Neural di ff erentiation of choroid plexus epithelial cells: role of human traumatic cerebrospinal fl uid
Elham Hashemi, Yousef Sadeghi,, Abbas Aliaghaei, Afsoun Seddighi, Abbas Piryaei, Mehdi Eskandarian Broujeni, Fatemeh Shaerzadeh, Abdollah Amini, Ramin Pouriran
1 Department of Anatomy and Cell Biology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2 Shohada Tajrish Neurosurgical Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3 Department of Stem Cells and Regenerative Medicine, Faculty of Medical Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
4 Molecular Medicine Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
5 School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Neural di ff erentiation of choroid plexus epithelial cells: role of human traumatic cerebrospinal fl uid
Elham Hashemi1, Yousef Sadeghi1,*, Abbas Aliaghaei1, Afsoun Seddighi2, Abbas Piryaei1, Mehdi Eskandarian Broujeni3, Fatemeh Shaerzadeh4, Abdollah Amini1, Ramin Pouriran5
1 Department of Anatomy and Cell Biology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2 Shohada Tajrish Neurosurgical Center of Excellence, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3 Department of Stem Cells and Regenerative Medicine, Faculty of Medical Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
4 Molecular Medicine Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
5 School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
How to cite this article:Hashemi E, Sadeghi Y, Aliaghaei A, Seddighi A, Piryaei A, Broujeni ME, Shaerzadeh F, Amini A, Pouriran R (2017) Neural di ff erentiation of choroid plexus epithelial cells: role of human traumatic cerebrospinal fl uid. Neural Regen Res 12(1):84-89.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
As the key producer of cerebrospinal fluid (CSF), the choroid plexus (CP) provides a unique protective system in the central nervous system. CSF components are not invariable and they can change based on the pathological conditions of the central nervous system.e purpose of the present study was to assess the e ff ects of non‐traumatic and traumatic CSF on the di ff erentiation of multipotent stem‐like cells of CP into the neural and/or glial cells. CP epithelial cells were isolated from adult male rats and treated with human non‐traumatic and traumatic CSF. Alterations in mRNA expression ofNestinand microtubule‐associat‐ed protein (MAP2), as the speci fi c markers of neurogenesis, and astrocyte marker glial fi brillary acidic protein (GFAP) in cultured CP epithelial cells were evaluated using quantitative real‐time PCR.e data revealed that treatment with CSF (non‐traumatic and traumatic) led to increase in mRNA expression levels ofMAP2andGFAP. Moreover, the expression of Nestin decreased in CP epithelial cells treated with non‐traumatic CSF, while treatment with traumatic CSF signi fi cantly increased its mRNA level compared to the cells cultured only in DMEM/F12 as control. It seems that CP epithelial cells contain multipotent stem‐like cells which are inducible under pathological conditions including exposure to traumatic CSF because of its compositions.
nerve regeneration; choroid plexus; cerebrospinal fl uid; stem cells; Nestin; microtubule-associated protein 2; glial fi brillary acidic protein; neurogenesis; central nervous system; neural regeneration
Accepted: 2016-12-27
Introduction
Well‐delineated function of choroid plexus (CP) is the pro‐duction of cerebrospinal fl uid (CSF). As a matter of fact, CP epithelial cells secrete a wide range of proteins and signaling molecules into the CSF in which they are essential for devel‐opment of the brain and its preservation against damaging elements (Strazielle and Ghersi‐Egea, 2000). Furthermore, CSF not only guards the central nervous system (CNS) physically by providing a fl uid cushion, but also protects the brain chemically and immunologically (Skipor and Thiery, 2008).
Particularly, CSF contents are considerably similar to blood plasma, however, it appears to contain less protein concentration and some modi fi ed amount of electrolytes, vitamins and growth factors (Hühmer et al., 2006). It is quite important to mention that CSF components are not invariable and based on the pathological conditions of the CNS, the composition of the CSF can change (McLean et al., 2006). According to the reports, there has been a growing concern for developing novel biomarkers for neurological diseases using biological components of CSF (Craig‐Schapiro et al., 2011). Indeed, the protein level of neurogranin, a calmodulin‐binding postsynaptic pro‐tein which regulates synaptic plasticity, increases in CSF of Alzheimer’s disease patients (Janelidze et al., 2016). In addition to application of CSF for diagnostic tests, it has been reported that due to the brain‐derived proteins, CSF can be a source of neurotrophins and growth factors alongside its protein profile originated from the plasma (Patterson et al., 1993; Romeo et al., 2005). Huttner et al. (2008) reported that human CSF contains membrane par‐ticle‐associated prominin‐1/CD133, a somatic stem cell marker. Furthermore, variations in the protein pro fi le and content of CSF have been documented in distinct neuro‐pathological situations indicating that the protein pro fi le of CSF signi fi cantly changes based on the condition (Nor‐dinet al., 2007).
Unlike the traditional view, identification and isolation of neural stem cells (NSCs) from adult CNS not only indicates the developmental abilities of adult brain, but also opens acontemporary therapeutic window for neurological disorders. As a matter of fact, the distinct molecular mechanisms by which undifferentiated neural stem cells progress into the di ff erentiated functional neurons have not been fully under‐stood. Absolutely, a series of components of the neurogenic niche are needed for NSC differentiation including cell‐cell interaction and secretory factors (Wurmser et al., 2004; Walker et al., 2009; Gattazzo et al., 2014). Due to the exis‐tence of various neurotrophic, mitogenic, and morphogenic factors, CSF could be considered as a fundamental media providing proper signals for prenatal and postnatal neuro‐genesis (Guerra et al., 2015).
The purpose of the present study was to evaluate the in‐fluence of non‐traumatic (normal) and traumatic CSF on neurogenesis markersNestin, microtubule‐associated pro‐tein (MAP2) and glial fi brillary acidic protein (GFAP) in the cultured CP epithelial cells.
Materials and Methods
Isolation and culture of CP epithelial cells
CP epithelial cells were isolated and cultured according to the protocol as described previously (Aliaghaei et al., 2014). Brie fl y, adult male albino Wistar rats (Pasteur insti‐tute, Tehran, Iran), weighing 150–200 g, were decapitated under deep anesthesia and their brains were removed (n= 8). All the procedures described in this study were consis‐tent with theGuide for the Care and Use of Laboratory Animals(National Institutes of Health Publication No. 80‐23, revised 1996), approved by the Animal Care Committee of Shahid Beheshti University of Medical Sciences, Iran. CP tissues were removed from the ventricles and incubated with 0.25% trypsin solution for 20 minutes at 37°C. Aer‐wards, the fetal bovine serum (FBS) (10%) was added and following the centrifugation, the pellets were transferred into a culture media containing DMEM/F12, FBS (10%), and antibiotic. After reaching the maximal confluency (about 2 weeks), CP epithelial cells were stained immuno‐cytochemically with antibody against transthyretin (TTR), a CP epithelial cell marker and visualized with a fl uores‐cence microscope (Olympus, IX71, Japan). CP epithelial cells were cultured in DMEM/F12 media (Sigma‐Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, penicillin and streptomycin (1%) for treatment (Figure 1).
CSF collection and treatment
Adult male patients with acute traumatic brain injury, aged 30–45 years, were included in this study.ey were admitted to the Department of Neurosurgery of Shohada Tajrish Hos‐pital, Tehran, Iran within 48 hours aer the onset of symp‐toms.e normal CSF was obtained from patients who did not su ff er from any neurological disorders and were selected from the other departments of Shohada Tajrish Hospital. The study protocol was approved by the local ethics com‐mittee (Ethics code 9020878), and written informed consent was obtained from each participant or from the close family members prior to enrollment. Ten patients with severe trau‐ matic brain injury were included in this study. All patients had a Glasgow Coma Score ≤ 8 and showed abnormal imag‐ing findings including obvious midline shift, effacement of the lateral ventricle and shape of hemorrhage. Intracranial hematomas were surgically evacuated if it was needed. Af‐ter successful surgery, the patients were transferred to the intensive care unit (ICU) and treated according to the stan‐dardized protocol.e CSF was drainedviaintraventricular catheter (IVC) when intracranial pressure (ICP) exceeded 15 mmHg; furthermore, CSF samples were screened for bacte‐rial infection every 3 days to ensure the absence of any in‐fection factors. Catheters were removed when ICP was con‐stantly remained below 15 mmHg for 24 hours. All patients were fed by gastric tube; nonetheless, no parenteral feeding was needed. Distinctly, in the control group, the CSF samples were collected by lumbar puncture from seven patients who received spinal anesthesia. These patients were checked by an independent medical doctor to rule out any neurological diseases through monitoring the recent medication. A sub‐sample of each CSF sample was sent to a clinical laboratory for routine CSF diagnostics.
CSF samples (6—8 mL for each) were collected using poly‐propylene tubes. The samples were filtered and split into 1 mL aliquots and stored at –80°C prior to use. CP epithelial cells were treated with normal CSF and/or traumatic CSF (volume ratio of CSF to DMEM/F12 medium = 4:1, respec‐tively). In the control group, CP epithelial cells were cultured only with DMEM/F12.
Reverse transcription polymerase chain reaction
The total RNA in CP epithelial cells was extracted using a high pure RNA isolation kit according to the manufac‐turer’s instructions (Roche, Basel, Switzerland). RNA was quanti fi ed according to the absorbance at 260 nm using an Nanodrop ND‐1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Then, 1 μg of total RNA was transcribed into cDNA using murine leukemia virus (MuLV) reverse transcriptase (Fermentas, Walthman, MA, USA) in the presence of random hexamers and RNase inhibitor. Aerwards, the obtained cDNA was ampli fi ed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 40 seconds and extension at 72°C for 45 seconds using specific primers (able 1). PCR products were size fractionated by 1.5% agarose gel electrophoresis and the images were analyzed for the density of DNA bands using US‐Scan‐It Gel Analysis Software (Skill Scientific Inc., Orem, Utah, USA).
Quantitative real-time PCR
For quantitative real‐time PCR (qPCR) analysis, specific primers forTTR,Nestin,GFAP,MAP2and hypoxanthine phosphoribosyl transferase 1 (HPRT1) genes were used (able 1).e reactions were performed using Super SYBR Green qPCR masterMix 2X (Yektatajhiz, Iran) on a Ro‐tor‐GeneTM6000 real‐time PCR machine (Corbett Research, Qiagen, Germany). The initial denaturation was done at 95°C for 5 minutes following 45 cycles of denaturation at95°C for 10 seconds, under primer speci fi c conditions (able 1), eventually extension was performed at 72°C for 20 seconds. Comparative quantitation was performed between selected groups using REST 2009 (Relative Expression So‐ware Tool, Qiagen) based on Pair Wise Fixed Reallocation Randomization Test©.
able 1 R-PCR and qPCR primer sequences

able 1 R-PCR and qPCR primer sequences
Primer Sequence Size (bp) Annealing Accession HPRT Forward: AGG CCA GAC TTT GTT GGA TT Reverse: GCT TTT CCA CTT TCG CTG AT 119 60°C, 15 seconds NM_012583.2 TTR Forward: CCT GGG GGT GCT GGA GAA T Reverse: ATG GTG TAG TGG CGA TGA C 317 61°C, 20 seconds NM_012681.2 GFAP Forward: GGT GGA GAG GGA CAA TCT CA Reverse: CCA GCT GCT CCT GGA GTT CT 233 61°C, 20 seconds NM_017009.2 Nestin Forward: CCT CAA GAT GTC CCT TAG TCT G Reverse: TCC AGA AAG CCA AGA GAA GC 114 60°C, 20 seconds NM_001308239.1 MAP2 Forward: CAA ACG TCA TTA CTT TAC AAC TTG A Reverse: CAG CTG CCT CTG TGA GTG AG 122 60°C, 20 seconds NM_013066.1

Figure 1 Schematic fl owchart of experimental procedure.
Statistical analysis
All data are represented as the mean ± SEM.e comparison between groups was done by one‐way analysis of variance (ANOVA) followed by a speci fi cpost-hoctest (Tukey’s mul‐tiple comparison test). Statistical analysis was performed us‐ing REST 2009 (Relative Expression Soware Tool, Qiagen, Hilden, Germany) based on Pair Wise Fixed Reallocation Randomization Test (Pfafflet al., 2002). A level ofP< 0.05 was considered statistically signi fi cant.

Figure 2 Cultured choroid plexus (CP) epithelial cells and the e ff ect of normal and traumatic cerebrospinal fl uid (CSF) onTTRmRNA expression in CP epithelial cells.
Results
Three days after culture of CP epithelial cells, round cells were detected in the fl asks. Two weeks later, the cells cov‐ered the whole surface of the flasks. Cells were polygonal and had the appearance of epithelial cells. As shown in Figure 2, immunocytochemistry data showed that these cells were immunopositive forTTR, a marker for CP epithelial cells (Herbert et al., 1986). Furthermore, mRNA expressionofTTRin cells exposed to normal and traumatic CSF de‐creased obviously than that in the control cells (P< 0.001) (Figure 2D).

Figure 3 E ff ect of normal and traumatic cerebral spinal fl uid (CSF) onNestin(A) and microtubule-associated protein (MAP2; B) mRNA expression in choroid plexus epithelial cells.
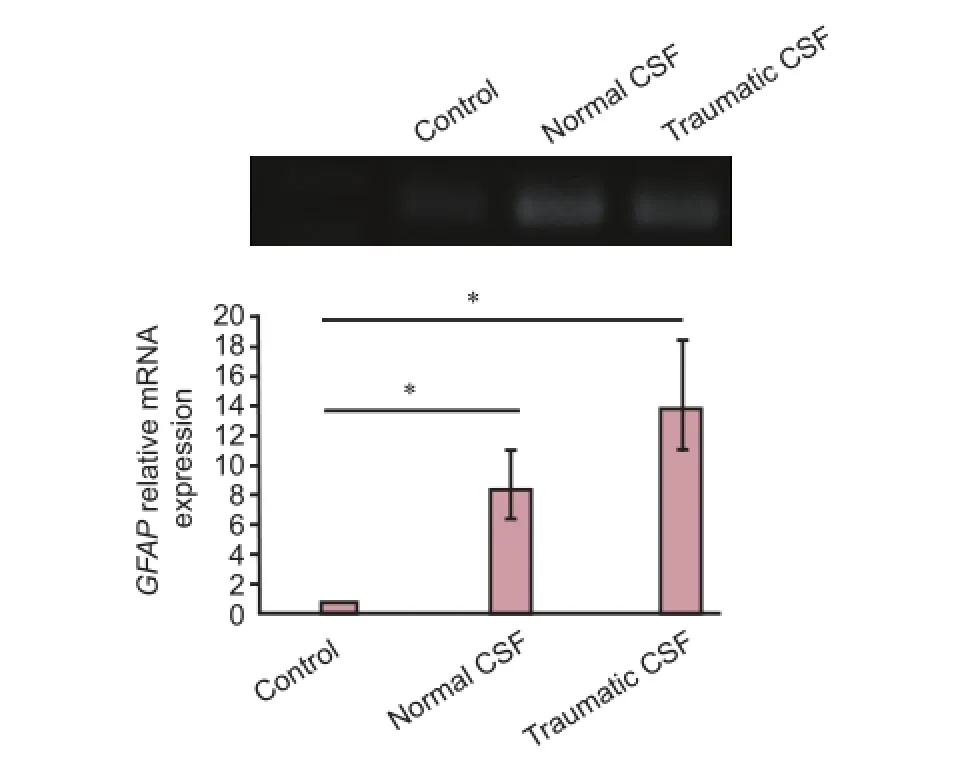
Figure 4 E ff ect of normal and traumatic cerebrospinal fl uid (CSF) on mRNA expression of glial fi brillary acidic protein (GFAP) in choroid plexus epithelial cells.
E ff ect of traumatic CSF on mRNA expression ofNestinandMAP2in CP epithelial cells
Nestinhas been recognized as a marker for neural stem/pro‐genitor cells (Suzuki et al., 2010).e expression ofNestinin CP epithelial cells incubated with traumatic CSF increased remarkably compared to that in control CP epithelial cells cultured with DMEM/F12. mRNA level ofNestinin cells exposed to normal CSF decreased signi fi cantly than that in control cells (P< 0.001). mRNA expression ofNestinin the presence of traumatic CSF in CP epithelial cells was 1.3‐fold greater than that in the control cells (P< 0.05) and 10‐ fold higher than that in the cells exposed to normal CSF (P<0.05; Figure 3A).e mRNA level ofMAP2in CP epithelial cells incubated with normal and traumatic CSF signi fi cantly increased than that in the control cells (P< 0.05). mRNA ex‐pression ofMAP2was nearly 7.7‐fold (P< 0.05) and 7.5‐fold (P< 0.05) greater in cells exposed to normal and traumatic CSF separately than that in the control cells (Figure 3B).
GFAP is a specific marker for astroglial cells (Lewis et al., 1984). As shown in Figure 4, mRNA expression ofGFAPin CP epithelial cells exposed to normal and traumatic CSF in‐creased compared to that in control cells. mRNA expression level ofGFAPin cells exposed to normal and traumatic CSF was 8.4‐fold (P< 0.05) and 13.8‐fold (P< 0.05) greater than that in control cells, separately.
Discussion
Despite the fact that all CNS forming cells excepting mi‐croglia are originated from a common source located in the ventricular zone, following birth and throughout life, neuro‐genesis processes continue in two particular regions in adult mammalian brains: the subventricular zone lining the walls of the lateral ventricles and the subgranular zone of hippo‐campal dentate gyrus (Alvarez‐Buylla and Garcia‐Verdugo, 2002; Gage, 2002). In addition, several lines of evidence have denoted the generation of new functional neurons from pre‐cursor cells in other parts of the brain including the cortex, amygdala, hypothalamus, striatum, and substantia nigra (Guerra et al., 2015).e present fi ndings demonstrated that traumatic human CSF induced cultured CP epithelial cells into neural di ff erentiation.
CSF composition probably has a significant influence on self‐renewal, proliferation, and development of NSCs in the subventricular zone since the subventricular zone is locat‐ed in the CSF‐filled lateral ventricle. Farivar et al. (2015) reported that CSF at proper concentrations increases the mRNA expression ofNestin,MAP2andGFAPwhich lead to the neural di ff erentiation of mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord (a gelatinous connective tissue from the umbilical cord).
As a marker of neural stem and neural progenitor cells,Nestin‐expressing cells are directly involved in regeneration and self‐renewing of NSCs. Once cells became di ff erentiated,Nestinexpression decreases which is frequently accompa‐nied by overexpression ofGFAPand neuro fi laments (Wiese et al., 2004). Our findings suggest that down‐regulation ofNestinmRNA in combination with up‐regulation ofGFAPmRNA in normal CSF‐treated CP epithelial cells were likely attributed to induction of cell di ff erentiation. Up‐regulation ofNestinmRNA by traumatic CSF in CP epithelial cells could be due to induction of stem/progenitor cells which are engaged in active proliferation. In term of CSF composi‐tions, several reports have indicated a long list of biologically active proteins, peptides and neurotransmitters that are secreted into the CSF through various mechanisms (Johan‐son et al., 2008; Rodríguez et al., 2010). Biologically active compounds are secreted into CSF by the circumventricular organs including subcommissural organ, pineal body, CP and median eminence (Johanson et al., 2008). Accumulative evidence indicates that microvesicles containing signaling and proteins are released into the CSF by cells located in the ventricular walls (Chiasserini et al., 2014). CSF provides a platform for glial di ff erentiation of neural stem cells (Kiiski et al., 2013).e protective e ff ects of growth factors includ‐ing brain‐derived neurotrophic factor, nerve growth factor, and basic fibroblast growth factor on neuronal survival and differentiation have been conclusively well known. CSF consists of brain‐borne and blood‐borne signals and molecules targeting distinct regions within the brain that eventually leads to development, di ff erentiation and protec‐tion of neuronal cells. Under stress condition, some factors released by CSF drive brain cells including epithelial cells toward stem/progenitor cells, which is likely considered as a potential therapy of CSF for some brain disorders. Be‐cause CSF exhibits protective effects on the brain, so it is not surprising that traumatic CSF has potential to trigger some signals in PC epithelial cells, which lead to neuronal development and differentiation. As mentioned above, CSF is a heterogeneous and highly dynamic fluid and its molecular composition changes from lateral ventricles to third and fourth ventricles as well under di ff erent physio‐logical and pathological conditions (Johanson et al., 2008; Rodríguez et al., 2010). Pathological conditions including traumatic brain injury and neuroin fl ammation likely a ff ect CSF composition. Traumatic brain injury has been shown to be associated with elevated level of monocyte chemoat‐tractant protein‐1 in CSF (Sempleet al., 2010). Hong et al. (2015) reported that activity of monocyte chemoattractant protein‐1 contributes to an increase in neurogenesis and it also decreases neuroinflammation in Niemann‐Pick type C disease mouse brain. Decimo et al. (2011) reported that the number ofNestin‐positive cells in PC epithelial cells increased aer spinal cord injury.us, it can be assumed that traumatic injury causes secretion of some molecules into the CSF, which provide brain protection and induce the proliferation and development of brain stem cells. Ac‐tually, our data revealed that under stress condition, CNS cells likely secrete some compounds into the CSF to induce CP epithelial cells to di ff erentiate into stem/progenitor cells as con fi rmed by elevated expression of neuronal markers.erefore, the composition of traumatic CSF possibly pro‐motes the regeneration and self‐renewal of injured brain tissue. However, there is a need to identify the proteins, neurotrophins and bioactive molecules that secreted into the traumatic CSF.
Taken together, CP epithelial cells contain multipotent stem‐like cells, which are quite similar to other known brain regions with neurogenic features. Traumatic CSF triggers the proliferation and differentiation of CP epithelial cells into neural progenitor cells which lead to tissue regeneration.e present data provide a platform for future studies regarding targeting CP epithelial cells with therapeutic potential for a wide variety of human brain disorders.
Author contributions:YS and Aliaghaei A conceived and designed the experiments. EH performed the experiments. Amini A, MEB, and YS analyzed the data. AS, AP, and Amini A contributed to reagents/materials/analysis tools. Aliaghaei A, FS and RP wrote the paper. All authors approved the fi nal version of this paper.
Con fl icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:
Aliaghaei A, Khodagholi F, Ahmadiani A (2014) Conditioned media of choroid plexus epithelial cells induces Nrf2‐activated phase II an‐tioxidant response proteins and suppresses oxidative stress‐induced apoptosis in PC12 Cells. J Mol Neurosci 53:617‐625.
Alvarez‐Buylla A, Garcia‐Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22:629‐634.
Chiasserini D, van WeeringJR, PiersmaSR, PhamTV, MalekzadehA, TeunissenCE, de Wit H, Jiménez CR (2014) Proteomic analysis of cerebrospinal fl uid extracellular vesicles: a comprehensive dataset. J Proteomics 106:191‐204.
Craig‐Schapiro R, Kuhn M, Xiong C, Pickering EH, Liu J, Misko TP, Perrin RJ, Bales KR, Soares H, Fagan AM, Holtzman DM (2011) Multiplexed immunoassay panel identi fi es novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PLoS One 6:e18850.
Decimo I, Bifari F, Rodriguez FJ, Malpeli G, Dolci S, Lavarini V, Pretto S, Vasquez S, Sciancalepore M, Montalbano A, Berton V, Krampera M, Fumagalli G (2011) Nestin‐ and doublecortin‐positive cells reside in adult spinal cord meninges and participate in injury‐induced paren‐chymal reaction. Stem Cells 29:2062‐2076.
Farivar S, Mohamadzadeh Z, Shiari R, Fahimzad A (2015) Neural di ff erentiation of human umbilical cord mesenchymal stem cells by cerebrospinal fl uid. Iran J Child Neurol 9:87‐93.
Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22:612‐613.
Gattazzo F, Urciuolo A, Bonaldo P (2014) Extracellular matrix: a dy‐namic microenvironment for stem cell niche. Biochim Biophys Acta 1840:2506‐2519.
Guerra MM, González C, Caprile T, Jara M, Vío K, Muñoz RI, Rodrí‐guez S, Rodríguez EM (2015) Understanding how the subcommis‐sural organ and other periventricular secretory structures contribute via the cerebrospinal fl uid to neurogenesis. Front Cell Neurosci 9:408.
Hühmer AF, Biringer RG, Amato H, Fonteh AN, Harrington MG (2006) Protein analysis in human cerebrospinal fl uid: physiological aspects, current progress and future challenges. Dis markers 22:3‐26.
Herbert J, Wilcox JN, Pham KT, Fremeau RT Jr, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA (1986) Transthyretin: a choroid plexus‐speci fi c transport protein in human brain.e 1986 S. Weir Mitchell Award. Neurology 36:900‐911.
Hong YR, Lee H, Park MH, Lee JK, Lee JY, Suh HD, Jeong MS, Bae JS, Jin HK (2015) CCL2 induces neural stem cell proliferation and neuronal di ff erentiation in Niemann‐Pick type C mice. J Vet Med Sci 77:693‐699.
Huttner HB, Janich P, Köhrmann M, Jászai J, Siebzehnrubl F, Blümcke I, Suttorp M, Gahr M, Kuhnt D, Nimsky C, Krex D, Schackert G, Löwenbrück K, Reichmann H, Jüttler E, Hacke W, Schellinger PD, Schwab S, Wilsch‐Bräuninger M, Marzesco AM, et al.e stem cell marker prominin‐1/CD133 on membrane particles in human cere‐brospinal fl uid o ff ers novel approaches for studying central nervous system disease. Stem Cells 26:698‐705.
Janelidze S, Hertze J, Zetterberg H, Landqvist Waldö M, Santillo A, Blennow K, Hansson O (2016) Cerebrospinal fl uid neurogranin and YKL‐40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neu‐rol 3:12‐20.
Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silver‐berg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10.
Kiiski H, Aanismaa R, Tenhunen J, Hagman S, Yla‐Outinen L, Aho A, Yli‐Hankala A, Bendel S, Skottman H, Narkilahti S (2013) Healthy human CSF promotes glial fifferantiation of hESC‐derived neural cells while retaining spontaneous activity in existing neuronal net‐works. Biol Open 2:605‐612.
Lewis SA, Balcarek JM, Krek V, Shelanski M, Cowan NJ (1984) Se‐quence of a cDNA clone encoding mouse glial fi brillary acidic pro‐tein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A 81:2743‐2746.
McLean BN, Luxton RW, Thompson EJ (1990) A study of immuno‐globulin G in the cerebrospinal fl uid of 1007 patients with suspected neurological disease using isoelectric focusing and the log IgG‐index. Brain 113:1269‐1289.
Nordin C, Gupta RC, Sjödin I (2007) Cerebrospinal fl uid amino acids in pathological gamblers and healthy controls. Neuropsychobiology 56:152‐158.
Patterson SL, Grady MS, Bothwell M (1993) Nerve growth factor and a fi broblast growth factor‐like neurotrophic activity in cerebrospinal fl uid of brain injured human patients. Brain Res 605:43‐49.
Pfaffl MW, Horgan GW, Demp fl e L (2002) Relative expression soware tool (REST) for group‐wise comparison and statistical analysis of rel‐ative expression results in real‐time PCR. Nucleic Acids Res 30:e36.
Rodríguez EM, Blázquez JL, Guerra M (2010)e design of barriers in the hypothalamus allows the median eminence and the arcuate nu‐cleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fl uid. Peptides 31:757‐776.
Romeo MJ, Espina V, Lowenthal M, Espina BH, Petricoin EF 3rd, Liotta LA (2005) CSF proteome: a protein repository for potential biomark‐er identi fi cation. Expert Rev Proteomics 2:57‐70.
Semple BD, Bye N, Rancan M, Ziebell JM, Morganti‐Kossmann MC (2010) Role of CCL2 (MCP‐1) in traumatic brain injury (TBI): ev‐idence from severe TBI patients and CCL2−/−mice. J Cereb Blood Flow Metab 30:769‐782.
Strazielle N, Ghersi‐Egea JF (2000) Choroid plexus in the central ner‐vous system: biology and physiopathology. J Neuropathol Exp Neu‐rol 59:561‐574.
Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H (2010)e neural stem/progenitor cell marker nestin is expressed in proliferative en‐dothelial cells, but not in mature vasculature. J Histochem Cytochem 58:721‐730.
Walker MR, Patel KK, Stappenbeck TS (2009) The stem cell niche. J Pathol 217:169‐180.
Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasova KV, Wersto RP, Boheler KR, Wobus AM (2004) Nestin expression—A property of multi‐lineage progenitor cells? Cell Mol Life Sci 61:2510‐2522.
Wurmser AE, Palmer TD, Gage FH (2004) Cellular interactions in the stem cell niche. Science 304:1253‐1255.
Copyedited by Li CH, Song LP, Zhao M
Yousef Sadeghi, M.D., Ph.D., dr.ysadeghi@yahoo.com.
10.4103/1673-5374.198989
*< class="emphasis_italic">Correspondence to: Yousef Sadeghi, M.D., Ph.D., dr.ysadeghi@yahoo.com.
orcid: 0000-0002-0889-2555 (Yousef Sadeghi)
- 中国神经再生研究(英文版)的其它文章
- Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration
- Information for Authors -Neural Regeneration Research
- A new computational approach for modeling diffusion tractography in the brain
- Celebration of the 10thAnniversary of Neural Regeneration Research
- Terapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury
- Blood microRNAs as potential diagnostic markers for hemorrhagic stroke

