rhesus monkey. American Journal of Primatology, 19(2): 111-117.
Champoux M, Metz B, Suomi SJ. 1989b. Rehousing NonreproductiveRhesus Macaques with Weanlings: I. Behavior of Adults toward Weanlings. Laboratory Primate Newsletter, 28(4): 1-4.
Champoux M, Metz B, Suomi SJ. 1991. Behavior of nursery/peer-reared and mother-reared rhesus monkeys from birth through 2 years of age. Primates, 32(4): 509-514.
Champoux M, Byrne E, Delizio R, Suomi SJ. 1992. Motherless mothers revisited: rhesus maternal behavior and rearing history. Primates, 33(2): 251-255.
Cicchetti D, Rogosch FA. 2001. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology, 13(3): 677-693. Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. 2010. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development, 81(1): 252-269.
Clarke AS. 1993. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Developmental Psychobiology, 26(8): 433-446.
Clarke AS, Hedeker DR, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. 1996. Rearing experience and biogenic amine activity in infant rhesus monkeys. Biological Psychiatry, 40(5): 338-352.
Clarke AS, Kraemer GW, Kupfer DJ. 1998. Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Research, 79(2): 91-104.
Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. 1999. Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biological Psychiatry, 46(2): 221-228. Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, Mccaffery J, Hitsman B, Niaura R, Clark CR, MacFarlane A, Bryant R, Gordon E, Williams LM. 2006. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59(10): 975-982.
Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. 2007. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse & Neglect, 31(3): 211-229. Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. 1996. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America, 93(4): 1619-1623.
Coplan JD, Smith ELP, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Rosenblum LA. 2001. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biological Psychiatry, 50(3): 200-204.
Coplan JD, Altemus M, Mathew SJ, Smith ELP, Sharf B, Coplan PM, Kral JG, Gorman JM, Owens MJ, Nemeroff CB, Rosenblum LA. 2005. Synchronized maternal-infant elevations of primate CSF CRF concentrations in response to variable foraging demand. CNS Spectrums, 10(7): 530-536.
Cotter D, Mackay D, Landau S, Kerwin R, Everall I. 2001. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Archives of General Psychiatry, 58(6): 545-553.
Craig IW. 2005. The role of monoamine oxidase A, MAOA, in the aetiology of antisocial behaviour: the importance of gene-environment interactions. Novartis Foundation Symposium, 268: 227-237; discussion 237-241, 242-253. (请核对页码)
Cross HA, Harlow HF. 1965. Prolonged and progressive effects of partial isolation on the behavior of macaque monkeys. Journal of Experimental Research in Personality, 1(1): 39-49.
Curley JP, Mashoodh R, Champagne FA. 2011. Epigenetics and the origins of paternal effects. Hormones and Behavior, 59(3): 306-314.
Cushing BS, Kramer KM. 2005. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neuroscience & Biobehavioral Reviews, 29(7): 1089-1105.
Cutuli JJ, Wiik KL, Herbers JE, Gunnar MR, Masten AS. 2010. Cortisol function among early school-aged homeless children. Psychoneuroendocrinology, 35(6): 833-845.
Czoty PW, Gould RW, Nader MA. 2009. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). Journal of Neuroendocrinology, 21(1): 68-76.
Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. 2013. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9): 1858-1873.
Davis M. 1992. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15: 353-375.
Day J, Savani S, Krempley BD, Nguyen M, Kitlinska JB. 2016. Influence of paternal preconception exposures on their offspring: through epigenetics to phenotype. American Journal of Stem Cells, 5(1): 11-18.
De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. 1994. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. The Journal of Clinical Endocrinology and Metabolism, 78(2): 249-255.
De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. 1999. Developmental traumatology part II: brain development. Biological Psychiatry, 45(10): 1271-1284.
De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. 2001. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry, 50(4): 305-309.
De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. 2002. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry, 52(11): 1066-1078.
De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, Mccrory EJ. 2013. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry, 54(1): 105-112.
De Weerth C, Zijl RH, Buitelaar JK. 2003. Development of cortisol circadian rhythm in infancy. Early Human Development, 73(1-2): 39-52.
Dettling A, Pryce CR, Martin RD, Döbeli M. 1998. Physiological Responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi's monkeys (Callimico goeldii). Developmental Psychobiology, 33(1): 21-31.
Dettling AC, Feldon J, Pryce CR. 2002a. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology Biochemistry and Behavior, 73(1): 259-269.
Dettling AC, Feldon J, Pryce CR. 2002b. Repeated parental deprivation inthe infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biological Psychiatry, 52(11): 1037-1046. Dettmer AM, Ruggiero AM, Novak MA, Meyer JS, Suomi SJ. 2008. Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta). Developmental Psychobiology, 50(4): 418-422.
Dettmer AM, Novak MA, Suomi SJ, Meyer JS. 2012. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology, 37(2): 191-199.
Devinsky O, Morrell MJ, Vogt BA. 1995. Contributions of anterior cingulate cortex to behaviour. Brain, 118(1): 279-306.
DeVore I. 1990. Introduction: current studies on primate socioecology and evolution. International Journal of Primatology, 11(1): 1-5.
Dickmeis T, Weger BD, Weger M. 2013. The circadian clock and glucocorticoids – interactions across many time scales. Molecular and Cellular Endocrinology, 380(1-2): 2-15.
Diorio J, Meaney MJ. 2007. Maternal programming of defensive responses through sustained effects on gene expression. Journal of Psychiatry and Neuroscience, 32(4): 275-284.
Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. 2006. Foster children's diurnal production of cortisol: an exploratory study. Child Maltreatment, 11(2): 189-197.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME. 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386(6627): 824-827.
Drevets WC, Savitz J, Trimble M. 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8): 663-681.
Eastman RF, Mason WA. 1975. Looking behavior in monkeys raised with mobile and stationary artificial mothers. Developmental Psychobiology, 8(3): 213-221.
Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. 2011. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine, 165(12): 1069-1077.
Eichenbaum H, Otto T, Cohen NJ. 1992. The hippocampus—what does it do? Behavioral and Neural Biology, 57(1): 2-36.
Eichenbaum H, Schoenbaum G, Young B, Bunsey M. 1996. Functional organization of the hippocampal memory system. Proceedings of the National Academy of Sciences of the United States of America, 93(24): 13500-13507.
Eichenbaum H. 2001. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behavioural Brain Research, 127(1-2): 199-207.
Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. 2004. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry, 9(10): 908-915. Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. 2008. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology, 33(2): 227-237.
Erwin J, Mitchell G, Maple T. 1973. Abnormal behavior in non-isolate-reared rhesus monkeys. Psychological Reports, 33(2): 515-523.
Essex MJ, Klein MH, Cho E, Kalin NH. 2002. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biological Psychiatry, 52(8): 776-784.
Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, Liberzon I. 2016. Childhood cumulative risk exposure and adult amygdala volume and function. Journal of Neuroscience Research, 94(6): 535-543.
Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. 2000. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcoholism: Clinical and Experimental Research, 24(5): 644-650.
Farrington DP, Loeber R. 2000. Epidemiology of juvenile violence. Child and Adolescent Psychiatric Clinics of North America, 9(4): 733-748.
Fekete JM, Norcross JL, Newman JD. 2000. Artificial turf foraging boards as environmental enrichment for pair-housed female squirrel monkeys. Journal of the American Association for Laboratory Animal Science, 39(2): 22-26.
Feng XL, Wang LN, Yang SC, Qin DD, Wang JH, Li CL, Lv LB, Ma YY, Hu XT. 2011. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America, 108(34): 14312-14317.
Fenoglio KA, Brunson KL, Baram TZ. 2006. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Fronters in Neuroendocrinology, 27(2): 180-192.
Fernald LCH, Gunnar MR. 2009. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science & Medicine, 68(12): 2180-2189.
Fisher PA, Gunnar MR, Chamberlain P, Reid JB. 2000. Preventive intervention for maltreated preschool children: impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child & Adolescent Psychiatry, 39(11): 1356-1364.
Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. 2006. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Annals of the New York Academy of Sciences, 1094: 215-225.
Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. 2007. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology, 32(8-10): 892-905.
Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. 2002. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacology Biochemistry and Behavior, 73(1): 61-75.
Flinn MV, England BG. 1997. Social economics of childhood glucocorticoid stress response and health. American Journal of Physical Anthropology, 102(1): 33-53.
Floeter MK, Greenough WT. 1979. Cerebellar plasticity: modification of Purkinje cell structure by differential rearing in monkeys. Science, 206(4415): 227-229.
Fonberg E. 1988. Dominance and aggression. International Journal of Neuroscience, 41(3-4): 201-213.
Francis D, Diorio J, Liu D, Meaney MJ. 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science, 286(5442): 1155-1158.
Galea LAM, Mcewen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons inresponse to chronic restraint stress. Neuroscience, 81(3): 689-697.
Gallagher M, Chiba AA. 1996. The amygdala and emotion. Current Opinion in Neurobiology, 6(2): 221-227.
Garrett JE, Wellman CL. 2009. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience, 162(1): 195-207.
Gershon A, Sudheimer K, Tirouvanziam R, Williams LM, O'hara R. 2013. The long-term impact of early adversity on late-life psychiatric disorders. Current Psychiatry Reports, 15: 352.
Gilmer WS, McKinney WT. 2003. Early experience and depressive disorders: human and non-human primate studies. Journal of Affective Disorders, 75(2): 97-113.
Gluck JP, Harlow HF, Schiltz KA. 1973. Differential effect of early enrichment and deprivation on learning in the rhesus monkey (Macaca mulatta). Journal of Comparative and Physiological Psychology, 84(3): 598-604.
Gluck JP, Sackett GP. 1974. Frustration and self-aggression in social isolate rhesus monkeys. Journal of Abnormal Psychology, 83(3): 331-334. Gluck JP, Sackett GP. 1976. Extinction deficits in socially isolated rhesus monkeys (Macaca mulatta). Developmental Psychology, 12(2): 173-174.
Gluckman PD, Hanson MA. 2004. Living with the past: evolution, development, and patterns of disease. Science, 305(5691): 1733-1736.
Gluckman PD, Hanson MA, Beedle AS. 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. American Journal of Human Biology, 19(1): 1-19.
Goff B, Tottenham N. 2015. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS Spectrums, 20(4): 337-345. Goldfoot DA, Wallen K, Neff DA, McBrair MC, Goy RW. 1984. Social influences on the display of sexually dimorphic behavior in rhesus monkeys: isosexual rearing. Archives of Sexual Behavior, 13(5): 395-412.
Gonzalez A, Jenkins JM, Steiner M, Fleming AS. 2009. The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology, 34(1): 76-86.
Goo GP, Sassenrath EN. 1980. Persistent adrenocortical activation in female rhesus monkeys after new breeding groups formation. Journal of Medical Primatology, 9(6): 325-334.
Goosen C. 1981. Abnormal behavior patterns in rhesus monkeys: symptoms of mental disease. Biological Psychiatry, 16(8): 697-716.
Gorman JM, Mathew S, Coplan J. 2002. Neurobiology of early life stress: nonhuman primate models. Seminars in Clinical Neuropsychiatry, 7(2): 96-103.
Gos T, Bock J, Poeggel G, Braun K. 2008. Stress-induced synaptic changes in the rat anterior cingulate cortex are dependent on endocrine developmental time windows. Synapse, 62(3): 229-232.
Griffin GA, Harlow HF. 1966. Effects of three months of total social deprivation on social adjustment and learning in the rhesus monkey. Child Development, 37(3): 533-547. (与下条参考文献重复, 请核对)
Griffin GA, Harlow HF. 1966. Effects of three months of total social deprivation on social adjustment and learning in the rhesus monkey. Child Development, 37(3): 533-547.
Gröger N, Matas E, Gos T, Lesse A, Poeggel G, Braun K, Bock J. 2016. The transgenerational transmission of childhood adversity: behavioral, cellular, and epigenetic correlates. Journal of Neural Transmission, 123(9): 1037-1052.
Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. 1995. Neonatal stress reactivity: predictions to later emotional temperament. Child Development, 66(1): 1-13.
Gunnar MR, Morison SJ, Chisholm K, Schuder M. 2001. Salivary cortisol levels in children adopted from romanian orphanages. Development and Psychopathology, 13(3): 611-628.
Gunnar MR, Vazquez DM. 2001. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology, 13(3): 515-538.
Gustafsson PE, Janlert U, Theorell T, Hammarström A. 2010. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology, 35(4): 613-623.
Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22): 7466-7472. Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. 2015. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry, 77(4): 314-323.
Harlow HF. 1958. The nature of love. American Psychologist, 13(12): 673-685.
Harlow HF, Zimmermann RR. 1959. Affectional response in the infant monkey: orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science, 130(3373): 421-432. Harlow HF. 1962. The heterosexual affectional system in monkeys. American Psychologist, 17(1): 1-9.
Harlow HF, Harlow M. 1962. Social deprivation in monkeys. Scientific American, 207(5): 136-147.
Harlow HF, Rowland GL, Griffin GA. 1964. The effect of total social deprivation on the development of monkey behavior. Psychiatric Research Reports, 19: 116-135.
Harlow HF, Dodsworth RO, Harlow MK. 1965. Total social isolation in monkeys. Proceedings of the National Academy of Sciences of the United States of America, 54(1): 90-97.
Harlow HF, Harlow MK. 1965. The affectional systems. In: Schrier AJ, Harlow HF, Stollnitz F. Behavior of Nonhuman Primates. New York: Academic Press, 287-334.
Harlow HF, Joslyn WD, Senko MG, Dopp A. 1966. Behavioral aspects of reproduction in primates. Journal of Animal Science, 25(S): 49-65.
Harlow HF, Suomi SJ. 1971a. Production of depressive behaviors in young monkeys. Journal of Autism and Childhood Schizophrenia, 1(3): 246-255. Harlow HF, Suomi SJ. 1971b. Social recovery by isolation-reared monkeys. Proceedings of the National Academy of Sciences of the United States of America, 68(7): 1534-1538.
Hart H, Rubia K. 2012. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience, 6: 52.
Hart J G, Gunnar M, Cicchetti D. 1995. Salivary cortisol in maltreated children: evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology, 7(1): 11-26.
Heim C, Ehlert U, Hellhammer DH. 2000a. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25(1): 1-35.
Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. 2000b. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA, 284(5): 592-597.
Heim C, Nemeroff CB. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry, 49(12): 1023-1039.
Heinrichs SC, Menzaghi FE, Merlo Pich E, Britton KT, Koob GF. 1995. The role of CRF in behavioral aspects of stress. Annals of the New York Academy of Sciences, 771: 92-104.
Hennessy MB, Kaplan JN. 1982. Influence of the maternal surrogate on pituitary-adrenal activity and behavior of infant squirrel monkeys. Developmental Psychobiology, 15(5): 423-431.
Herman JP, Prewitt CMF, Cullinan WE. 1996. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews™ in Neurobiology, 10(3-4): 371-394.
Herman JP, Cullinan WE. 1997. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences, 20(2): 78-84.
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology, 24(3): 151-180.
Higley JD, Hasert MF, Suomi SJ, Linnoila M. 1991. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America, 88(16): 7261-7265.
Higley JD, Suomi SJ, Linnoila M. 1992. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry, 32(2): 127-145.
Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. 1996a. CSF testosterone and 5-
HIAA correlate with different types of aggressive behaviors. Biological Psychiatry, 40(11): 1067-1082.
Higley JD, Suomi SJ, Linnoila M. 1996b. A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism: Clinical & Experimental Research, 20(4): 643-650.
Hinde RA, Spencer-Booth Y, Bruce M. 1966. Effects of 6-day maternal deprivation on rhesus monkey infants. Nature, 210(5040): 1021-1023.
Hinde RA, Spencer-Booth Y. 1967. The behaviour of socially living rhesus monkeys in their first two and a half years. Animal Behaviour, 15(1): 169-196.
Hinde RA, McGinnis L. 1977. Some factors influencing the effects of temporary mother-infant separation: some experiments with rhesus monkeys. Psychological Medicine, 7(2): 197-212.
Hotchkiss CE, Paule MG. 2003. Effect of pair-housing on operant behavior task performance by rhesus monkeys. Journal of the American Association for Laboratory Animal Science, 42(4): 38-41.
Houston SM, Herting MM, Sowell ER. 2014. The neurobiology of childhood structural brain development: conception through adulthood. In: Andersen SL, Pine DS. The Neurobiology of Childhood: Current Topics in Behavioral Neurosciences. Berlin Heidelberg: Springer, 16: 3-17.
Howell BR, Grand AP, Mccormack KM, Shi YD, Laprarie JL, Maestripieri D, Styner MA, Sanchez MM. 2014. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Developmental Psychobiology, 56(8): 1735-1746.
Hoyer C, Richter H, Brandwein C, Riva MA, Gass P. 2013. Preconceptional paternal exposure to a single traumatic event affects postnatal growth of female but not male offspring. Neuroreport, 24(15): 856-860.
Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. 2006. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience, 26(17): 4638-4643.
Insel TR. 1992. Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology, 17(1): 3-35.
Jablonka E, Lamb MJ. 2002. The changing concept of epigenetics. Annals of the New York Academy of Sciences, 981: 82-96.
Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Perez O, Gonzalez-Castaneda R, Garcia-Estrada J, Luquin S. 2010. Responses of glial cells to stress and glucocorticoids. Current Immunology Reviews, 6(3): 195-204.
Javanbakht A, King AP, Evans GW, Swain JE, Angstadt M, Phan KL, Liberzon I. 2015. Childhood Poverty Predicts Adult Amygdala and Frontal Activity and Connectivity in Response to Emotional Faces. Frontiers in Behavioral Neuroscience, 9: 154.
Jawahar MC, Murgatroyd C, Harrison EL, Baune BT. 2015. Epigenetic alterations following early postnatal stress: a review on novel aetiological mechanisms of common psychiatric disorders. Clinical Epigenetics, 7: 122. Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, Toth SL, Thomas KM. 2015. Long-term consequences of childhood maltreatment: altered amygdala functional connectivity. Development and Psychopathology, 27(4Pt2): 1577-1589.
Johnson LR, Farb C, Morrison JH, McEwen BS, Ledoux JE. 2005. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience, 136(1): 289-299.
Jung-Testas I, Baulieu EE. 1998. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. The Journal of Steroid Biochemistry and Molecular Biology, 65(1-6): 243-251.
Kaemingk K, Reite M. 1987. Social environment and nocturnal sleep: studies in peer-reared monkeys. Sleep, 10(6): 542-550.
Kagan J, Reznick JS, Snidman N. 1988. Biological bases of childhood shyness. Science, 240(4849): 167-171.
Kaplan J. 1974. Growth and behavior of surrogate-reared squirrel monkeys. Developmental Psychobiology, 7(1): 7-13.
Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. 2009. What is an "adverse" environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biological Psychiatry, 65(9): 770-777.
Kaufman IC, Rosenblum LA. 1967. The reaction to separation in infant monkeys: anaclitic depression and conservation-withdrawal. Psychosomatic Medicine, 29(6): 648-675.
Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, Wells W, Ryan ND. 1997. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry, 42(8): 669-679.
Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. 2004. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America, 101(49): 17316-17321.
Kessler RC, Davis CG, Kendler KS. 1997. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine, 27(5): 1101-1119.
Kessler RC. 2003. Epidemiology of women and depression. Journal of Affective Disorders, 74(1): 5-13.
Kessler RC, Wang PS. 2008. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annual Review of Public Health, 29: 115-129.
Kikusui T, Mori Y. 2009. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocrinology, 21(4): 427-431.
Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL. 2013. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46): 18442-18447.
Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. 2006. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry, 11(10): 903-913.
Kinnally EL, Lyons LA, Abel K, Mendoza S, Capitanio JP. 2008. Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes, Brain and Behavior, 7(4): 481-486.
Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. 2010. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes, Brain and Behavior, 9(6): 575-582.
Kinnally EL, Capitanio JP. 2015. Paternal early experiences influence infant development through non-social mechanisms in Rhesus Macaques. Frontiers in Zoology, 12(S1): S14.
Kitayama N, Quinn S, Bremner JD. 2006. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of Affective Disorders, 90(2-3): 171-174.
Klein B, Gorter JW, Rosenbaum P. 2013. Diagnostic shortfalls in early childhood chronic stress: a review of the issues. Child: Care, Health and Development, 39(6): 765-771.
Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. 2001. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology, 13(3): 695-719.
Koford CB. 1963. Rank of mothers and sons in bands of rhesus monkeys. Science, 141(3578): 356-357.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. 2008. Central amygdala glucocorticoid receptor action promotes fearassociated CRH activation and conditioning. Proceedings of the National Academy of Sciences of the United States of America, 105(33): 12004-12009.
Korosi A, Naninck EFG, Oomen CA, Schouten M, Krugers H, Fitzsimons C, Lucassen PJ. 2012. Early-life stress mediated modulation of adult neurogenesis and behavior. Behavioural Brain Research, 227(2): 400-409. Koyama N. 1967. On dominance rank and kinship of a wild Japanese monkey troop in Arashiyama. Primates, 8(3): 189-216.
Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, Mcbreen M, Stranahan AM, Gould E. 2005. Experience induces structural and biochemical changes in the adult primate brain. Proceedings of the National Academy of Sciences of the United States of America, 102(48): 17478-17482.
Kraemer GW, McKinney WT. 1979. Interactions of pharmacological agents which alter biogenic amine metabolism and depression: an analysis of contributing factors within a primate model of depression. Journal of Affective Disorders, 1(1): 33-54.
Kraemer GW, Ebert MH, Lake CR, McKinney WT. 1983. Amphetamine challenge: effects in previously isolated rhesus monkeys and implications for animal models of schizophrenia. Progress in Clinical and Biological Research, 131: 199-218.
Kraemer GW, Ebert MH, Lake CR, McKinney WT. 1984. Hypersensitivity to d-amphetamine several years after early social deprivation in rhesus monkeys. Psychopharmacology, 82(3): 266-271.
Krohg K, Hageman I, Jørgensen MB. 2008. Corticotropin-releasing factor (CRF) in stress and disease: a review of literature and treatment perspectives with special emphasis on psychiatric disorders. Nordic Journal of Psychiatry, 62(1): 8-16.
Kumar S, Sachar K, Huber J, Weingarten DP, de Vellis J. 1985. Glucocorticoids regulate the transcription of glycerol phosphate dehydrogenase in cultured glial cells. The Journal of Biological Chemistry, 260(27): 14743-14747.
Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, Kreppner J, Rutter M, Sonuga-Barke E. 2010. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from the English and Romanian Adoptee (ERA) study. Journal of Child Psychology and Psychiatry, 51(7): 755-762.
Kwak HR, Lee JW, Kwon KJ, Park JI, Chun W, Kim SS, Lee HJ. 2008. Neuronal architecture of the hippocampus and the cerebral cortex in rats experiencing maternal social separation. Clinical Psychopharmacology and Neuroscience, 6(2): 65-70.
Ladd CO, Owens MJ, Nemeroff CB. 1996. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology, 137(4): 1212-1218.
Laudenslager ML, Boccia ML, Berger CL, Gennaro-Ruggles MM, McFerran B, Reite ML. 1995. Total cortisol, free cortisol, and growth hormone associated with brief social separation experiences in young macaques. Developmental Psychobiology, 28(4): 199-211.
Law AJ, Pei Q, Feldon J, Pryce CR, Harrison PJ. 2009a. Gene expression in the anterior cingulate cortex and amygdala of adolescent marmoset monkeys following parental separations in infancy. The International Journal of Neuropsychopharmacology, 12(6): 761-772.
Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Pryce CR, Harrison PJ. 2009b. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology, 34(6): 1381-1394.
LeDoux J. 2003. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology, 23(4-5): 727-738.
Leliavski A, Dumbell R, Ott V, Oster H. 2015. Adrenal clocks and the role ofadrenal hormones in the regulation of circadian physiology. Journal of Biological Rhythms, 30(1): 20-34.
Lenroot RK, Giedd JN. 2006. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30(6): 718-729.
Leventopoulos M, Rüedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. 2007. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Research, 1142: 119-126.
Levine S, Wiener SG. 1988. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology, 13(1-2): 143-154.
Levine S. 2000. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. European Journal of Pharmacology, 405(1-3): 149-160.
Levine S, Mody T. 2003. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neuroscience & Biobehavioral Reviews, 27(1-2): 83-89.
Lim MM, Young LJ. 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior, 50(4): 506-517.
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277(5332): 1659-1662.
Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. 2000. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. Journal of Neuroendocrinology, 12(1): 5-12.
Loman MM, Gunnar MR. 2010. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews, 34(6): 867-876.
Lovejoy CO. 1981. The origin of man. Science, 211(4480): 341-350.
Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. 2013. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12): 1135-1142.
Lukkes JL, Burke AR, Zelin NS, Hale MW, Lowry CA. 2012. Post-weaning social isolation attenuates c-Fos expression in GABAergic interneurons in the basolateral amygdala of adult female rats. Physiology & Behavior, 107(5): 719-725.
Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA. 2013. Development × environment interactions control tph2 mRNA expression. Neuroscience, 237: 139-150.
Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. 2007. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain and Cognition, 65(3): 209-237.
Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6): 434-445.
Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Séguin JR. 2011. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America, 108(34): 14324-14329.
Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. American Journal of Primatology, 60(1): 1-15.
Lutz C, Tiefenbacher S, Meyer J, Novak M. 2004. Extinction deficits in male rhesus macaques with a history of self-injurious behavior. American Journal of Primatology, 63(2): 41-48.
Lutz CK, Novak MA. 2005. Environmental enrichment for nonhuman primates: theory and application. ILAR Journal, 46(2): 178-191.
Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. 2007. Early predictors of selfbiting in socially-housed rhesus macaques (Macaca mulatta). American Journal of Primatology, 69(5): 584-590.
Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. 2000. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. Journal of Neuroendocrinology, 12(8): 723-728.
Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, Teicher MH. 2016. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behavioural Brain Research, 308: 83-93.
Maestripieri D, Wallen K, Carroll KA. 1997. Infant abuse runs in families of group-living pigtail macaques. Child Abuse & Neglect, 21(5): 465-471.
Maestripieri D, Carroll KA. 1998. Risk factors for infant abuse and neglect in group-living rhesus monkeys. Psychological Science, 9(2): 143-145.
Maestripieri D. 2005. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America, 102(27): 9726-9729.
Maestripieri D, Mccormack K, Lindell SG, Higley JD, Sanchez MM. 2006. Influence of parenting style on the offspring's behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behavioural Brain Research, 175(1): 90-95.
Magariños AM, McEwen BS, Flügge G, Fuchs E. 1996. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. Journal of Neuroscience, 16(10): 3534-3540.
Majer M, Nater UM, Lin JMS, Capuron L, Reeves WC. 2010. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurology, 10: 61.
Manns JR, Eichenbaum H. 2006. Evolution of declarative memory. Hippocampus, 16(9): 795-808.
Martin LJ, Spicer DM, Lewis MH, Gluck JP, Cork LC. 1991. Social deprivation of infant rhesus monkeys alters the chemoarchitecture of the brain: I. Subcortical regions. Journal of Neuroscience, 11(11): 3344-3358. Mason WA, Sponholz RR. 1963. Behavior of Rhesus Monkeys Raised in Isolation. Journal of Psychiatric Research, 1(4): 299-306.
Mason WA. 1968. Early social deprivation in the nonhuman primates: implications for human behavior. In: Glass DS. Environmental Influences. New York: Rockefeller University Press, 70-100.
Mason WA, Berkson G. 1975. Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys: a study with artificial mothers. Developmental Psychobiology, 8(3): 197-211.
Maughan B, McCarthy G. 1997. Childhood adversities and psychosocial disorders. British Medical Bulletin, 53(1): 156-169.
McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. 2009. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior,55(4): 538-547.
McCrory E, De Brito SA, Viding E. 2011. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers in Psychiatry, 2: 48.
McEwen BS. 1999. Stress and hippocampal plasticity. Annual Review of Neuroscience, 22: 105-122.
McEwen BS. 2003. Early life influences on life-long patterns of behavior and health. Mental Retardation and Developmental Disabilities Research Reviews, 9(3): 149-154.
Meaney MJ. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience, 24: 1161-1192.
Meaney MJ, Szyf M. 2005. Maternal care as a model for experiencedependent chromatin plasticity? Trends in Neurosciences, 28(9): 456-463. Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. 1995. Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. The American Journal of Psychiatry, 152(6): 907-913.
Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, Sonuga-Barke EJS. 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, 50(8): 943-951.
Meyer JS, Novak MA, Bowman RE, Harlow HF. 1975. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Developmental Psychobiology, 8(5): 425-435.
Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. 2009. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America, 106(34): 14716-14721.
Miller RE, Mirsky IA, Caul WF, Sakata T. 1969. Hyperphagia and polydipsia in socially isolated rhesus monkeys. Science, 165(3897): 1027-1028.
Miller-Butterworth CM, Kaplan JR, Barmada MM, Manuck SB, Ferrell RE. 2007. The serotonin transporter: sequence variation in Macaca fascicularis and its relationship to dominance. Behavior Genetics, 37(5): 678-696.
Miller-Butterworth CM, Kaplan JR, Shaffer J, Devlin B, Manuck SB, Ferrell RE. 2008. Sequence variation in the primate dopamine transporter gene and its relationship to social dominance. Molecular Biology and Evolution, 25(1): 18-28.
Miska EA, Ferguson-Smith AC. 2016. Transgenerational inheritance: models and mechanisms of non-DNA sequence-based inheritance. Science, 354(6308): 59-63.
Mitchell GD. 1968. Persistent behavior pathology in rhesus monkeys following early social isolation. Folia Primatologica, 8(2): 132-147.
Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge JZ, Schroeder CM, van Hoesen GW. 2007. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. The Journal of Comparative Neurology, 500(1): 134-165.
Morey RA, Haswell CC, Hooper SR, De Bellis MD. 2016. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology, 41(3): 791-801.
Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. 2000. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. American Journal of Primatology, 52(3): 115-131.
Musholt K, Cirillo G, Cavaliere C, Rosaria Bianco M, Bock J, Helmeke C, Braun K, Papa M. 2009. Neonatal separation stress reduces glial fibrillary acidic protein- and S100β-immunoreactive astrocytes in the rat medial precentral cortex. Developmental Neurobiology, 69(4): 203-211.
Nagy C, Turecki G. 2015. Transgenerational epigenetic inheritance: an open discussion. Epigenomics, 7(5): 781-790.
Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, Delaney D, Ernst M, Fox NA, Suomi SJ, Winslow JT, Pine DS. 2009. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biological Psychiatry, 66(7): 702-704.
Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. 2005. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biological Psychiatry, 57(2): 167-172.
Newton AW, Vandeven AM. 2009. Update on child maltreatment. Current Opinion in Pediatrics, 21(2): 252-261.
Newton AW, Vandeven AM. 2010. Child abuse and neglect: a worldwide concern. Current Opinion in Pediatrics, 22(2): 226-233.
Nichols NR, Agolley D, Zieba M, Bye N. 2005. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Research Reviews, 48(2): 287-301.
Novak MA, Suomi SJ. 1991. Social interaction in nonhuman primates: an underlying theme for primate research. Laboratory Animal Science, 41(4): 308-314.
Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. 1998. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. American Journal of Primatology, 46(3): 213-227.
Novak MFSX, Sackett GP. 1997. Pair-rearing infant monkeys (Macaca nemestrina) using a "rotating-peer" strategy. American Journal of Primatology, 41(2): 141-149.
O'Callaghan JP, Brinton RE, McEwen BS. 1989. Glucocorticoids regulate the concentration of glial fibrillary acidic protein throughout the brain. Brain Research, 494(1): 159-161.
Ohmura Y, Yoshioka M. 2009. The roles of corticotropin releasing factor (CRF) in responses to emotional stress: is CRF release a cause or result of fear/anxiety? CNS & Neurological Disorders - Drug Targets, 8(6): 459-469. Öngür D, Drevets WC, Price JL. 1998. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America, 95(22): 13290-13295.
Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. 2008. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience, 156(4): 1103-1110.
Oomen CA, Girardi CEN, Cahyadi R, Verbeek EC, Krugers H, Joëls M, Lucassen PJ. 2009. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One, 4(1): e3675.
Opitz B. 2014. Memory function and the hippocampus. Frontiers of Neurology and Neuroscience, 34: 51-59.
Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu SJ, Brasic JR. 2014. History of childhood adversity is positively associated with ventral striataldopamine responses to amphetamine. Psychopharmacology, 231(12): 2417-2433.
Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. 2004. Prospective investigation of stress inoculation in young monkeys. Archives of General Psychiatry, 61(9): 933-941.
Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. 2005. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biological Psychiatry, 57(8): 848-855.
Parker KJ, Buckmaster CL, Lindley SE, Schatzberg AF, Lyons DM. 2012. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. International Journal of Behavioral Development, 36(1), doi: 10.1177/0165025411406864.
Payne C, Machado CJ, Bliwise NG, Bachevalier J. 2010. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus, 20(8): 922-935
Pesonen AK, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DIW, Barker DJP, Eriksson JG, Kajantie E. 2010. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology, 35(5): 758-767.
Phelps EA. 2006. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology, 57: 27-53.
Pirkola S, Isometsä E, Aro H, Kestilä L, Hämäläinen J, Veijola J, Kiviruusu O, Lönnqvist J. 2005. Childhood adversities as risk factors for adult mental disorders: results from the Health 2000 study. Social Psychiatry and Psychiatric Epidemiology, 40(10): 769-777.
Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. 2005. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology, 30(12): 2192-2204.
Provençal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, Bennett AJ, Pierre PJ, Friedman DP, Côté SM, Hallett M, Tremblay RE, Suomi SJ, Szyf M. 2012. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. Journal of Neuroscience, 32(44): 15626-15642.
Provençal N, Booij L, Tremblay RE. 2015. The developmental origins of chronic physical aggression: biological pathways triggered by early life adversity. The Journal of Experimental Biology, 218(Pt 1): 123-133.
Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. 2004a. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Annals of the New York Academy of Sciences, 1032: 245-249.
Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. 2004b. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biological Psychiatry, 56(2): 72-79. Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Rélo A. 2005a. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. European Journal of Neuroscience, 21(6): 1521-1535.
Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. 2005b. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neuroscience & Biobehavioral Reviews, 29(4-5): 649-674.
Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. 2011. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology, 214(1): 33-53.
Raleigh MJ, Brammer GL, McGuire MT. 1983. Male dominance, serotonergic systems, and the behavioral and physiological effects of drugs in vervet monkeys (Cercopithecus aethiops sabaeus). Progress in Clinical and Biological Research, 131: 185-197.
Raleigh MJ, Brammer GL, McGuire MT, Yuwiler A. 1985. Dominant social status facilitates the behavioral effects of serotonergic agonists. Brain Research, 348(2): 274-282.
Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. 1991. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Research, 559(2): 181-190.
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry, 47(9): 769-776.
Reite M, Short RA. 1978. Nocturnal sleep in separated monkey infants. Archives of General Psychiatry, 35(10): 1247-1253.
Reite M, Kaufman CI, Pauley DJ, Stynes AJ. 1974. Depression in infant monkeys: physiological correlates. Psychosomatic Medicine, 36(4): 363-367.
Richards JE, Xie WZ. 2015. Brains for all the ages: structural neurodevelopment in infants and children from a life-span perspective. Advances in Child Development and Behavior, 48: 1-52.
Richert KA, Carrion VG, Karchemskiy A, Reiss AL. 2006. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depression and Anxiety, 23(1): 17-25.
Rilling JK, Winslow JT, O'brien D, Gutman DA, Hoffman JM, Kilts CD. 2001. Neural correlates of maternal separation in rhesus monkeys. Biological Psychiatry, 49(2): 146-157.
Rinne-Albers MAW, van der Werff SJA, van Hoof MJ, van Lang ND, Lamers-Winkelman F, Rombouts SA, Vermeiren RRJM, van der Wee NJA. 2016. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: a DTI study. European Child & Adolescent Psychiatry, 25(8): 869-878.
Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. 2002. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular Psychiatry, 7(6): 609-616.
Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. Journal of Neuroscience, 33(21): 9003-9012.
Romens SE, Casement MD, McAloon R, Keenan K, Hipwell AE, Guyer AE, Forbes EE. 2015. Adolescent girls' neural response to reward mediates the relation between childhood financial disadvantage and depression. Journal of Child Psychology and Psychiatry, 56(11): 1177-1184.
Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. 2009a. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science, 12(1): 61-72. Rommeck I, Gottlieb DH, Strand SC, McCowan B. 2009b. The effects of four nursery rearing strategies on infant behavioral development in rhesusmacaques (Macaca mulatta). Journal of the American Association for Laboratory Animal Science, 48(4): 395-401.
Rommeck I, Capitanio JP, Strand SC, McCowan B. 2011. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta). American Journal of Primatology, 73(7): 692-701.
Rosenblum LA, Paully GS. 1984. The effects of varying environmental demands on maternal and infant behavior. Child Development, 55(1): 305-314.
Rosenblum LA, Andrews MW. 1994. Influences of environmental demand on maternal behavior and infant development. Acta Paediatrica, 83(S397): 57-63.
Rosenblum LA, Coplan JD, Friedman S, Bassoff T, Gorman JM, Andrews MW. 1994. Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biological Psychiatry, 35(4): 221-227.
Roustit C, Renahy E, Guernec G, Lesieur S, Parizot I, Chauvin P. 2009. Exposure to interparental violence and psychosocial maladjustment in the adult life course: advocacy for early prevention. Journal of Epidemiology & Community Health, 63(7): 563-568.
Roy MA, Wolf RH, Martin LN, Rangan SR, Allen WP. 1978. Social and reproductive behaviors in surrogate-reared squirrel monkeys (Saimiri sciureus). Laboratory Animal Science, 28(4): 417-421.
Rüedi-Bettschen D, Zhang WN, Russig H, Ferger B, Weston A, Pedersen EM, Feldon J, Pryce CR. 2006. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. European Journal of Neuroscience, 24(10): 2879-2893.
Ruppenthal GC, Walker CG, Sackett GP. 1991. Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages. American Journal of Primatology, 25(2): 103-113.
Rutter M. 2007. Resilience, competence, and coping. Child Abuse & Neglect, 31(3): 205-209.
Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. 2007. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience, 27(12): 3295-3304.
Sackett GP. 1967. Some persistent effects of different rearing conditions on preadult social behavior of monkeys. Journal of Comparative and Physiological Psychology, 64(2): 363-365.
Sackett GP. 1969. The persistence of abnormal behaviour in monkeys following isolation rearing. International Psychiatry Clinics, 6(1): 3-37.
Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. 1998. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research, 812(1-2): 38-49.
Sánchez MM, Young LJ, Plotsky PM, Insel TR. 2000. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience, 20(12): 4657-4668.
Sánchez MM, Ladd CO, Plotsky PM. 2001. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology, 13(3): 419-449. Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. 2005. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry, 57(4): 373-381.
Sanchez MM. 2006. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones and Behavior, 50(4): 623-631.
Sapolsky RM, Krey LC, McEwen BS. 1985. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. Journal of Neuroscience, 5(5): 1222-1227.
Schneider ML, Suomi SJ. 1992. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavior and Development, 15(2): 155-177.
Schweizer S, Walsh ND, Stretton J, Dunn VJ, Goodyer IM, Dalgleish T. 2016. Enhanced emotion regulation capacity and its neural substrates in those exposed to moderate childhood adversity. Social Cognitive and Affective Neuroscience, 11(2): 272-281.
Seay B, Hansen E, Harlow HF. 1962. Mother-infant separation in monkeys. Journal of Child Psychology and Psychiatry, 3(3-4): 123-132.
Seay B, Alexander BK, Harlow HF. 1964. Maternal behavior of socially deprived Rhesus monkeys. The Journal of Abnormal and Social Psychology, 69(4): 345-354.
Shannon C, Champoux M, Suomi SJ. 1998. Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology, 46(4): 311-321.
Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. 2005. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. American Journal of Psychiatry, 162(9): 1658-1664.
Sheridan M, Drury S, McLaughlin K, Almas A. 2010. Early institutionalization: neurobiological consequences and genetic modifiers. Neuropsychology Review, 20(4): 414-429.
Shishkina GT, Kalinina TS, Bulygina VV, Lanshakov DA, Babluk EV, Dygalo NN. 2015. Anti-apoptotic protein Bcl-xL expression in the midbrain raphe region is sensitive to stress and glucocorticoids. PLoS One, 10(12): e0143978.
Shohamy D, Turk-Browne NB. 2013. Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology. General, 142(4): 1159-1170.
Siegel SJ, Ginsberg SD, Hof PR, Foote SL, Young WG, Kraemer GW, Mckinney WT, Morrison JH. 1993. Effects of social deprivation in prepubescent rhesus monkeys: immunohistochemical analysis of the neurofilament protein triplet in the hippocampal formation. Brain Research, 619(1-2): 299-305.
Singh M, D’Souza L, Singh M. 1992. Hierarchy, kinship and social interaction among Japanese monkeys (Macaca fuscata). Journal of Biosciences, 17(1): 15-27.
Smagin GN, Dunn AJ. 2000. The role of CRF receptor subtypes in stressinduced behavioural responses. European Journal of Pharmacology, 405(1-3): 199-206.
Spencer-Booth Y, Hinde RA. 1971. The effects of 13 days maternal separation on infant rhesus monkeys compared with those of shorter and repeated separations. Animal Behaviour, 19(3): 595-605.
Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ,Higley JD, Goldman D, Barr CS. 2007. Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Development and Psychopathology, 19(4): 977-987.
Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. 2009. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry, 66(6): 658-665.
Sprague DS. 1998. Age, dominance rank, natal status, and tenure among male macaques. American Journal of Physical Anthropology, 105(4): 511-521.
Stavisky RC, Adams MR, Watson SL, Kaplan JR. 2001. Dominance, cortisol, and behavior in small groups of female cynomolgus monkeys (Macaca fascicularis). Hormones and Behavior, 39(3): 232-238.
Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. 1997. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine, 27(4): 951-959.
Stell M, Riesen A. 1987. Effects of early environments on monkey cortex neuroanatomical changes following somatomotor experience: effects on layer III pyramidal cells in monkey cortex. Behavioral Neuroscience, 101(3): 341-346.
Struble RG, Riesen AH. 1978. Changes in cortical dendritic branching subsequent to partial social isolation in stumptailed monkeys. Developmental Psychobiology, 11(5): 479-486.
Suomi SJ, Harlow HF, Kimball SD. 1971. Behavioral effects of prolonged partial social isolation in the rhesus monkey. Psychological Reports, 29(3S): 1171-1177.
Suomi SJ. 1973. Surrogate rehabilitation of monkeys reared in total social isolation. Journal of Child Psychology and Psychiatry, 14(1): 71-77.
Suomi SJ. 1978. Maternal behavior by socially incompetent monkeys: neglect and abuse of offspring. Journal of Pediatric Psychology, 3(1): 28-34. Suomi SJ, Harlow HF, Novak MA. 1974. Reversal of social deficits produced by isolation rearing in monkeys. Journal of Human Evolution, 3(6): 527-534.
Suomi SJ, Mineka S, DeLizio RD. 1983. Short- and long-term effects of repetitive mother-infant separations on social development in rhesus monkeys. Developmental Psychology, 19(5): 770-786.
Suomi SJ, Ripp C. 1983. A history of mother-less mother monkey mothering at the University of Wisconsin Primate Laboratory. In: Reite M, Caine N. Child Abuse: The Nonhuman Primate Data. New York: Alan R Liss, 50-78.
Suomi SJ. 1991. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Foundation Symposium, 156: 171-183; discussion 183-188. (请核对页码)
Suomi SJ. 1997. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin, 53(1): 170-184.
Suomi SJ. 2005. Mother–infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Human Development, 48(1-2): 67-79.
Takahashi H. 2002. Changes of dominance rank, age, and tenure of wild Japanese macaque males in the kinkazan a troop during seven years. Primates, 43(2): 133-138.
Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. 1997. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences, 821: 160-175.
Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. 2004. Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry, 56(2): 80-85.
Thomaes K, Dorrepaal E, Draijer N, De Ruiter MB, Van Balkom AJ, Smit JH, Veltman DJ. 2010. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. The Journal of Clinical Psychiatry, 71(12): 1636-1644.
Thomas BW, Champoux M, Suomi SJ, Gunnar MR. 1995. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Developmental Psychobiology, 28(5): 257-267.
Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. 2009. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage, 47(S2): T66-T71.
Tottenham N, Sheridan MA. 2009. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3: 68.
Tottenham N, Hare TA, Quinn BT, Mccarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1): 46-61.
Treadway MT, Grant MM, Ding ZH, Hollon SD, Gore JC, Shelton RC. 2009. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One, 4(3): e4887.
Tsang AH, Barclay JL, Oster H. 2014. Interactions between endocrine and circadian systems. Journal of Molecular Endocrinology, 52(1): R1-16.
Tsang AH, Astiz M, Friedrichs M, Oster H. 2016. Endocrine regulation of circadian physiology. Journal of Endocrinology, 230(1): R1-R11.
Uylings HBM, van Eden CG. 1991. Qualitative and quantitative comparison of the prefrontal cortex in rat and in Primates, including humans. Progress in Brain Research, 85: 31-62.
Vaiserman A. 2015a. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clinical Epigenetics, 7: 96.
Vaiserman AM. 2015b. Epigenetic programming by early-life stress: evidence from human populations. Developmental Dynamics, 244(3): 254-265.
van Haarst AD, Oitzl MS, de Kloet ER. 1997. Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochemical Research, 22(11): 1323-1328.
van Harmelen AL, van Tol MJ, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BWJH, Elzinga BM. 2010. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry, 68(9): 832-838.
Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, López JF, Levine S. 2006. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Research, 1121(1): 83-94. Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. 2006. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience, 140(1): 355-365.
Vielkind U, Walencewicz A, Levine JM, Bohn MC. 1990. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes.Journal of Neuroscience Research, 27(3): 360-373.
Volavka J, Bilder R, Nolan K. 2004. Catecholamines and aggression: the role of COMT and MAO polymorphisms. Annals of the New York Academy of Sciences, 1036: 393-398.
Wakefield CL, Levine MS. 1985. Early postnatal development of basolateral amygdala in kitten: a Golgi morphometric analysis. Brain Research Bulletin, 14(2): 159-167.
Wall VL, Fischer EK, Bland ST. 2012. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiology & Behavior, 107(3): 440-450.
Wallen K, Goldfoot DA, Goy RW. 1981. Peer and maternal influences on the expression of foot-clasp mounting by juvenile male rhesus monkeys. Developmental Psychobiology, 14(4): 299-309.
Wang X, Xie H, Cotton AS, Duval ER, Tamburrino MB, Brickman KR, Elhai JD, Ho SS, McLean SA, Ferguson EJ, Liberzon I. 2016. Preliminary Study of Acute Changes in Emotion Processing in Trauma Survivors with PTSD Symptoms. PLoS One, 11(7): e0159065.
Washburn DA, Rumbaugh DM. 1991. Impaired performance from brief social isolation of rhesus monkeys (Macaca mulatta): a multiple video-task assessment. Journal of Comparative Psychology, 105(2): 145-151.
Watamura SE, Donzella B, Kertes DA, Gunnar MR. 2004. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Developmental Psychobiology, 45(3): 125-133. Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8): 847-854.
Weaver ICG. 2007. Epigenetic programming by maternal behavior and pharmacological intervention Nature versus nurture: let's call the whole thing off. Epigenetics, 2(1): 22-28.
White SF, Costanzo ME, Blair JR, Roy MJ. 2015. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage: Clinical, 7: 19-27.
Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. 2003. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology, 28(5): 910-918.
Woon FL, Hedges DW. 2008. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus, 18(8): 729-736.
Worlein JM, Sackett GP. 1997. Social development in nursery-reared pigtailed macaques (Macaca nemestrina). American Journal of Primatology, 41(1): 23-35.
Worlein JM. 2014. Nonhuman primate models of depression: effects of early experience and stress. ILAR Journal, 55(2): 259-273.
Wright JC. 1983. The effects of differential rearing on exploratory behavior in puppies. Applied Animal Ethology, 10(1-2): 27-34.
Wright PC. 1990. Patterns of paternal care in primates. International Journal of Primatology, 11(2): 89-102.
Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. 2003. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America, 100(15): 9039-9043.
Yuan TF, Li A, Sun X, Ouyang H, Campos C, Rocha NBF, Arias-Carrión O, Machado S, Hou GL, So KF. 2016. Transgenerational inheritance of paternal neurobehavioral phenotypes: stress, addiction, ageing and metabolism. Molecular Neurobiology, 53(9): 6367-6376.
Zarrow MX, Philpott JE, Denenberg VH. 1970. Passage of14C-4-corticosterone from the rat mother to the foetus and neonate. Nature, 226(5250): 1058-1059.
Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. 2006. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology, 73(1): 72-89.
Zhang TY, Meaney MJ. 2010. Epigenetics and the environmental regulation of the genome and its function. Annual Review of Psychology, 61: 439-466. Zheng J, Xiao XH, Zhang Q, Yu M. 2014. DNA methylation: the pivotal interaction between early-life nutrition and glucose metabolism in later life. British Journal of Nutrition, 112(11): 1850-1857.
Ziabreva I, Schnabel R, Braun K. 2000. Parental deprivation induces N-methyl-D-aspartate-receptor upregulation in limbic brain areas of Octodon degus: protective role of the maternal call. Neural Plasticity, 7(4): 233-244. Ziabreva I, Poeggel G, Schnabel R, Braun K. 2003a. Separation-induced receptor changes in the hippocampus and amygdala of Octodon degus: influence of maternal vocalizations. Journal of Neuroscience, 23(12): 5329-5336.
Ziabreva I, Schnabel R, Poeggel G, Braun K. 2003b. Mother's voice "buffers" separation-induced receptor changes in the prefrontal cortex of octodon degus. Neuroscience, 119(2): 433-441.
Received: 01 December 2016; Accepted: 30 December 2016
Foundation items: This research was supported by Hainan special fund project for science and technology (KJHZ2015-20)
*Corresponding author, E-mail: bozhangpp@foxmail.com
10.13918/j.issn.2095-8137.2017.002
- Zoological Research的其它文章
- New Year Address of Zoological Research
- In Memory of Academician Er-Mi Zhao (1930-2016)
- Does mRNA structure contain genetic information for regulating co-translational protein folding?
- Expression of pIgR in the tracheal mucosa of SHIV/SIV-infected rhesus macaques
- Nest survival rate of Reeves’s pheasant (Syrmaticus reevesii) based on artificial nest experiments
- Engrafted newborn neurons could functionally integrate into the host neuronal network

