State-of-art modelling of in fl ammatory astrocyte-synapse interactions in injury and amyotrophic lateral sclerosis
State-of-art modelling of in fl ammatory astrocyte-synapse interactions in injury and amyotrophic lateral sclerosis
Different outcomes of astrocyte inflammatory signalling in injury and neurodegeneration: It is emerging that astrocytes have a signi fi cant impact on the neuronal network by modulating synaptic connections and neuronal viability in both normal and pathological states.is provides a novel insight into pathomech‐anistic discoveries and therapeutics. It has been proposed that activation of the innate immune system and concomitant astro‐cytic and microglial in fl ammatory responses are contributors to synapse and neuronal dysfunction in neurodegenerative disorders (Heneka et al., 2014). On the contrary, current studies (Ander‐son et al., 2016) and our own work (Tyzack et al., 2014) indicate that astrocytic response in moderate traumatic injury induced in fl ammation promotes neuronal viability and synaptic recovery. This poses the question how beneficial and harmful aspects of in fl ammatory astrocyte signaling are altered in neurodegenerative diseases. Another central issue is how this e ff ects synaptic connec‐tions then, which is an emerging theme in early pathogenesis in neurodegenerative conditions. Do astrocytes exacerbate synaptic dysfunction or impair plasticity? Discovery in this fi eld had been hampered by the lack of cell‐type speci fi c experimental methods and human disease models suitable for exploring precise molec‐ular events. Recent advances now provide an unprecedented op‐portunity to single out astrocyte mediated mechanisms relevant to degeneration or recovery of neuronal networks.
Astrocyte mediated synaptic plasticity in traumatic injury-induced inflammation: The recently developed “RiboTag”mouse system has brought a promising new prospective in providing a molecular profile of individual cell‐types in ani‐mal models.is allows the pull‐down of mRNAs by cell‐type speci fi c ribosomal tags, helping to de fi ne translational changes in communicating cells from whole tissue samples. Using this approach in combination with astrocyte‐specific gene manip‐ulation, a breakthrough study by the Sofroniew group has re‐versed the long‐standing negative view that astrocytes activated by in fl ammatory cues impede repair in injury (Anderson et al., 2016).ey have also elegantly proven that astrocytes require in fl ammatory pathway activation through signal transducer and activator of transcription‐3 (STAT3) to promote the regrowth of axonal terminals in moderate spinal cord damage. Our own studies (Tyzack et al., 2014) have revealed that this same path‐way is required for the recovery of central axon terminals onto motor neurons (MNs) as part of the re‐arrangement of viable neuronal networks. In particular, we have shown a novel mech‐anism of structural synaptic plasticity, which is governed by STAT3 dependent re‐expression and release of a synaptogenic molecule, thrombospondin‐1 by astrocytes (Tyzack et al., 2014). Increasing evidence suggest that not only structural, but func‐tional plasticity is also dependent on the astrocytic in fl amma‐tory response. Under normal conditions, astrocytes in accord with microglia balance synaptic and neuronal excitationviaglutamate, adenosine, D‐serine release, glutamate and extracel‐lular potassium concentration and the postsynaptic glutamate receptor subunit composition (Haydon and Nedergaard, 2014). One of the most studied inflammatory synapse modulationviaastrocytes is mediated by tumor necrosis factor (TNF)‐α.is facilitates excitatory synaptic transmission by inducing the traffic of α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid‐receptors (AMPAR) to the postsynaptic cell membrane and also by astrocytic release of glutamate that potentiates presynaptic activityviatheir N‐methyl‐D‐aspartate receptors (NMDAR) (Habbas et al., 2015). While it is now clear that bal‐anced synaptic excitation facilitates neuronal survival, it is less understood how astrocyte mediated responses limit detrimental neuronal overactivation in the inflammatory processes of re‐generation. Functional compensatory mechanisms dependent on increased astrocytic glutamate uptake or initial structural synapse removal (stripping) have been long proposed as pro‐tective events following neuronal injury. Recent advances show that synapse stripping involves pro‐in fl ammatory astrocyte and microglial activation, involving a complement (C1q) mediated process (Stevens et al., 2007). It transpires that synaptic recovery involves di ff erent glial in fl ammatory pathways, though this still not adequately explored. Our recent study indicates that STAT3 signaling during in fl ammation is not responsible for synapse re‐moval but instead shis the balance towards a recovery process (Tyzack et al., 2014). Understanding the triggers and master regulators in this injury‐related astrocyte response may there‐fore bring us closer to understand how to modify glial in fl am‐mation so as to restore the neuronal network in neurotrauma and other neurological diseases (Figure 1).
Inflammatory mediators in amyotrophic lateral sclerosis (ALS) and perturbed astrocyte-synapse interactions: ALS is a rapidly progressive and fatal neurological disease with es‐sentially no e ff ective treatment, which a ff ects motor and other neuronal populations and their synapses, leading to muscle weakness and to a variable degree of cognitive dysfunction. It appears that apart from intrinsic damage to MNs, non‐cell autonomous processes, such as in fl ammation are conducive to neuronal degeneration. But it is less clear how these processes a ff ect synaptic input onto MNs, which is known to be a ff ected from the early stages in ALS.is is of immense relevance as the balance of neuronal excitation in fl uences neuronal viability in neurodegeneration. Limited evidence suggests that there is an overall loss of synaptic input with some degree of potential plasticity and overactivation of surviving neurons (Matsumoto et al., 1994).e latter may have a synergistic e ff ect on motor neuron (MN) activation along with the increased extrasynaptic excitation and intrinsic hyperexcitability (Wainger et al., 2014). Apart from microglia, astrocytes are most likely to contribute to this process in light of their intimate communication with synapses and their involvement in inflammatory activation. An elegant study using cell‐speci fi c translational pro fi ling in a mouse ALS model has directly demonstrated increased pro‐in‐fl ammatory signaling in astrocytes from an early disease stage (Sun et al., 2015). In particular, nuclear factor (NF)‐kappa B (κB) appears to be a master regulator in ALS related in fl amma‐tion, featuring in neuronal pathology (Ikiz et al., 2015), which points to the importance of interleukin (IL)‐6 and TNF‐α. These mediators signal through the NF‐κB pathway and are increased in the brains and spinal cords of patients. Experi‐mental examples from traumatic CNS injuries imply that they would lead to astrocyte mediated synapse overactivation and progressive stripping in ALS. Indeed, there is now evidence that pro‐inflammatory mediators through NF‐κB exacerbate MN overactivation. Then hyperexcitability may lead to ER stress (Wainger et al., 2014) and to other degeneration‐pro‐moting pathways.is raises an important and therapeutically relevant question: why is in fl ammatory signaling is over‐rep‐resented in astrocytes in ALS? Is it predominantly an extrinsic in fl uence on astrocytes or do they lose their compensatory or anti‐in fl ammatory properties observed in the recovery phase of traumatic MN injuries?e answers may certainly guide us towards identifying more sophisticated targets for anti‐in fl am‐matory therapies that have been promising in animal models but have so far failed in human clinical trials.us there is an urgent need to integrate data fromin vivomouse models andin vitrohuman patient derived induced pluripotent stem cell‐based disease systems to allow more precise elucidations for the role of astrocytes in synaptic and neuronal dysfunction.
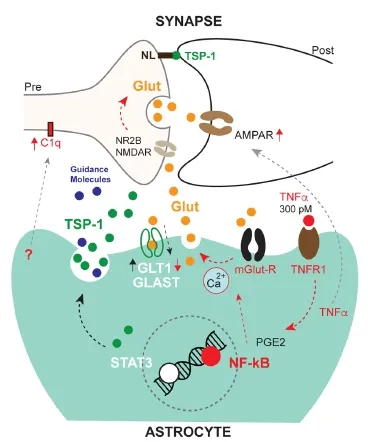
Figure 1 E ff ects of astrocyte mediated in fl ammatory responses on synapses.
Conclusion and future perspective: Restorative neuroscience has been for long borrowing ideas from injury systems char‐acterized by regenerative events. Now with the development of state‐of‐art tools providing high‐throughput cell‐type spe‐cific molecular data, master regulators of glial inflammatory response may be more precisely dissected in this paradigm. Embarking on this path, it should be further addressed what aspects of detrimental or beneficial inflammatory signalling are dysregulated in ALS, which could a ff ect the neuronal net‐work.e use of cell‐type speci fi c screening approaches in both mouse and human models now may help pathway speci fi c revi‐sions of anti‐in fl ammatory strategies in ALS.
András Lakatos*
John van Geest Centre for Brain Repair, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom; Department of Neurology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Trust, Cambridge, United Kingdom
*Correspondence to: András Lakatos, M.D., Ph.D., AL291@cam.ac.uk.
Accepted:2017-01-18
orcid: 0000-0002-1301-2292
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew M V (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195‐200.
Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, Suter T, Volterra A (2015) Neu‐roinflammatory TNFα impairs memory via astrocyte signaling. Cell 163:1730‐1741.
Haydon PG, Nedergaard M (2014) How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol 7:a020438.
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neuro‐degenerative disease. Nat Rev Immunol 14:463‐477.
Ikiz B, Alvarez MJ, Ré DB, Le Verche V, Politi K, Lotti F, Phani S, Pradhan R, Yu C, CroGF, Jacquier A, Henderson CE, Califano A, Przedborski S (2015)e regulatory machinery of neurodegeneration in in vitro models of amyotrophic lateral sclerosis. Cell Rep 12:1‐11.
Matsumoto S, Goto S, Kusaka H, Ito H, Imai T (1994) Synaptic pathology of spinal anterior horn cells in amyotrophic lateral sclerosis: an immunohis‐tochemical study. J Neurol Sci 125:180‐185.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Sta ff ord B, Sher A, Litke AM, Lambris JD, Smith SJ, John SWM, Barres BA (2007)e classical complement cascade mediates CNS synapse elimination. Cell 131:1164‐1178.
Sun S, Sun Y, Ling SC, Ferraiuolo L, McAlonis‐Downes M, Zou Y, Drenner K, Wang Y, Ditsworth D, Tokunaga S, Kopelevich A, Kaspar BK, Lagier‐Tou‐renne C, Cleveland DW (2015) Translational pro fi ling identi fi es a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1‐mediated ALS. Proc Natl Acad Sci U S A 112:E6993‐7002.
Tyzack GE, Sitnikov S, Barson D, Adams‐Carr KL, Lau NK, Kwok JC, Zhao C, Franklin RJM, Karadottir RT, Fawcett JW, Lakatos A (2014) Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3‐regulated TSP‐1 expression. Nat Commun 5:4294.
Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SSW, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, Brown RH, Cudkowicz ME, Bean BP, Eggan K, Woolf CJ (2014) Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient‐derived motor neurons. Cell Rep 7:1‐11.
10.4103/1673-5374.198977
How to cite this article:Lakatos A (2017) State-of-art modelling of inflammatory astrocyte-synapse interactions in injury and amyotrophic lateral sclerosis. Neural Regen Res 12(1):75-76.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration
- Information for Authors -Neural Regeneration Research
- A new computational approach for modeling diffusion tractography in the brain
- Celebration of the 10thAnniversary of Neural Regeneration Research
- Terapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury
- Blood microRNAs as potential diagnostic markers for hemorrhagic stroke

