Real-time and wearable functional electrical stimulation system for volitional hand motor function control using the electromyography bridge method
Hai-peng Wang, Zheng-yang Bi, Yang Zhou, Yu-xuan Zhou, Zhi-gong Wang,, Xiao-ying Lv,
1 Institute of RF‐ & OE‐ICs, Southeast University, Nanjing, Jiangsu Province, China
2 State Key Lab of Bioelectronics, Southeast University, Nanjing, Jiangsu Province, China
3 Co‐innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
Real-time and wearable functional electrical stimulation system for volitional hand motor function control using the electromyography bridge method
Hai-peng Wang1, Zheng-yang Bi2, Yang Zhou1, Yu-xuan Zhou2, Zhi-gong Wang1,3,*, Xiao-ying Lv2,3
1 Institute of RF‐ & OE‐ICs, Southeast University, Nanjing, Jiangsu Province, China
2 State Key Lab of Bioelectronics, Southeast University, Nanjing, Jiangsu Province, China
3 Co‐innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
How to cite this article:Wang HP, Bi ZY, Zhou Y, Zhou YX, Wang ZG, Lv XY (2017) Real-time and wearable functional electrical stimulation system for volitional hand motor function control using the electromyography bridge method. Neural Regen Res 12(1):133-142.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Graphical Abstract
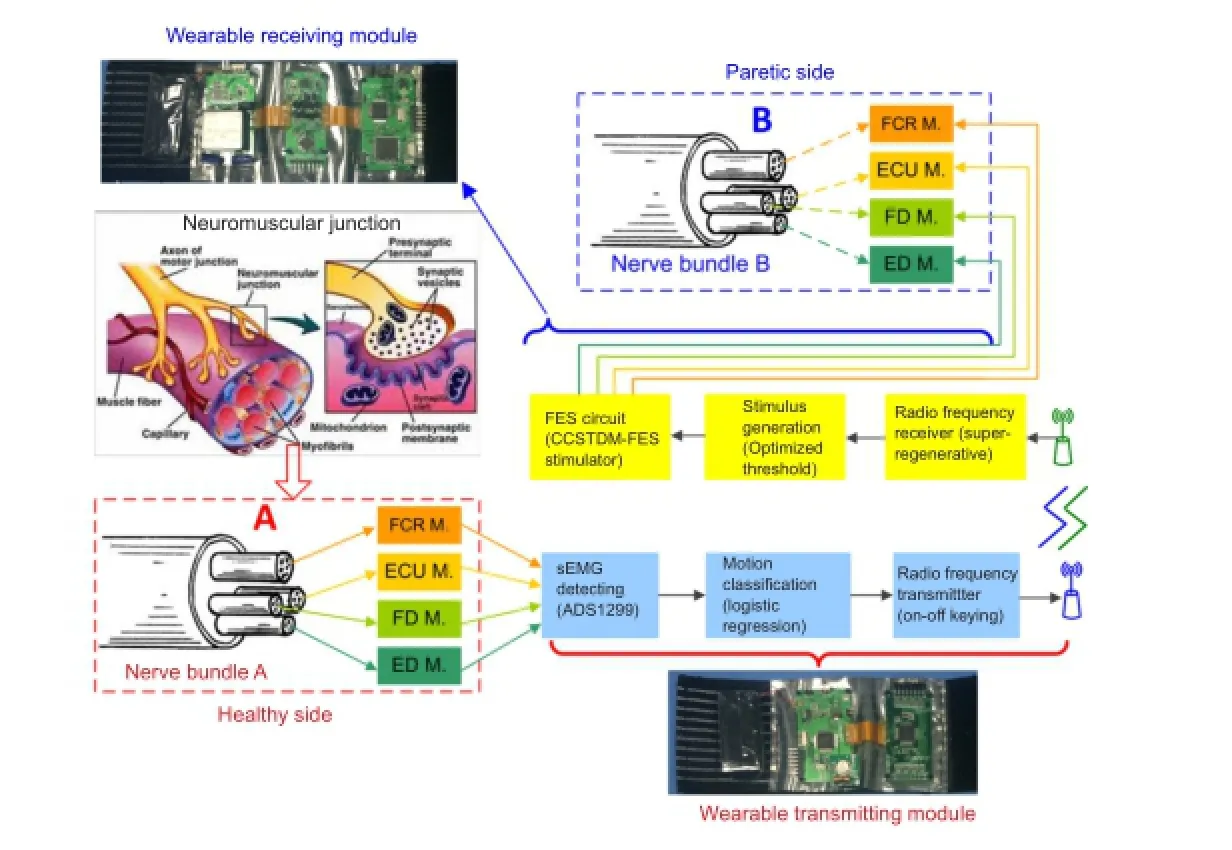
A novel prototype system of volitional hand motor function rebuilding system for paralysis rehabilitation: real-time and wearable “electromyography bridge”
orcid: 0000-0002-9203-4683 (Zhi-gong Wang)
Voluntary participation of hemiplegic patients is crucial for functional electrical stimulation therapy. A wearable functional electrical stim‐ulation system has been proposed for real‐time volitional hand motor function control using the electromyography bridge method.rough a series of novel design concepts, including the integration of a detecting circuit and an analog‐to‐digital converter, a miniaturized functional electrical stimulation circuit technique, a low‐power super‐regeneration chip for wireless receiving, and two wearable armbands, a pro‐totype system has been established with reduced size, power, and overall cost. Based on wrist joint torque reproduction and classi fi cation experiments performed on six healthy subjects, the optimized surface electromyography thresholds and trained logistic regression classi fi er parameters were statistically chosen to establish wrist and hand motion control with high accuracy. Test results showed that wrist fl exion/ extension, hand grasp, and fi nger extension could be reproduced with high accuracy and low latency.is system can build a bridge of in‐formation transmission between healthy limbs and paralyzed limbs, e ff ectively improve voluntary participation of hemiplegic patients, and elevate e ffi ciency of rehabilitation training.
nerve regeneration; functional electrical stimulation; logistic regression; rehabilitation of upper-limb hemiplegia; electromyography control; wearable device; stroke; frequency-modulation stimulation; hand motion; circuit and system; real-time; neural regeneration
Introduction
Functional electrical stimulation (FES) has been introduced as a neurorehabilitation method to arti fi cially activate senso‐ry and motor systems following central nervous system dis‐ease or injury, such as spinal cord injury and stroke (Popović, 2014; Shen et al., 2016; Wade and Gorgey, 2016). The first noninvasive FES system was used for foot drop correction of hemiplegic patients by Liberson et al. (1961). Many novel FES systems have been designed as surface or implantable stimulation systems for controlling arms and hands (Saxena et al., 1995; Ijzerman et al., 1996; Kilgore et al., 1997; Knut‐son et al., 2012; Hara et al., 2013).
Based on the success of volitional control of FES, our group previously designed an FES system for restoring mo‐tor function in post‐stroke hemiplegic patients (Huang et al., 2014). In that system, a frequency‐modulation stimulation algorithm based on surface EMG (sEMG) and the support vector machine model were used. However, sEMG thresh‐olds need to be carefully chosen and force reproduction per‐formance has not yet been established.e system is also too di ffi cult to wear and remove.
The specific objectives of this paper were: (1) to use sta‐tistical experiments and analyses to optimize the primary parameter “sEMG thresholds” of the frequency‐modulation stimulation generation algorithm formerly proposed by our group and to verify the force reproduction performance; (2) to develop a low‐complexity algorithm based on logistic re‐gression for hand movement classi fi cation achieved by these sEMG thresholds; (3) to develop a wireless and wearable FES system using the EMG‐bridge method for real‐time volition‐ al hand motor function control, and to assess the feasibility of this system in real‐time control of four hand movements.is novel system is a wearable EMG‐bridge system that is distributedviaa contralateral sEMG‐controlled FES system providing more convenience to use at home.e size, power, and overall cost have been significantly reduced compared with the previous prototype (Huang et al., 2014).
Subjects and Methods
Wearable EMG-bridge system overview
System hardware description
An analog front‐end ADS1299 (Texas Instruments Inc., Dallas, TX, USA) with single‐di ff erential sEMG sensors was selected to integrate the sEMG signal detecting circuit and analog‐to‐digital converter. The ADS1299 had eight low‐noise programmable gain amplifiers and eight 24‐bit ana‐log‐to‐digital converters, which could support a maximum of eight channel sEMG signal acquisitions. Aer sending the digitalized sEMG signals to the MCU, they were band‐pass fi ltered from 20 to 500 Hz and ampli fi ed with a gain of 1,000.A fully integrated super‐regenerative receiver chip operating in the 335/433 MHz band was selected as the radio frequen‐cy receiver for its simplicity and e ffi ciency in low‐power and short‐range communication, as previously described by our group (Wang et al., 2012) and realized in a 0.35‐μm com‐plementary metal oxide semiconductor process. Similarly, an on‐off keying circuit with an operating frequency that matched the radio frequency receiver was used as the radio frequency transmitter.
Optimization of sEMG thresholds
Subjects
All volunteers were recruited by posting posters on bill‐boards at Southeast University of China. Six healthy sub‐jects (five males and one female; 26.3 ± 2.9 years of age) participated in the study. Each subject was asked to refrain from strenuous exercise of the upper extremities for at least 24 hours before the study. All subjects provided written in‐formed consent before participation, and this study was ap‐proved by the Human Subjects Review Board of Southeast University.
Experiment protocol 1:orque reproduction test
The main target muscles in this test were the flexor carpi radialis muscle and extensor carpi ulnaris muscle. Isometric wrist joint torque was acquired using a custom‐made device (Zhou et al., 2016).e sEMG and torque signals were sam‐pled with a 16‐bit analog‐to‐digital converter acquisition board PCI‐6220 (National Instruments Inc., Austin, TX, USA) at a frequency of 1 kHz. A self‐designed PC‐based software was used to record data for off‐line analysis and provided visual feedback to the subject.

In Equation (1), MAVkrepresents the mean absolute value of sEMG data (x(i)) in the kth channel for 2‐second contrac‐tion movement (Phinyomark et al., 2012), andrkwas ac‐quired using the ratio parameterNkas presented in Equation (2).
According to the signal noise ratio of the acquired sEMG and Equation (1) and (2), three sEMG thresholds were selected with a ratio of 60%, 90%, and 120% of the mean absolute value, which means thatNkequaled 0.6, 0.9, and 1.2.e mean absolute value was calculated based on three 5‐second segments of sEMG extracted from the voluntary test motion, which elicited 30% maximal voluntary contrac‐tion. Aerwards, the stimulating pulse trains of varying fre‐quency were generated from the sEMG signals with di ff erent thresholds, and this step was conducted using Matlab 2014 soware (MathWorks Inc., Natick, MA, USA) on a personal computer with an Intel CPU (Core i3, 2.3‐GHz) and 6‐GB memory (Figure 2c).e generated stimulating pulse trains were then stored in the MCU fl ash memory of the wearable receiving module.e MCU delivered the trigger pulses tothe FES circuit in a real‐time manner with a time window of 1 ms.e receiving module was used to stimulate the fl exor carpi radialis/extensor carpi ulnaris muscles, and the corre‐sponding stimulated torque signals were recorded (Figure 2d) to determine the optimized sEMG threshold.e stimu‐lation procedure in each subject was also conducted at least 24 hours after the previous steps. The stimulating current used in the experiment was adjusted to induce a 30% maxi‐mal voluntary contraction of wrist motion using a simulat‐ing pulse train (500 μs, 50 Hz).
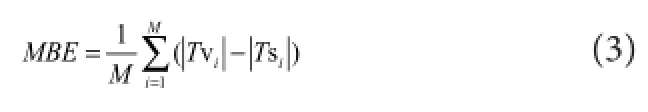
whereTv represented voluntary torque andTs represented stimulated torque.
Logistic regression for hand motion classification: four classes of hand motions, plus the no‐motion class were investigated in this study, because they were the most com‐monly employed upper‐limb motions in clinical rehabilita‐tion studies (Li et al., 2010; Knutson et al., 2012; Craven et al., 2015).e fi ve classes were: wrist extension, wrist fl ex‐ion, fi nger extension, hand grasp, and no motion. It is im‐portant to note that the above‐described motions were two degrees of freedom, while the logistic regression algorithm was used for one degree of freedom motion classification and control. Therefore, the other one degree of freedom motion and control could only be realized by changing the detecting and stimulating electrodes.us, sEMG data for logistic regression classifier training and testing were de‐scribed as follows: (1) wrist motion data were acquired, and two sEMG data channels were detected using bipolar sur‐face Ag/AgCl ECG electrodes (Junkang Medical Supplies., Shanghai, China) that were spaced 2 cm apart.ese were placed on the forearm above the wrist flexor (flexor carpi radialis) and extensor (extensor carpi ulnaris), and a refer‐ence electrode was placed on the lecranon. (2) Two sEMG data channels that correlated with hand motions were sim‐ilarly collected, with electrodes placed on the fi nger fl exor (flexor digitorum) and extensor (extensor digitorum). (3) Data were obtained from six healthy subjects; each was asked to perform three repetitions of each dynamic motion class for a 2‐second contraction with up‐ramping force ex‐ertion and a 2‐second relaxation.e fi rst two data sets were used for training and the last set was used for testing. Data were also sampled by ADS1299 in the EMG‐bridge system at a rate of 1 kHz and were digitally fi ltered at 20—500 Hz, with a digital gain of 1,000.
In this logistic regression classi fi er, the voltage amplitude of the sEMG signal was selected as the feature. For each mo‐tion class, the feature set was constructed on each of the two channels, which satis fi ed the following equations:

represented theithsampled sEMG signal amplitude data vector containing channel Ch1 and Ch2,y(i)represented each motion result.en, the suitable data (x(i),y(i)) was collected into the corresponding supervised feature set and fed into the classi fi er.e remaining data were labelled as no‐motion class.e hypothesis functionhθ(x)and cost functionJ(θ)of the logistic regression were calculated as follows:e parameterθcould be solved by a gradient descent ofJ(θ)with the training data. In the testing phase, the classi fi cation result (d(j)) was obtained by Equation (8) when the testing data were fed into the classi fi er. For example, ‘Ch1_muscle’meant that the recognized movement was related to the ago‐nist motion muscle where CH1 electrodes placed.
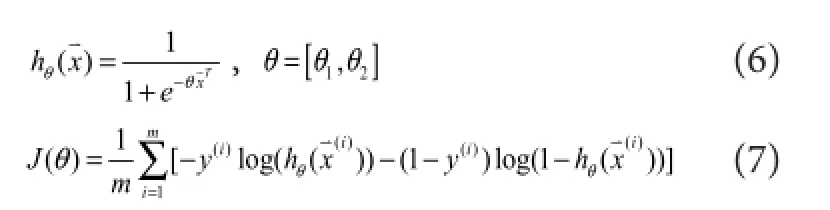
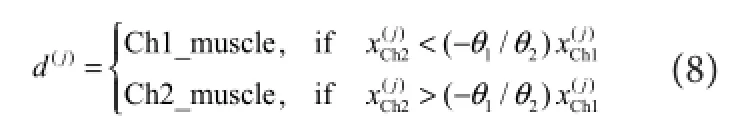
Experiment protocol 2: Motion classi fi cation o ff-line test
The data sets from the four motion classes were collected from all six subjects as described above. To pre‐verify the logistic regression classifier performance in the real‐time EMG‐bridge system, the mean optimized sEMG thresholds acquired from the above torque reproduction test of six subjects were used for the wrist fl exion/extension and hand grasp/finger extension classifications. The performance of the trained classifier in identifying a movement was mea‐sured byclassi fi cation accuracy (CA), which was de fi ned as the number of correctly classified samples divided by the total number of testing samples (Li et al., 2010).e logistic regression classi fi er parameters (θ) were averaged over data sets of all subjects to calculate the overall CA. The off‐line analysis was conducted using Matlab 2014 soware.
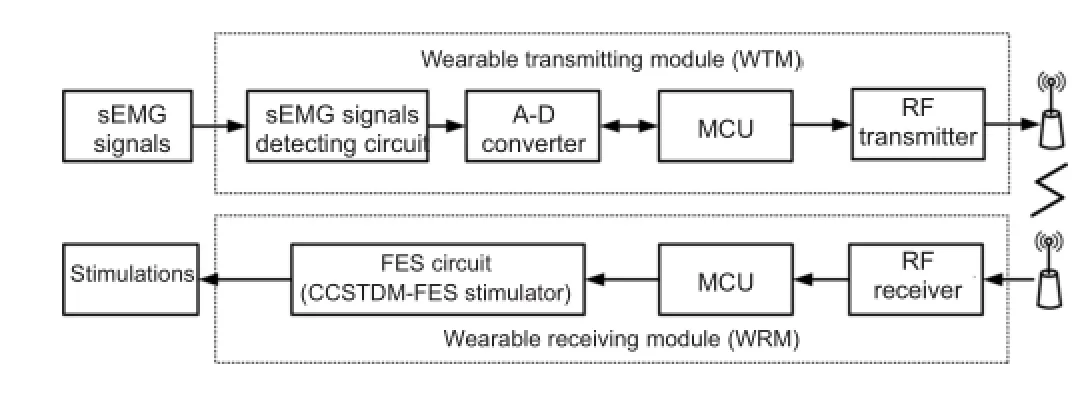
Figure 1 Block diagram of the wearable EMG-bridge system for volitional hand motor function control.
Wearable design and implementation: Photographs of the wearable EMG‐bridge system prototype are shown in Figure 3. The wearable transmitting module and the wearable re‐ceiving module were both fi xed with a stretchable armband for ease of wear. The maximum stretchable length of the armband was 30 cm, which was maintained to adapt to the upper extremity of most individuals (22—28 cm). Addition‐ally, a soanti‐static fi lm was tailored and assembled to the top of the armband to prevent electrostatic interference. In the wearable transmitting module, there were two primary printed circuit boards consisting of an ADS1299 printed cir‐cuit board and a controller‐printed circuit board.e sizes of the two printed circuit boards were 30 × 60 × 10 mm3and 25 × 60 × 10 mm3, respectively. In the wearable receiving module, there were three main printed circuit boards that included a high‐voltage power‐printed circuit board, a FES circuit‐printed circuit board and a controller‐printed circuit board, which were all fi xed with equal distance between to the armband. The dimension of each printed circuit board was 30 × 60 × 10 mm3, 25 × 60 × 10 mm3, and 30 × 60 × 10 mm3. Two 32‐cm custom‐made cables based on the flex‐ible‐printed circuit were used to connect the three circuit boards.e wearable transmitting module and the wearable receiving module were supplied by a 12‐V and 500‐mAh rechargeable Li‐battery. Simultaneously, the radio frequency transmitter and receiver‐printed circuit boards were a min‐iaturized design with an area of 2.5 × 3 cm2and 1.3 × 1.1 cm2. Figure 3B shows the receiver‐printed circuit board with an 8‐pin packaged super‐regenerative receiver chip, and the photomicrograph of this chip in the 0.35‐μm complementa‐ry metal oxide semiconductor is presented in Figure 3C.e area of the chip was 550 × 775 μm2, and the maximum data rate was 20 kbps.
Experiment protocol 3: Real-time sEMG bridging test
The mean optimized sEMG thresholds and the averaged trained logistic regression classi fi er metrics (θ) in the o ff‐line test were used for the real‐time volitional sEMG bridging motion control test.e six healthy subjects were randomly divided into three experimental pairs. To simulate the stroke patient using the proposed EMG‐bridge system, two subjects acted as the role of the controller and the controlled within each pair, and then these roles were exchanged for another repetition. For each controller, the wearable transmitting module was worn on the right forearm, and for each con‐trolled subject, the wearable receiving module was applied in the same position. The real‐time bridging test consisted of two procedures.e fi rst step was real‐time wrist fl exion/ extension control, and the second was for the hand grasp/ fi nger extension control.
A customized hand motion tracking system based on the Leap Motion sensor (Leap Ltd., San Francisco, CA, USA) and PC software (Visual C#) was developed for measuring wrist and fi nger joint angles in the controlling and controlled limbs. In our tracking system, the measured wrist joint angle was defined as the angle between hand and forearm. The fi ve‐ fi nger joint angle was represented as the mean metacar‐ pophalangeal joint (MPJ) angles of the fi ve fi ngers (5F‐MPJ).ree metrics were used to quantify real‐time performance.enormalized cross-correlation(ρxy) was de fi ned as the sim‐ilarity, synchronism, or functional coupling of three mea‐sured angle trajectories, which was calculated as:

Figure 2 Flowchart of the wrist joint torque reproduction test using stimulating pulse generation algorithm based on the sEMG threshold.
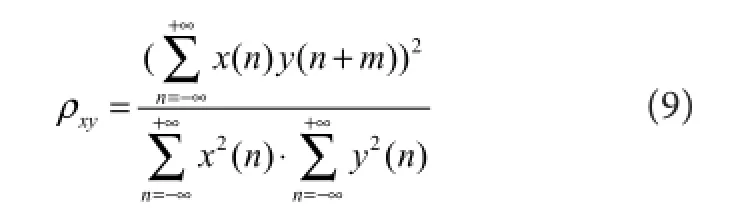
wherex(n)andy(n)were the joint angle trajectories of the controller and the controlled, respectively. The variablemcorrelated with the metricdelay time(Td), which was the time offset of the controlled trajectory compared with the controlling trajectory.e last metricmotion completion rate (MCR)was defined as the percentage of successfully com‐pleted motions.
Statistical analysis
Statistical analysis was performed using SPSS statistics 19.0 software (IBM corp., Chicago, IL, USA) and the paired Wilcoxon signed‐rank test.e results are expressed as the mean ± SD.P≤ 0.05 was considered signi fi cantly di ff erent.
Results
Selection of three sEMG thresholds from the torque reproduction test results
The assessing metrics (R,RMSE, andMBE) for each movement trial using the stimulation generation algorithm with three different ratios are presented in Figure 5. The meanRvalue across the six subjects and four behaviors was 0.80 ± 0.07 for TH_60% protocol (Nk= 60%), 0.84 ± 0.08 for TH_90% protocol (Nk= 90%), and 0.86 ± 0.08 for TH_120% protocol (Nk= 120%).e corresponding aver‐ageRMSEvalues were 20.7 ± 4.8%, 20.1 ± 4.6%, and 21.1 ± 6.0%, respectively.e corresponding average MBE values were 0.02 ± 0.10, 0.07 ± 0.08, and 0.10 ± 0.08, respectively. Additionally, the TH_90% protocol showed significantly higherRvalues for wrist fl exion behaviors (Z= 2.201,P= 0.028) and fast wrist extension (Z= 2.023,P= 0.043) than the TH_60% protocol, and slightly (not signi fi cantly) high‐erRvalues than the TH_60% protocol for the slow wrist ex‐tension.e TH_120% protocol showed signi fi cantly higher R values for the slow wrist extension (Z= 2.201,P= 0.028), fast wrist extension (Z= 2.023,P= 0.043), and fast wrist fl exion (Z= 1.992,P= 0.046) compared with the TH_60% protocol, and slightly higherRvalues than the TH_60% protocol for wrist fl exion with slow trail (Figure 5A). For the four‐type wrist behaviors, no significant differences were found inRMSEvalues between the three protocols, with the exception that theRMSEvalue obtained for the fast wrist fl exion movement generated using the TH_60% protocol was signi fi cantly lower than in the TH_90% pro‐tocol (Z= 1.992,P= 0.046) (Figure 5B). Simultaneously, the MBE result for the TH_120% protocol showed signi fi‐cantly higher negative offset from the target torque (P<0.05), which indicated that the TH_120% protocol did not produce su fficient torque to trace the target compared with the TH_60% protocol (Figure 5C). Although the TH_120% protocol also showed a slightly higher bias from the tar‐get, and considering the trade‐off between the similarity and o ff set with the target force, the TH_90% protocol was selected as the threshold‐de fi nition method for the stimula‐tion generation and classi fi cation.
Motion classi fi cation o ff-line test result in subjects with the optimized sEMG threshold and logistic regression classi fi er
According to the torque reproduction test above, the mean thresholds were computed across the six subjects as 0.030 V for wrist movements. The hand motion thresholds were also de fi ned as 90% of the sEMG mean absolute value, but without force tracing test.e mean thresholds for the hand grasp/ fi nger extension were 0.105 V and 0.130 V, respective‐ly.able 2 presents the trained logistic regression classi fi er parameter (–θ1/–θ2) and the corresponding performance metric CA in the off‐line analysis. Then, the average pa‐rameter (–θ1/–θ2) across the six subjects was selected as the trained logistic regression classifier, and the total CA for all datasets from the six subjects was analyzed o ff‐line. Figure 6 illustrates the scatter plot of the sEMG signals of two channels and decision boundary using the trained logistic regression classi fi er with optimizedθand the above‐de fi ned thresholds. TheCAof the wrist flexion/extension across the total six subjects was 99.92% using the parameter (–θ1/θ2=1.05), and theCAof the hand grasp/ fi nger extension was 98.90% using the parameter (–θ1/θ2=1.01).
Real-time sEMG bridging test results in subjects with the wearable functional electrical stimulation system
Discussion
We present a novel sEMG‐controlled FES system, which can enhance volitional hand motor function control of FES induced muscle contractions during different target move‐ments. The method presents two advantages over previous systems. (1) Aer statistically analyzing and selecting an op‐timized sEMG amplitude threshold from six healthy subjects, the low‐complexity algorithm was based on logistic regression for hand movement classification, which allowed for more accuracy and reliability compared with the multi‐channel sys‐tem. (2) Using a series of novel design concepts, including the integration of a detecting circuit and analog‐to‐digital con‐verter, a miniaturized FES circuit technique, a low‐power su‐per‐regeneration chip for wireless receiving, and two wearable armbands, we were able to create a prototype system that was signi fi cantly reduced in size, power, and overall cost, making it wearable and suitable for rehabilitation at home. Simultane‐ously, the adoption of a Li‐battery as a power supply helped to ensure greater safety of use (Cook et al., 2015).
able 1 Subject parameters of wrist joint in torque reproduction test
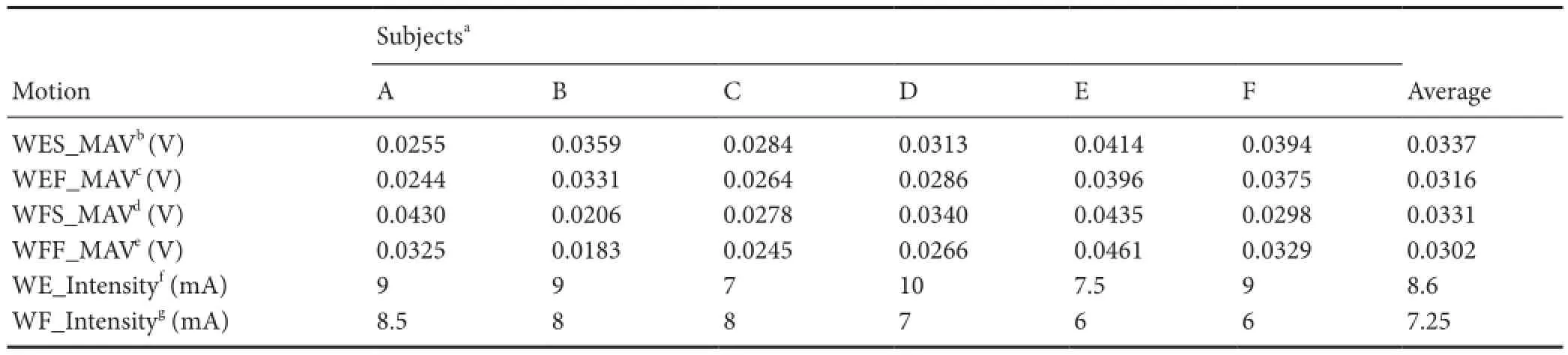
able 1 Subject parameters of wrist joint in torque reproduction test
aSubjects A—F;bWES_MAV,cWEF_MAV,dWFS_MAV, andeWFF_MAV represent the mean absolute values (MAVs) of sEMG signals that correspond with the four wrist test behaviors, including slow/fast wrist extension (WES/WEF), and slow/fast wrist fl exion (WFS/WFF). Each MAV was calculated based on three 5‐second segments of sEMG extracted from each voluntary test behavior that elicited a 30% maximal voluntary contraction.e sEMGs were acquired from wrist extensor (slow trial), wrist extensor (fast trial), wrist fl exor (slow trial), and wrist fl exor (fast trial), separately.fWE_Intensity andgWF_Intensity represent stimulation intensity of wrist extension and fl exion task, respectively.
SubjectsaMotion A B C D E F Average WES_MAVb(V) 0.0255 0.0359 0.0284 0.0313 0.0414 0.0394 0.0337 WEF_MAVc(V) 0.0244 0.0331 0.0264 0.0286 0.0396 0.0375 0.0316 WFS_MAVd(V) 0.0430 0.0206 0.0278 0.0340 0.0435 0.0298 0.0331 WFF_MAVe(V) 0.0325 0.0183 0.0245 0.0266 0.0461 0.0329 0.0302 WE_Intensityf(mA) 9 9 7 10 7.5 9 8.6 WF_Intensityg(mA) 8.5 8 8 7 6 6 7.25
able 3 Measured metrics of the real-time bridging test using the wearable EMG-bridge system
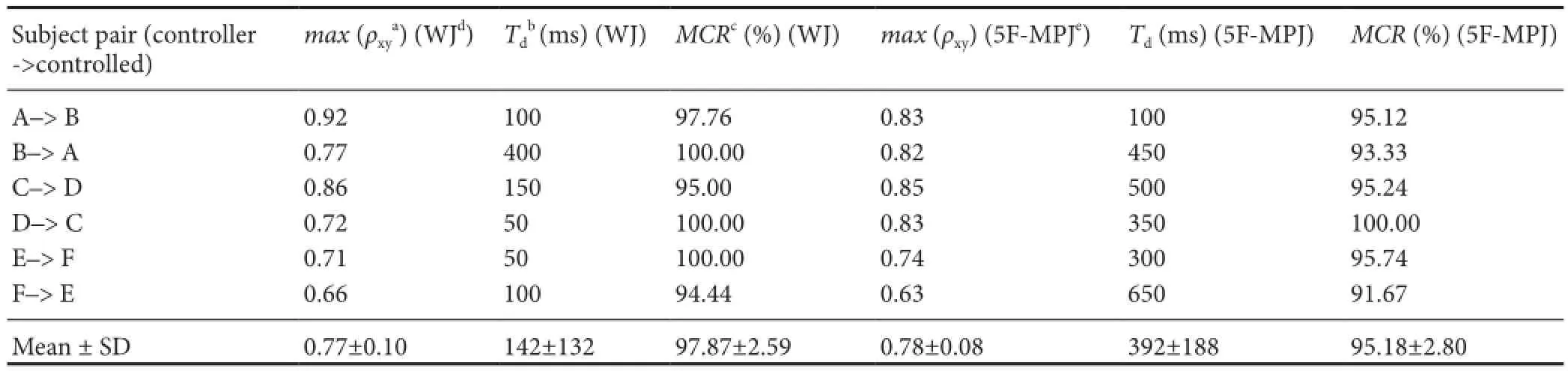
able 3 Measured metrics of the real-time bridging test using the wearable EMG-bridge system
aρxy: Normalized cross‐correlation of two measured angle trajectories, which was calculated as Equation (9).bTd: Delay time o ff set of the controlled trajectory compared with the controlling trajectory.cMCR: Motion completion rate, which is de fi ned as the percentage of successfully completed motions.dWJ: Wrist joint angle, which is de fi ned as the angle between hand and forearm.e5F‐MPJ: Mean metacarpophalangeal joint angles from the fi ve fi ngers.
Subject pair (controller‐>controlled)max(ρxya) (WJd)Tdb(ms) (WJ)MCRc(%) (WJ)max(ρxy) (5F‐MPJe)Td(ms) (5F‐MPJ)MCR(%) (5F‐MPJ) A–> B 0.92 100 97.76 0.83 100 95.12 B–> A 0.77 400 100.00 0.82 450 93.33 C–> D 0.86 150 95.00 0.85 500 95.24 D–> C 0.72 50 100.00 0.83 350 100.00 E–> F 0.71 50 100.00 0.74 300 95.74 F–> E 0.66 100 94.44 0.63 650 91.67 Mean ± SD 0.77±0.10 142±132 97.87±2.59 0.78±0.08 392±188 95.18±2.80
able 2 Parameter of classifier & performance metrics for motion classi fi cation o ff-line analysis

able 2 Parameter of classifier & performance metrics for motion classi fi cation o ff-line analysis
aOptimizedθmeans using the average parameter (–θ1/θ2) across six subjects as the trained logistic regression classifier and analysis of total classification accuracy (CA) for all datasets from six subjects.bCA represents motion classification accuracy of the off‐line trained classi fi er.
Subject CA (%) (hand)–θ1/θ2(wrist) CAb(%) (wrist)–θ1/θ2(hand) A 0.975 100.00 1.072 99.73 B 0.926 100.00 0.926 99.35 C 1.100 99.60 1.008 100.00 D 1.200 99.70 1.043 96.35 E 1.025 99.96 1.080 99.96 F 1.052 100.00 0.949 100.00 Optimizedθa1.050 99.92 1.010 98.90
This proposed wearable EMG‐bridge system helped to overcome the limitations of previous systems (Huang et al., 2014) that had many wires and were cumbersome to wear. It is also interesting that this system can be used in a healthy subject without the use of a ground ring (Huang et al., 2014).e subject can successfully control contralateral wrist and fingers through the use of a wearable transmitting module worn on the upper extremity and a wearable receiving mod‐ule applied to the other limb, as previously described (Knut‐son et al., 2012). This was mainly possible because of the contribution of the CCTDM‐FES circuit design (Wang et al., 2015), which used the multiplexer circuit to quickly estab‐lish a short connection between two stimulating electrodes and reduce the impact of the stimulation artifact.erefore, it could be applied to upper‐extremity hemiplegia to assist with rehabilitation at home, if positioning of the electrodes on the skin can be properly made (Knutson et al., 2012). Stroke patients do not typically require stimulation to the wrist (or fi nger) fl exor, so this system could assist patients in the stimulation of the wrist (or fi nger) extensor by changing the electrode positions according to markings and photos.
Nevertheless, there are still several aspects worth noting from this study:
(1) Because the above experiments were performed on healthy subjects and not impaired subjects aer stroke, the musculoskeletal physiology is di ff erent between healthy and impaired subjects with stroke or spinal cord injury (Ha‐fer‐Macko et al., 2008; Biering‐Sørensen et al., 2009), so we cannot completely infer feasibility of this proposed wearable EMG‐bridge system. However, we believe that it can provide excellent performance for contralaterally controlled FES applications (Knutson et al., 2014), because the healthy side of a patient with upper‐extremity hemiplegia is similar tohealthy subjects. Further clinical research should be con‐ducted in various patients to test the robustness of the wear‐able EMG‐bridge system.

Figure 3 Prototype wearable EMG-bridge system.
(2) According to results from the above‐described wrist joint torque reproduction test, the stimulating pulse gener‐ation algorithm we used could result in the stimulated force tracing the voluntary force to a certain degree. However, at the force peak point shown in Figure 4, it signi fi cantly de‐teriorated performance. During voluntary contractions, the central nervous system modulates muscle force by adjusting the number of recruited motor units (recruitment coding) and the activation frequency (rate coding) (Kamen and Du, 1999). Analogously in the FES, the stimulation intensity (amplitude and pulse width) and frequency can be modu‐lated to control the muscle force. Kesar et al. (2008) report‐ed that frequency modulation showed better performance for both peak forces and force‐time integrals in response to the fatiguing trains than pulse duration modulation, while producing similar levels of muscle fatigue.erefore, the frequency modulation was selected for our stimulating pulse generation algorithm, but the frequency modulation with fixed intensity was responsible for the deterioration in force tracing performance, which is in accordance with previous results (Binder‐Macleod and McDermond, 1992) showing that varying any stimulation parameters independently results in a nonlinear force response. Ad‐ditionally, both recruitment and rate coding strategies more effectively prevent fatigue than the use of indepen‐dent modulation alone (Johnson and Fuglevand, 2011). A pulse width and frequency co‐modulation stimulation strategy based on sEMG time‐domain features was recently proposed by our group (Zhou et al., 2016). Although the co‐modulation strategy exhibited better voluntary force re‐production and more fatigue resistance than the traditional sEMG proportional control strategy, the high complexity of computation and transplantation to the wearable system requires further development.
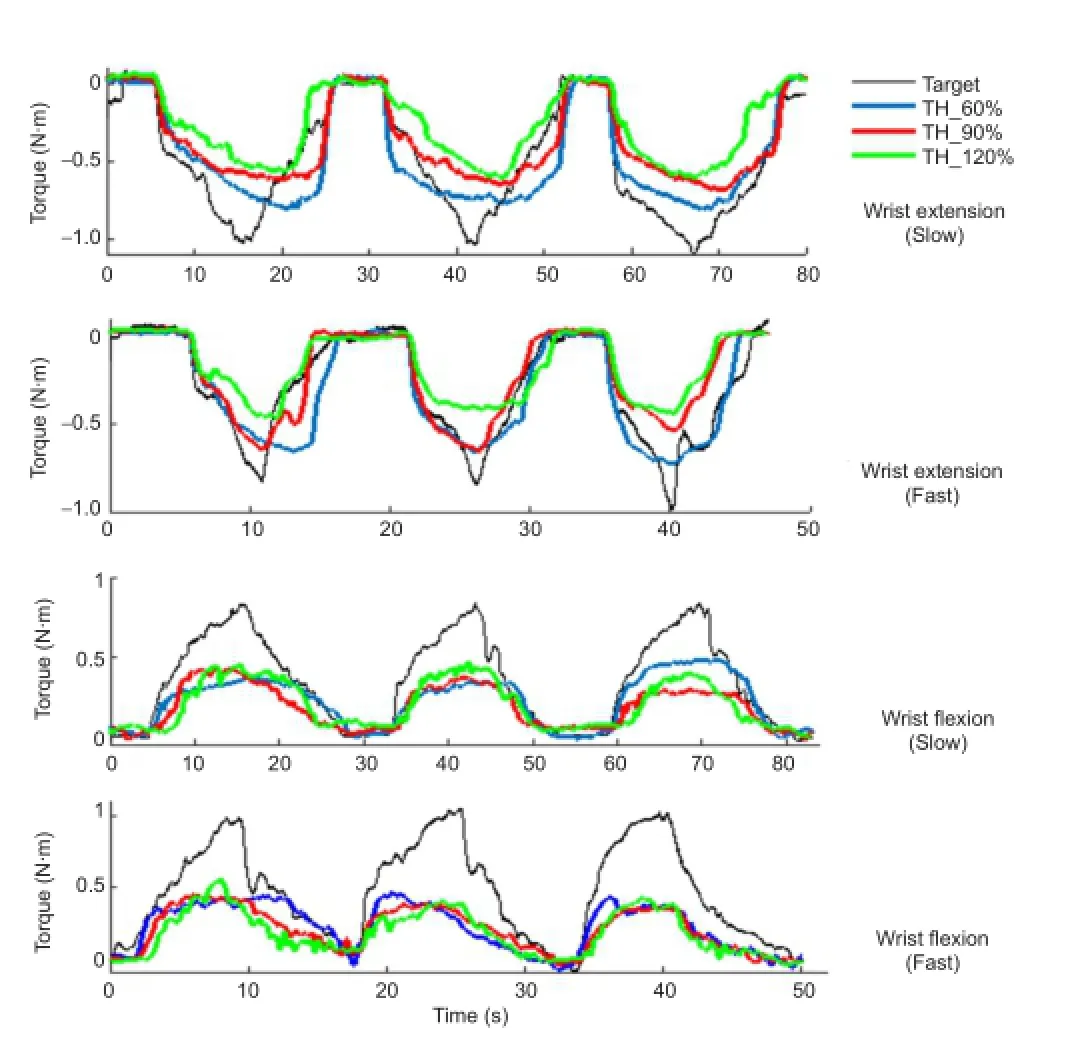
Figure 4 Representative wrist torque trajectories of voluntary and stimulated using stimulation generation algorithms by selecting three ratios (Nk= 60%, 90%, and 120%) of surface electromyography mean absolute value in each movement trial for the thresholds (Subject B).
(3) In our current system, only one agonist muscle corre‐sponding with speci fi c movement was chosen, whereas in reality, the smooth, continuous, and functional voluntary movement was performed by the agonist, stabilizer, and synergist together. Additionally, the most typical need for stroke survivors is fi nger and thumb extension, rather than wrist extension. Therefore, methods such as non‐negative matrix factorization (Jiang et al., 2009, 2014) for extracting time‐invariant synergies in multi‐channel sEMG and multi‐pad electrode surface stimulation system (Malešević et al., 2012; Popović‐Maneski et al., 2013) are needed to realize multi‐degree of freedom bridging‐control movements.
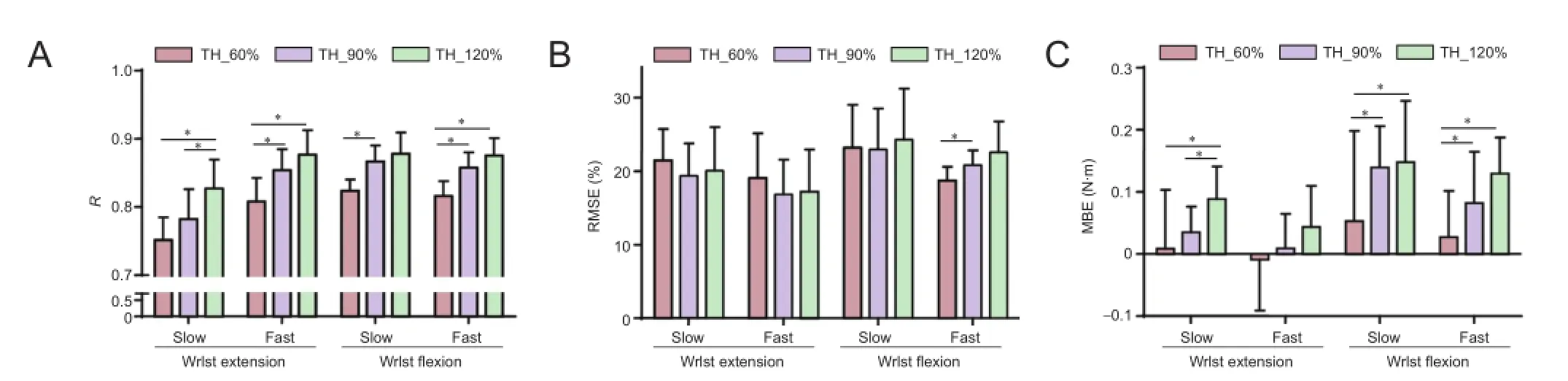
Figure 5ree metrics (R, RMSE, and MBE) values for the three di ff erent thresholds of isometric wrist torque reproduction test.

Figure 6 Scatter plot of the two channels surface electromyography signals and decision boundary using the trained logistic regression classi fi er with optimizedθand thresholds (Nk= 90%) for o ff-line motion classi fi cation performed on datasets from six subjects.
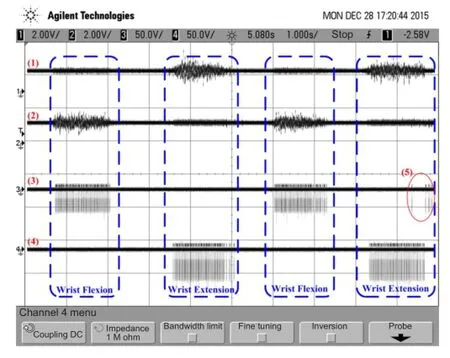
Figure 7 Representative measured waveforms of signals in the real-time bridging test using the wearable EMG-bridge system.
In summary, the present study proposed a wearable EMG‐bridge system for real‐time volitional hand motor function control to improve voluntary participation of hemiplegic patients. Movement of the controller’s hand can be regener‐ated on the hand under control with high accuracy and low latency. For future studies, the feasibility of wearable EMG‐bridge systems should be tested on hemiplegic patients using a contralaterally controlled FES paradigm to establish voli‐tional participation of paralyzed patients.

Figure 8 Representative results of the joint angles in the real-time bridging test using the wearable EMG-bridge system.
Declaration of participant consent:obtained all appropriate participant consent forms. In the form the participant(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal.e participants understand that their names and initials will not be published and due e ff orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgments:We would like to thank Chong-Yao Xu of Guangdong University of Science and Technology, China and Yu-Ting Su of Nanjing SQ Medical Device Corporation, China for their technical help.
Author contributions:HPW designed the study, manufactured the circuit, analyzed the data, and wrote the paper. ZYB and YZ designed and manufactured the circuits. YXZ performed the experiment and designed the motion assessment instruments. ZGW and XYL gave guidance to the design, developed the experimental method, and revised this paper. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:
Barsi GI, Popovic DB, Tarkka IM, Sinkjær T, Grey MJ (2008) Cortical excitability changes following grasping exercise augmented with electrical stimulation. Exp Brain Res 191:57‐66.
Biering‐Sørensen B, Kristensen IB, Kjaer M, Biering‐Sørensen F (2009) Muscle aer spinal cord injury. Muscle Nerve 40:499‐519.
Binder‐Macleod SA, McDermond LR (1992) Changes in the force‐fre‐quency relationship of the human quadriceps femoris muscle follow‐ing electrically and voluntarily induced fatigue. Physer 72:95‐104.
Cauraugh JH, Kim SB, Duley A (2005) Coupled bilateral movements and active neuromuscular stimulation: intralimb transfer evidence during bimanual aiming. Neurosci Lett 382:39‐44.
Cook AJ, Gargiulo GD, Lehmann T, Hamilton TJ (2015) Open platform, eight‐channel, portable bio‐potential and activity data logger for wear‐able medical device development. Electron Lett 51:1641‐1643.
Craven BC, Hadi SC, Popovic MR (2015) Functional Electrical Stimu‐lationerapy: Enabling Functionrough Reaching and Grasping. In: International Handbook of Occupationalerapy Interventions (Söderback I, ed), pp 587‐605. Cham: Springer International Pub‐lishing.
Hafer‐Macko CE, Ryan AS, Ivey FM, Macko RF (2008) Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev 45:261‐272.
Hara Y, Obayashi S, Tsujiuchi K, Muraoka Y (2013)e e ff ects of elec‐tromyography‐controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clin Neurophysiol 124:2008‐2015.
Huang ZH, Wang ZG, Lv XY, Zhou YX, Wang HP, Zong SH (2014) A novel functional electrical stimulation‐control system for restoring motor function of post‐stroke hemiplegic patients. Neural Regen Res 9:2102‐2110.
Ijzerman MJ, Sto ff ers TS, Klatte M, in’t Groen FA, Snoek GJ, Vorsteveld JH, Nathan RH, Hermens HJ (1996)e NESS Handmaster orthosis: restoration of hand function in C5 and stroke patients by means of electrical stimulation. J Rehabil Sci 9:86‐89.
Jiang N, Englehart KB, Parker PA (2009) Extracting simultaneous and proportional neural control information for multiple‐DOF prosthe‐ses from the surface electromyographic signal. IEEE Trans Biomed Eng 56:1070‐1080.
Jiang N, Rehbaum H, Vujaklija I, Graimann B, Farina D (2014) Intui‐tive, online, simultaneous, and proportional myoelectric control over two degrees‐of‐freedom in upper limb amputees. IEEE Trans Neural Syst Rehabil Eng 22:501‐510.
Johnson LA, Fuglevand AJ (2011) Mimicking muscle activity with elec‐trical stimulation. J Neural Eng 8:016009.
Kamen G, Du DC (1999) Independence of motor unit recruitment and rate modulation during precision force control. Neuroscience 88:643‐653.
Kesar T, Chou LW, Binder‐Macleod SA (2008) Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol 18:662‐671.
Kilgore KL, Peckham PH, Keith MW,rope GB, Wuolle KS, Bryden AM, Hart RL (1997) An implanted upper‐extremity neuroprosthesis. Follow‐up of fi ve patients. J Bone Joint Surg Am 79:533‐541.
Knutson JS, Hisel TZ, Harley MY, Chae J (2009) A novel functional elec‐trical stimulation treatment for recovery of hand function in hemiple‐gia: 12‐week pilot study. Neurorehabil Neural Repair 23:17‐25.
Knutson JS, Harley MY, Hisel TZ, Makowski NS, Chae J (2014) Con‐tralaterally controlled functional electrical stimulation for recovery of elbow extension and hand opening aer stroke: a pilot case series study. Am J Phys Med Rehabil 93:528‐539.
Knutson JS, Harley MY, Hisel TZ, Hogan SD, Maloney MM, Chae J (2012) Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early‐phase randomized clinical trial in subacute stroke patients. Neurorehabil Neural Repair 26:239‐246.
Li G, Schultz AE, Kuiken TA (2010) Quantifying pattern recogni‐tion‐based myoelectric control of multifunctional transradial pros‐theses. IEEE Trans Neural Syst Rehabil Eng 18:185‐192.
Liberson WT, Holmquest HJ, Scot D, Dow M (1961) Functional elec‐trotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Reha‐bil 42:101‐105.
Lorrain T, Jiang N, Farina D (2011) In fl uence of the training set on the accuracy of surface EMG classi fi cation in dynamic contractions for the control of multifunction prostheses. J Neuroeng Rehabil 8:25.
Malešević NM, Maneski LZP, Ilić V, Jorgovanović N, Bijelić G, Keller T, Popović DB (2012) A multi‐pad electrode based functional electrical stimulation system for restoration of grasp. J Neuroeng Rehabil 9:66.
McGie SC, Zari ff a J, Popovic MR, Nagai MK (2015) Short‐term neu‐roplastic e ff ects of brain‐controlled and muscle‐controlled electrical stimulation. Neuromodulation 18:233‐240.
Nathan RH (1989) An FNS‐based system for generating upper limb function in the C4 quadriplegic. Med Biol Eng Comput 27:549‐556.
Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R (2003)‘Thought’‐‐control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett 351:33‐36.
Phinyomark A, Phukpattaranont P, Limsakul C (2012) Feature reduc‐tion and selection for EMG signal classification. Expert Syst Appl 39:7420‐7431.
Popović‐Maneski L, Kostić M, Bijelić G, Keller T, Mitrović S, Konstan‐tinović L, Popović DB (2013) Multi‐pad electrode for e ff ective grasp‐ing: design. IEEE Trans Neural Syst Rehabil Eng 21:648‐654.
Popović DB (2014) Advances in functional electrical stimulation (FES). J Electromyogr Kinesiol 24:795‐802.
Saxena S, Nikolić S, Popović D (1995) An EMG‐controlled grasping system for tetraplegics. J Rehabil Res Dev 32:17‐24.
Shen XY, Du W, Huang W, Chen Y (2016) Rebuilding motor function of the spinal cord based on functional electrical stimulation. Neural Regen Res 11:1327‐1332.
Thorsen R, Spadone R, Ferrarin M (2001) A pilot study of myoelec‐trically controlled FES of upper extremity. IEEE Trans Neural Syst Rehabil Eng 9:161‐168.
Wang H, Wang ZG, Wang R, Xu J, Fan WN (2012) RF front‐end for 315/433MHz super‐regenerative receiver. Dianlu yu Xitong Xuebao 17:15‐19.
Wade RC, Gorgey AS (2016) Skeletal muscle conditioning may be an effective rehabilitation intervention preceding functional electrical stimulation cycling. Neural Regen Res 11:1232‐1233.
Wang HP, Wang ZG, Lü XY, Huang ZH, Zhou YX (2015) Design of a pulse‐triggered four‐channel functional electrical stimulator using complementary current source and time division multiplexing out‐put method. Conf Proc IEEE Eng Med Biol Soc 2015:1671‐1674.
Zhou Y, X L, Wang Z, Huang Z, Yang J, Zhao X (2011) Surface myo‐electric signals decoding using the continuous wavelet transform singularity detection. Bioelectronics and Bioinformatics (ISBB), 2011 International Symposium on 2011:191‐194.
Zhou YX, Wang HP, Bao XL, Lü XY, Wang ZG (2016) A frequency and pulse‐width co‐modulation strategy for transcutaneous neuromus‐cular electrical stimulation based on sEMG time‐domain features. J Neural Eng 13:016004.
Copyedited by Cooper C, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.197139
Accepted: 2016-11-10
*Correspondence to: Zhi-gong Wang, Dr.-Ing., zgwang@seu.edu.cn.
- 中国神经再生研究(英文版)的其它文章
- Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration
- Information for Authors -Neural Regeneration Research
- A new computational approach for modeling diffusion tractography in the brain
- Celebration of the 10thAnniversary of Neural Regeneration Research
- Terapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury
- Blood microRNAs as potential diagnostic markers for hemorrhagic stroke

