Transfer of the extensor indicis proprius branch of posterior interosseous nerve to reconstruct ulnar nerve and median nerve injured proximally: an anatomical study
Pei-ji Wang, Yong Zhang, Jia-ju Zhao, Ju-pu Zhou, Zhi-cheng Zuo, Bing-bing Wu
Department of Hand and Foot Surgery, Second A ffiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
Transfer of the extensor indicis proprius branch of posterior interosseous nerve to reconstruct ulnar nerve and median nerve injured proximally: an anatomical study
Pei-ji Wang*,#, Yong Zhang#, Jia-ju Zhao, Ju-pu Zhou, Zhi-cheng Zuo, Bing-bing Wu
Department of Hand and Foot Surgery, Second A ffiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
How to cite this article:Wang PJ, Zhang Y, Zhao JJ, Zhou JP, Zuo ZC, Wu BB (2017) Transfer of the extensor indicis proprius branch of posterior interosseous nerve to reconstruct ulnar nerve and median nerve injured proximally: an anatomical study. Neural Regen Res 12(1):143-148. Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Graphical Abstract

Extensor indicis proprius (EIP) branch of posterior interosseous nerve transferr to reconstruct thenar branch of median nerve (BMN) and deep branch of ulnar nerve (DBUN)
#
orcid: 0000-0001-6131-2030 (Pei-ji Wang)
Proximal or middle lesions of the ulnar or median nerves are responsible for extensive loss of hand motor function.is occurs even when the most meticulous microsurgical techniques or nerve gras are used. Previous studies had proposed that nerve transfer was more e ff ec‐tive than nerve graing for nerve repair. Our hypothesis is that transfer of the posterior interosseous nerve, which contains mainly motor fi bers, to the ulnar or median nerve can innervate the intrinsic muscles of hands.e present study sought to investigate the feasibility of reconstruction of the deep branch of the ulnar nerve and the thenar branch of median nerve by transferring the extensor indicis proprius branch of the posterior interosseous nerve obtained from adult cadavers.e results suggested that the extensor indicis proprius branch of the posterior interosseous nerve had approximately similar diameters and number of fascicles and myelinated nerve fi bers to those of the deep branch of ulnar nerve and the thenar branch of the median nerve.ese con fi rm the feasibility of extensor indicis proprius branch of posterior interosseous nerve transfer for reconstruction of the deep branch of the ulnar nerve and the thenar branch of median nerve.is procedure could be a novel and e ff ective method for the functional recovery of the intrinsic muscles of hands aer ulnar nerve or median nerve injury.
nerve regeneration; posterior interosseous nerve; ulnar nerve; median nerve; extensor indicis proprius; thenar branch; nerve transfer; neural regeneration
Introduction
Proximal injuries of the ulnar nerve or median nerve are commonly seen in the clinic. Although sensation is restored in most cases, recovery of the motor function in the intrin‐sic muscles of the hand is usually poor, whether the injured nerve is repaired by traditional neurorrhaphy or nerve gras (Hundepool et al., 2015; Tang et al., 2016). This failure is principally the result of misconnection of the di ff erent func‐tional branches and misdirection of the regenerated nerve fi bers aer anastomosing of the injured nerve directly (Gor‐don et al., 2008). Another factor is the distance between the repair site and the receptor (intrinsic muscles of hand) is too far to be reinnervated. During the many months required for the regenerating axons to traverse this gap, the denervat‐ed muscles undergo irreversible atrophy and degeneration (Chao et al., 2013; Phillips et al., 2014). Prevention of intrin‐sic muscle atrophy and degeneration after proximal ulnar nerve or median nerve injury is still a challenging problem in the fi eld of hand surgery.
A traditional method of reconstructing intrinsic muscle satisfactorily is the dynamic method of tendon transfer (Seiler et al., 2013; Jia et al., 2015). However, it is di ffi cult to assess the tension on the tendon when it is surgically reat‐tached. Intrinsic palsy of the ulnar or median nerve is prob‐able with extrinsic palsy or tendon lesions suggesting tendon transfer is not feasible (Wang and Zhu, 1997).
Nerve transfer as a treatment has been described to re‐store function to the denervated muscle in peripheral nerve injuries. To the best of our knowledge, Wang et al. (1997) were the fi rst to study the feasibility of the transfer of the pronator quadratus branch of the anterior interosseous nerve (AION) to the thenar branch of the median nerve (TBMN) and the deep branch of the ulnar nerve (DBUN). They reported good results in a series of twenty patients and showed restoration of the functions of the intrinsic muscles. Studies on cadavers revealed that the DBUN, TBMN and AION had approximately the same number of axons and were of similar diameter, making it feasible to suture the nerves together satisfactorily. Transfer of the AION to the motor branch of the median and ulnar nerve should greatly reduce the delay in reinnervation of the in‐trinsic muscles and lead to an improved outcome (Wang and Zhu, 1997; Ustün et al., 2001).
In this study, we transfer the extensor indicis proprius (EIP) branch of the posterior interosseous nerve (PION) with the vascular pedicle and connect them to the DBUN and TBMN. This reconstruction was supported by microana‐tomical measurements and provides a novel method for the functional recovery of an intrinsic muscle of the hand.
Materials and Methods
Materials
Surgical simulation
Anastomotic patterns of the vascularized EIP branch of PION with the DBUN and TBMN were designed and sim‐ulated on the upper extremities of fresh adult cadaverous specimens.
Observation of microanatomy
The DBUN, TBMN, and EIP branch of PION were fully exposed under a microscope at 10× magnification (Shang‐hai Optical Instrument Factory, Shanghai, China).e EIP branch of PION was severed 9.5 ± 2.3 cm above the plane of the radial styloid process, at the level of the nerve entry point.e DBUN and the TBMN were severed 1.2 ± 0.8 cm and 6.0 ± 1.3 cm above the wrist respectively, after being separated atraumatically. The diameter of the nerve trunk, the number of myelinated nerve fi bers, the atraumatic and forced interfascicular dissection length, the distance between nerve entry point of the EIP branch of the PION to the proximal stump of the DBUN and TBMN after atraumatic dissection were all measured.
Osmium tetroxide staining
Statistical analysis
Data are expressed as the mean ± standard deviation. Sta‐tistical analyses were conducted using SPSS 17.0 statistics software (SPSS, Chicago, IL, USA). Differences in the an‐atomical data and the number of myelinated nerve fibers were analyzed by one‐way analysis of variance. If there was signi fi cant variation, thepost hocTukeyt‐test was used for the comparison between groups.P< 0.05 was considered statistically signi fi cant.

Figure 1 Myelinated nerve fi bers of the DBUN,BMN and the EIP branch of the PION (osmium tetroxide staining).
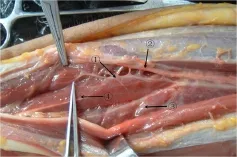
Figure 2 EIP branch of the PION and its concomitant vessels dissected in the forearm.

Figure 3erminal branch of the PION and its concomitant vessels dissected in the forearm.
Results
Anatomical characteristics of DBUN

Figure 4 Anastomosis of the EIP branch with terminal branch in the forearm.
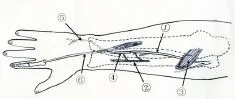
Figure 5 Schematic diagram of the reconstruction of the DBUN.
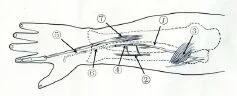
Figure 6 Schematic diagram of the reconstruction of theBMN.
At the wrist, the ulnar nerve crossed super fi cially to the fl ex‐or retinaculum at the radial side of the pisiform bone, where it divided into the super fi cial and deep branches. DBUN was a terminal, primarily motor branch of the ulnar nerve.e DBUN curved laterally to supply the hypothenar muscles and ran across the palm following the deep palmar arch, and then terminated in the abductor pollicis muscle. The trans‐verse diameter of the DBUN at the bifurcation was 2.04 ± 0.42 mm.e DBUN contained 2—5 fascicles with 1,342 ± 120 my‐elinated fi bers.e atraumatic dissection length of the super‐ fi cial and deep branches was 5.04 ± 2.02 cm, and the forced dissection length was 1.60 ± 1.30 cm. At 5.0—7.0 cm proximal portion after the bifurcation, the transverse diameter of the DBUN was 1.86 ± 0.40 mm (able 1 and Figure 1A).
Anatomical characteristics ofBMN
TBMN was the branch of the median nerve which innervat‐ed the intrinsic muscles of the thumb, such as abductor pol‐licis brevis, opponens pollicis, and the super fi cial head of the fl exor pollicis brevis. It passed distal to the transverse carpal ligament, and terminated in the opponens pollicis. The transverse diameter of the TBMN at the initial site was 1.62 ± 0.36 mm, whereas the transverse diameter of the TBMN at 3.0—4.0 cm proximal portion of the initial site was 1.58 ± 0.24 mm. The TBMN contained 2—4 fascicles with 1,088 ± 95 myelinated fi bers.e atraumatic dissectible length of the TBMN was 2.52 ± 0.60 cm, and the forced dissection lengthwas 0.50 ± 0.40 cm (able 1; Figure 1B).
able 1 Microanatomical parameters of the EIP branch of PION,BMN and DBUN

able 1 Microanatomical parameters of the EIP branch of PION,BMN and DBUN
Data are expressed as the mean ± SD ranging from minimum to maximum values (min—max) forn= 18 upper extremities. EIP: Extensor indicis proprius; PION: posterior interosseous nerve; TBMN: thenar branch of the median nerve; DBUN: deep branch of the ulnar nerve.
EIP branch of PION (n= 18) TBMN (n= 15) DBUN (n= 15) Fascicles counts (n) 1–3 2–4 2–5 Diameters (mm) 1.10±0.24(0.62–2.16) 1.62±0.36(0.73–2.46) 2.04±0.42(0.95–3.87) Myelinated fi bers (n) 618±76(439–931) 1,088±95(854–1267) 1,342±120(1041–1609) Atraumatic dissection length (cm) – 5.04±2.02(4.22–8.16) 2.52±0.60(1.43–4.89) Forced dissection length (cm) – 1.60±1.30(0.82–2.89) 0.50±0.40(0.31–0.93)
Anatomical features of EIP branch and terminal branch of posterior interosseous nerve
The terminal branches of the PION arose after the EIP branching, and descended under the extensor pollicis longus, formed a fusiform enlargement proximal to Lister’s tubercle, then traveled through the Lister’s tubercle to enter the carpus.e transverse diameter of the terminal branches of the PION at the initial site was 1.08 ± 0.14 mm, and 1.32 ± 0.22 mm at 0.5—0.8 cm proximal to Lister’s tubercle. In the distal 1/3 fore‐arm, the terminal branches of the PION were accompanied by dorsal perforating branches of the anterior interosseous artery at 6.34 ± 0.70 cm from the Lister’s tubercle (Figure 3).
Design and simulated transposition of the EIP branch
Surface projection and incision design
About 75.2% of the course of deep branch of radial nerves was on the line between the condylus lateralis humeri and the malleolus ulnaris, while the EIP branch was about 85.4%, similar to findings of Huang et al. (2002). Therefore, the surface projection of the EIP branch can be regarded as 3/5 distally of the line between the condylus lateralis humeri and the malleolus ulnaris when the forearm was pronated. Con‐sequently, we decided on a dorsal incision in the forearm and a carpometacarpal S‐shaped incision.
Dissection of the EIP branch and terminal branch of PION
The EIP branch was carefully dissected. Its concomitant blood vessels and a width of 0.5—1.0 cm fascia were pre‐served. It was then intersected 9.5 ± 2.3 cm above the malleolus radialis at the nerve entry point.e EIP branch with its concomitant vessels and fascia was freed from its distal to proximal end.e terminal branch was cut at the initial site with concomitant blood vessels and a width of 0.5—1.0 cm fascia preserved, and was dissected approxi‐mately 1.0 cm to the distal portion. The nerve and con‐comitant vessels were cut o ff at the level about 0.5—1.0 cm proximal to the Lister’s tubercle.e nerve was dissected to the proximal end to a length of 5.9 ± 0.8 cm, from where a tunnel to the volar aspect of forearm was made. After atraumatic dissection, the distance from the prox‐imal end of the DBUN or TBMN to the distal end of the transferred EIP branch was 3.40 ± 0.14 cm and 8.60 ± 0.56 cm, respectively.
Anastomosis of the EIP branch with DBUN or TBMN
The distal EIP branch was anastomosed to the proximal terminal branch of PION on the surface of the extensor pol‐licis longus.e DBUN was dissected atraumatically to the proximal end up to 6.0 ± 1.3 cm above the wrist where it was cut.e distal terminal branch of PION, through the tunnel, was anastomosed to the distal DBUN at the level 6.0 ± 1.3 cm above the volar side of the wrist.e thenar branch was dissected atraumatically and severed 1.2 ± 0.8 cm above the volar side of the wrist.e length of the gap between the dis‐tal terminal branch and the proximal thenar branch was 4.02 ± 0.96 cm.erefore, an autologous nerve was harvested to bridge the terminal branch to the thenar branch through the tunnel (Figures 4—6).
Discussion
It has heen demonstrated that the direct repair of injuries of the median nerve and ulnar nerve occurring proximally usually result in a poor functional outcome, with minimal recovery of intrinsic muscle function. The technique of transfer of the AION to reconstruct the motor component of the median and ulnar nerves has changed the prognosis of such lesions dramatically (Flores, 2011). Previous anatomical studies illustrated that the branch to the pronator quadratus of the AION was suitable for transfer to the motor fascicle of the ulnar and median nerves. Clinical studies reported good recovery of the function of the intrinsic muscles of the hands (Wang and Zhu, 1997; Wang et al., 1997). Based on these studies, we designed a novel method of transferring the EIP branch of the PION to reconstruct the TBMN and the DBUN.e purpose of this study was to investigate the feasibility of this technique.
The PION divides into multiple short branches, one of which is the EIP branch (Elgafy et al., 2000a, b). The EIPbranch is not easily injured because of its deep position in the forearm. All the nerve fi bers of the EIP branch are motor fi bers, so there should be no competition from sensory fi bers for the motor pathways during reinnervation. Therefore it can avoid “fault anastomosis” after anastomosing with the TBMN or terminal branch of PION. If the EIP branch is transferred, the functional loss will be minimal as the exten‐sor digitorum muscle can easily compensate for it. Regenera‐tion of a nerve with a good blood supply is better than those with a poor blood supply (Komatsu et al., 2013; Zhu et al., 2015).erefore, including a vascular pedicle in the transfer provides a good blood supply, bene fi tting nerve regeneration and supplying su ffi cient nutrition to the target muscle to en‐hance recovery of function.
The results of our anatomical studies demonstrated that the EIP branch of PION is su fficiently similar to either the TBMN or DBUN to allow direct suturing between them. Even though the number of myelinated nerve fi ber and the diameter of the EIP branch were slightly less than that of the DBUN and TBMN, it could be compensated during nerve regeneration through generating several lateral branches in the proximal end.is study has con fi rmed the feasibility of the EIP branch of PION transfer for reconstruction of the DBUN and TBMN anatomically.
There are limitations to the current study. Wang et al. (1997) reported that using a cutaneous nerve to bridge the defect of the pronator quadratus branch and the thenar branch resulted in a good functional recovery.is con fi rms that cutaneous nerve graft is feasible. In our study, the de‐fect length from the EIP branch to the proximal end of the DBUN or TBMN is respectively 3.40 ± 0.14 cm or 8.60 ± 0.56 cm.e terminal branch of PION is the sensory nerve which dominates sensation in the posterior region of the wrist and the dorsal hand (Liu et al., 2003; Ay et al., 2005; Jariwala et al., 2014).ere are no negative sensory consequences at the donor site following the transfer (Reissis et al., 1992; Inoue et al., 2002; Delclaux et al., 2014).erefore, using the terminal branch of the PION with the vascular pedicle to bridge the defects of the DBUN or TBMN in the same incision not only repairs the nerve defect, but also bene fi ts nerve regeneration. In reconstructing the thenar branch, the extra 4.02 ± 0.96 cm length of nerve defect has to be repaired by a cutaneous nerve gra. How that extra anastomosis in fl uences nerve re‐generation requires further study.
A transferred motor nerve can reinnervate the muscle of the acceptor nerve. However, the recovered function is dif‐ferent from its original one. Sunderland (1974) considered that the central nervous system can readjust to reassign its function.e use of a nerve that supplies co‐generic muscles would be preferable to facilitate the repair of the motor nerve injury.e function of EIP is similar to that of the intrinsic muscle. When transferring an EIP branch of the PION to re‐construct the function of intrinsic muscle, the new function can be developed by functional exercise, such as metacarpo‐phalangeal joint fl exion, interphalangeal joint extension and metacarpophalangeal joint fl exion of thumb.
In this study, we used light microscopy to quantify the number of myelinated nerve fibers in nerve branch cross sections. Recently, some studies comparing light and elec‐tron microscopy in the quantitative estimation of periph‐eral nerves have been performed. It was shown that small myelinated fibers are more easily detected with electron microscopy than with light microscopy (Geuna et al., 2000; Raimondo et al., 2009; Ronchi et al., 2014, 2015). In view of these studies, we suggest that light microscopy is a good starting point for the quantitative investigation of peripheral nerve regeneration. It is easier to perform, requires facilities available to everyone and is less expensive than electron mi‐croscopy. However, if signi fi cant di ff erences are not detect‐able with light microscopy, it may be necessary to compare and analyze the measurements with electron microscopy to detect any quantitative di ff erences due to the presence of very small regenerating fi bers or unmyelinated fi bers (Ronchi et al., 2015).
A couple of aspects are important to note as follows. (1) Sharp dissection of the small nerve should be performed un‐der the microscope. (2) To avoid damaging the nerve and its blood supply it is necessary to include a 0.5—1.0 cm of fascia tissue. (3) In the procedure of nerve transferring, the integri‐ty of tissues should be maintained to avoid separation of the nerve and vessels. (4) A plaster external fixation is needed for 1—2 week to reduce tension of the nerve. (5) Radial nerve injury on the upper arm or forearm is a contraindication of this surgical procedure. Further studies are needed to explore whether nerve transfer procedure is able to bene fi t patients.
In summary, this procedure has several distinct advan‐tages. Motor function is more likely to be reestablished with the use of a donor nerve that possesses mainly motor fi bers.e mean diameter and numbers of fi bers of the donor and recipient nerves are similar, and the loss of function is small and easily compensated for. It is feasible to reconstruct the DBUN and TBMN by transferring the EIP branch of the PION with its vascular pedicle. This could be a novel and e ff ective method for the recovery of function of the intrinsic muscles in hands aer ulnar nerve or median nerve injury.
Author contributions:PJW designed this study. YZ wrote the paper, collected and analyzed data. PJW and JPZ established experimental models and observed the anatomic features. JJZ performed histology staining, collected and analyzed data. ZCZ provided literature data. ZCZ and BBW were in charge of paper authorization. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:
Ay S, Apaydin N, Acar H, Akinci M, Piskin A, Tekdemir I, Elhan A (2005) Anatomic pattern of the terminal branches of posterior inter‐osseous nerve. Clin Anat 18:290‐295.
Chao T, Frump D, Lin M, Caiozzo VJ, Moza ff ar T, Steward O, Gupta R (2013) Matrix metalloproteinase 3 deletion preserves denervated motor endplates after traumatic nerve injury. Ann Neurol 73:210‐223.
Delclaux S, Aprédoaei C, Mansat P, Rongières M, Bonnevialle P (2014) Case report: Double nerve transfer of the anterior and posterior in‐terosseous nerves to treat a high ulnar nerve defect at the elbow. Chir Main 33:320‐324.
Elgafy H, Ebraheim NA, Yeasting RA (2000a)e anatomy of the pos‐terior interosseous nerve as a gra. J Hand Surg Am 25:930‐935.
Elgafy H, Ebraheim NA, Rezcallah AT, Yeasting RA (2000b) Posterior interosseous nerve terminal branches. Clin Orthop Relat Res:242‐251.
Flores LP (2011) Distal anterior interosseous nerve transfer to the deep ulnar nerve and end‐to‐side suture of the super fi cial ulnar nerve to the third common palmar digital nerve for treatment of high ulnar nerve injuries: experience in fi ve cases. Arq Neuropsiquiatr 69:519‐524.
Geuna S, Tos P, Battiston B, Guglielmone R (2000) Veri fi cation of the two‐dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fi bers in peripheral nerves. Ann Anat 182:23‐34.
Gordon T, Brushart TM, Chan KM (2008) Augmenting nerve regener‐ation with electrical stimulation. Neurol Res 30:1012‐1022.
Huang T, Liu DY, Qin JQ, Zhong SZ, Huo XK, Xiong SH, Yu L (2002) An anatomical study of muscular branches of radial nerve in the forearm. Zhonghua Guke Zazhi 22:158‐160.
Hundepool CA, Ultee J, Nijhuis TH, Houpt P, Jaquet JB, Spauwen PH, Hofman A, Ritt M, Kon M, Hovius SE (2015) Prognostic factors for outcome aer median, ulnar, and combined median‐ulnar nerve in‐juries: A prospective study. J Plast Reconstr Aesthet Surg 68:1‐8.
Inoue S, Ogino T, Tsutida H (2002) Digital nerve graing using the ter‐minal branch of posterior interosseous nerve: a report of three cases. Hand Surg 7:305‐307.
Jariwala A, Krishnan B, Soames R, Wigderowitz CA (2014) Important anatomical relationships of the posterior interosseous nerve in the distal forearm for surgical planning: a cadaveric study. J Wrist Surg 3:60‐63.
Jia YB, Liang ZH, Ren YZ, Han CX, Kong LY, Eerduntu (2015) Recon‐struction of anterior cruciate ligament with allogeneic tendon: a me‐dium‐term follow‐up. Zhongguo Zuzhi Gongcheng Yanjiu 19:6764‐6769.
Komatsu S, Wakabayashi T, Yamada K, Matsumoto K, Kimata Y, Kosa‐ka J (2013) Vascularized peripheral nerve graing promotes myelin‐ation of regrowing optic nerve. Neuroreport 24:566‐571.
Liu Y, Hong GX, Yang SH (2003) Microsurgery anatomy study and its clinical signi fi cance of the end part of the posterior interosseous nerve. Zhonghua Shouwaike Zazhi 19:58‐59.
Phillips BZ, Franco MJ, Yee A, Tung TH, Mackinnon SE, Fox IK (2014) Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury: case report and surgical technique. J Hand Surg Am 39:1358‐1362.
Raimondo S, Fornaro M, Di Scipio F, Ronchi G, Giacobini‐Robecchi MG, Geuna S (2009) Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: part II‐morphological techniques. Int Rev Neurobiol 87:81‐103.
Reissis N, Stirrat A, Manek S, Dunkerton M (1992) The terminal branch of posterior interosseous nerve: a useful donor for digital nerve graing. J Hand Surg Br 17:638‐640.
Ronchi G, Raimondo S, Geuna S, Gambarotta G (2015) New insights on the standardization of peripheral nerve regeneration quantitative analysis. Neural Regen Res 10:707‐709.
Ronchi G, Jager SB, Vaegter CB, Raimondo S, Giacobini‐Robecchi MG, Geuna S (2014) Discrepancies in quantitative assessment of normal and regenerated peripheral nerve fi bers between light and electron microscopy. J Peripher Nerv Syst 19:224‐233.
Seiler JGI, Desai MJ, Payne HS (2013) Tendon transfers for radial, me‐dian, and ulnar nerve palsy. J Am Acad Orthop Surg 21:675‐684.
Sunderland S (1974) The restoration of median nerve function after destructive lesions which preclude end‐to‐end repair. Brain 97:1‐14.
Tang YJ, Wu MH, Tai CJ (2016) Direct electrical stimulation on the injured ulnar nerve using acupuncture needles combined with reha‐bilitation accelerates nerve regeneration and functional recovery‐A case report. Complementer Med 24:103‐107.
Ustün ME, Oğün TC, Büyükmumcu M, Salbacak A (2001) Selective restoration of motor function in the ulnar nerve by transfer of the anterior interosseous nerve. An anatomical feasibility study. J Bone Joint Surg Am 83‐A:549‐552.
Wang Y, Zhu S (1997) Transfer of a branch of the anterior interosseus nerve to the motor branch of the median nerve and ulnar nerve. Chin Med J (Engl) 110:216‐219.
Wang Y, Zhu SX, Zhang BX (1997) Anatomical study and clinical application of transfer of pronator quadratus branch of anterior in‐terosseous nerve in the repair of thenar branch of median nerve and deep branch of ulnar nerve. Zhongguo xiufu Chongjian Waike Zazhi 11:335‐337.
Zhu Y, Liu S, Zhou S, Yu Z, Tian Z, Zhang C, Yang W (2015) Vascu‐larized versus nonvascularized facial nerve gras using a new rabbit model. Plast Reconstr Surg 135:331e‐339e.
Copyedited by Ann Dawes E, Maxwell R, Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.199007
Accepted: 2016-12-16
*Correspondence to: Pei-ji Wang, M.D., wangpeiji88@163.com.
- 中国神经再生研究(英文版)的其它文章
- Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration
- Information for Authors -Neural Regeneration Research
- A new computational approach for modeling diffusion tractography in the brain
- Celebration of the 10thAnniversary of Neural Regeneration Research
- Terapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury
- Blood microRNAs as potential diagnostic markers for hemorrhagic stroke

