Correction: Multi-site spinal stimulation strategies to enhance locomotion after paralysis
Correction: Multi-site spinal stimulation strategies to enhance locomotion after paralysis
Correction: Neural Regeneration Research, December, 2016; doi: 10.4103/1673‐5374.197131.
Due to the editorial o ffi ce’s error, a number of corrections were not made to the article prior to its publication; the publisher wishes to apologize to all concerned.e corrected version of the article ap‐pears in full below.
Multi-site spinal stimulation strategies to enhance locomotion after paralysis
With the advent of spinal cord epidural stimulation techniques,i.e., electrically enabled motor control (eEmc) in combination with activity dependent locomotor training, humans with traumatic complete sen‐sori‐motor paralysis are able to initiate voluntary leg movements and achieve gains in postural control, and bladder and sexual function (An‐geli et al., 2014). However, there are yet some technological barriers of eEmc for therapeutic purposes in humans that prevent weight bearing stepping. In this perspective, we highlight novel features of eEmc tech‐niques based off our recent work in spinalized rats and suggest their implementation in patients with a SCI for more meaningful functional motor outcomes.
In rodents, step‐like locomotor movements are generated when the spinal cord is stimulated at the rostral lumbar (L2) or sacral (S1) spinal segments (monopolar stimulation, with the reference electrode placed elsewhere in the body) (Ichiyama et al., 2005). Bipolar stimulation strategies that involve passing current between L2and S1(where elec‐trodes implanted over one of these segments is used as the reference electrode) have been most successful in eliciting robust stepping pat‐terns in the rodent (Shah et al., 2012). Frequencies of 40 Hz and pulse width of 0.2 ms have been widely adopted in almost all rodent studies. Similar to the rodent, in persons with a functionally complete SCI too, eEmc of the rostral or caudal spinal segments using bipolar con fi gura‐tion strategies and frequencies ranging from 30–40 Hz, pulse width of 0.2–0.5 ms has resulted in generating voluntary joint movements in the supine position.
These data collectively suggest that although the entire lumbosacral cord possesses rhythmogenic properties, the rostral lumbar and sacral cords are more robust in generating a motor output.e uniqueness of lumbar cord is most likely attributable to its greater potential in gener‐ating bursting rhythm and pattern of movement (McCrea and Rybak, 2008).e sacral cord, in contrast maintains its rhythmogenic capacity by direct activation of a ff erent fi bers and motor axons due to the com‐mon course of ascending a ff erent fi bers (nerve roots) around sacral seg‐ments. Additionally, ascending propriospinal circuits within the sacral cord terminate into and have an excitatory e ff ect on rostral lumbar lo‐comotor networks (Etlin et al., 2010). Given these unique features of the lumbosacral cord, an obvious scienti fi c inquiry is — what is the potential functional signi fi cance of the interactions of this input between the lum‐bar and sacral neuronal circuitries in de fi ning locomotor success? Will multi‐site eEmc strategies that adopt spatio‐temporal neuromodulation of the lumbar and sacral cords lead to more meaningful functional mo‐tor outcomes?
Given the relative preferential activation of rostral and caudal motor pools based on their topographical distribution along the spinal cord, the scienti fi c goal of multi‐site eEmc stimulation strategies is to spatial‐ ly and functionally activate a wide and discrete neuronal populations to synergistically in fl uence and modulate the excitability of sensorimotor pathways for an effective motor output. For example, in non‐injured human subjects, addition of stimulation at L1and/or at C5to an existing stimulation at T11immediately results in enhanced kinematics, inter‐limb coordination as well as EMG patterns in proximal and distal leg muscles. Sequential cessation of stimulation at C5and then at L1results in progressive degradation of the stepping pattern (Gerasimenko et al., 2015). Similarly, a stronger patterned EMG response from multiple leg muscles is observed with eEmc applied at multiple segments of the lumbosacral enlargement in contrast to localized individual segments in persons with a complete SCI (Angeli et al., 2014).
However, one of the limitations of multi‐site stimulation programs employed thus far, consists of stereotyped high‐frequency trains of elec‐trical pulses simultaneously delivered through multiple electrodes in the array.e e ff ects of alterations in parameters such as frequency of stimulation or the relative timing of stimulation pulses at distinct elec‐trode sites using independent monopolar con fi gurations on locomotor output have not been adequately explored. Because spinal locomotor related neural networks have varied functional and anatomical char‐acteristics, it seems reasonable to suggest that their selective activation using unique spatial and temporal stimulation con fi gurations will yield signi fi cant interactive e ff ects for locomotion regulation; thereby render‐ing multi‐site eEmc more conducive for translation to humans.
Recently, we tested the interactive e ff ects of di ff erent stimulation fre‐quencies and pulse intervals delivered at multiple spinal cord sites (in‐dependent monopolar stimulation at each stimulation site) in facilitat‐ing locomotion in spinal rats (Shah et al., 2016). We kept the frequency of stimulation at L2constant at 40 Hz and varied the frequency of stim‐ulation at S1( fi ve di ff erent frequencies) to allow rats spinalized at T10to step bipedally on a moving treadmill. Our goal was to target the rostral lumbar spinal cord and the sacral spinal cord for their unique capaci‐ties to generate bursting rhythmic patterns. Our data demonstrate that at 20 and 40 Hz frequencies of S1stimulation, and when stimulating the L2(40 Hz) and S1spinal segments independently, but with speci fi c inter stimulation time intervals, an obviously more robust stepping performance is observed in comparison to stimulation of lumbar or sacral segments alone. Noteworthily too, the stepping is achievable as early as three weeks aer the injury, with only six training sessions and without the use of a pharmacological agent (Shah et al., 2016). Stepping kinematics and coordinated locomotor EMG patterns of muscle acti‐vation throughout a step cycle are closer to pre‐injury levels when the independent source multi‐segmental stimulation is used.
Although use of speci fi c frequencies to elicit a locomotor response from independent eEmc at L2or S1has been previously reported; our data speci fi cally reveal that with the combined L240 Hz – S140 Hz and L240 Hz – S120 Hz sequences, a greater number of evoked responses are generated in a given time (Figure 1A–D); suggesting the need to ac‐tivate an optimal population of interneuronal networks or activate the same interneuronal pools more frequently for robust stepping to occur. Additionally, at the higher frequency, the presynaptic cell’s repeated and persistent stimulation of the postsynaptic cell most likely enhances syn‐aptic e ffi cacy to allow for consistent motor output (Hebbian learning).
Noticeably too, different interpulse intervals between the onset of L2and S1pulses elicit unique interactions in spinal evoked response in the muscle and this directly coincides with stepping ability (Figure 6 in Shah et al., 2016). Speci fi cally, near‐normal stepping is best attained when 1) the L2pulse is applied at 3—10 ms aer the onset of the S1pulse, [relative timing between stimulation pulses‐ condition 1] or when 2) the S1pulse is applied 0—7 msec aer the L2pulse, [condition 2] (Figure 1E, F). Our neurophysiology data demonstrate that in condition 1, the L2pulse modulates evoked response by S1pulse to result in a robust polysynaptic response; whereas in condition 2, the S1pulse signi fi cantly amplifies the evoked response elicited at L2. These findings lead us to suggest that the rostral lumbar segments play a greater role in generating stepping patterns; while sacral segments strongly facilitate the activity in‐duced by L2. Speci fi cally, for condition 1, eEmc at S1excites a larger pool of both lumbar and sacral neurons through the common course of as‐cending a ff erent fi bers (nerve roots) around sacral segments; while the L2pulse retains these excitability features of the evoked middle response (by S1) and engages a wider pool of neuronal networks (re fl ected in a poly‐synaptic response) that are crucial in generating an e ff ective locomotor pattern. For condition 2, eEmc at L2excites a ff erent nerves entering the cord and cord dorsum to initiate a rhythm and stepping pattern (Kiehn,2006). Subsequent eEmc at S1retrogradely activates spinal interneuronal pools, and through the propriospinal pathways that reside in the VII lamina of the sacral cord, can activate the lumbar locomotor related networks (see details in Shah et al., 2016). As such, the physiological mechanisms by which neural networks at lumbar and sacral interact are unique for the two segments. And depending on whether one segment is stimulated prior to the other, motor output is also altered.
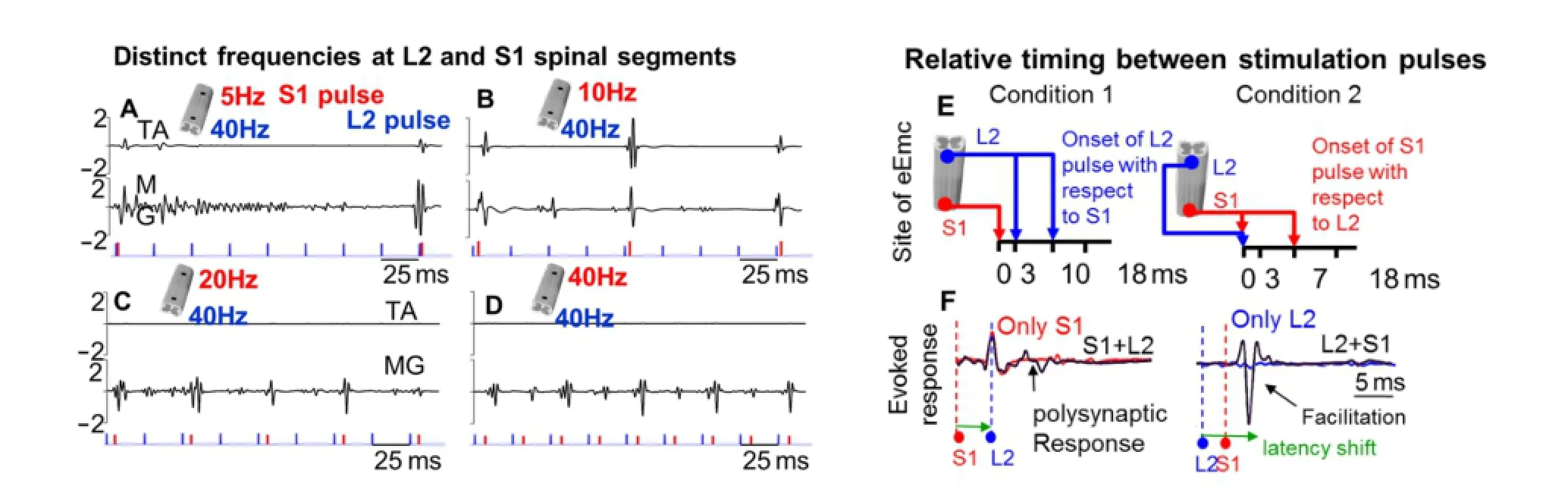
Figure 1 Multi-site stimulation that utilizes spatio-temporal independent monopolar stimulation strategies at L2and S1reveal unique e ff ects of change in frequency of stimulation and relative timing between stimulation pulses.
Collectively, in this brief perspective, we highlight the importance of incorporating a unique set of spatial and temporal variables delivered through multi‐site epidural stimulation to modulate spinal locomotor networks. An approach that capitalizes on 1) stimulation frequencies, 2) the site of stimulation, and 3) the relative timing between stimulation pulses, will eventually permit the complex interaction between excitatory and inhibitory circuits that are important for the generation of smooth locomotor output. Fabrication of multi‐electrode arrays that will allow such fl exibility might prove most e ff ective in regaining meaningful sen‐sorimotor function aer a SCI in humans.
Funding: This study was supported by Russian Foundation for Fundamental Research, No. 16-29-08173-o fi-m (YG), and the Russian Science Foundation, No. 14-45-00024 (YG). Support for data analysis, interpretation of results and publication cost was provided by the Craig H. Neilsen Foundation #338237 (PKS).
Prithvi K. Shah*, Yury Gerasimenko
Division of Rehabilitation Sciences, School of Health Technology and Management, Stony Brook University, Stony Brook, NY, USA; Departments of Physicalerapy and Neurobiology, Life Science Building, Stony Brook University, Stony Brook, NY, USA (Shah PK)
Department of Integrative Biology and Physiology, Charles E Young Dr, University of California, Los Angeles, CA, USA; Pavlov Institute of Physiology, St. Petersburg, Russia; Institute of Fundamental Medicine and Biology, Kazan Federal University, Kazan, Russia (Gerasimenko Y)
*Correspondence to: Prithvi K. Shah, Ph.D., Prithvi.Shah@stonybrook.edu. Accepted:2016-12-03
orcid: 0000-0001-6351-842X (Prithvi K. Shah)
doi: 10.4103/1673-5374.197131
How to cite this article:Shah PK, Gerasimenko Y (2017) Correction: Multi-site spinal stimulation strategies to enhance locomotion aer paralysis. Neural Regen Res 12(1):161-162.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ (2014) Altering spinal cord excitability enables voluntary movements aer chronic complete paralysis in humans. Brain 137:1394‐1409.
Dose F, Deumens R, Forget P, Taccola G (2016) Staggered multi‐site low‐frequency elec‐trostimulation e ff ectively induces locomotor patterns in the isolated rat spinal cord. Spinal Cord 54:93‐101.
Etlin A, Blivis D, Ben‐Zwi M, Lev‐Tov A (2010) Long and short multifunicular projec‐tions of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J Neurosci 30:10324‐10336.
Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR (2015) Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in non‐injured humans. J Neurophysiol 113:834‐842.
Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR (2005) Hindlimb step‐ping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 383:339‐344.
Kiehn O (2006) Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29:279‐306.
McCrea DA, Rybak IA (2008) Organization of mammalian locomotor rhythm and pat‐tern generation. Brain Res Rev 57:134‐146.
Sayenko DG, Atkinson DA, Floyd TC, Gorodnichev RM, Moshonkina TR, Harkema SJ, Edgerton VR, Gerasimenko YP (2015) E ff ects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci Lett 609:229‐234.
Shah PK, Sureddi S, Alam M, Zhong H, Roy RR, Edgerton VR, Gerasimenko Y (2016) Unique spatiotemporal neuromodulation of the lumbosacral circuitry shapes loco‐motor success aer spinal cord injury. J Neurotrauma 33:1709‐1723.
Shah PK, Gerasimenko Y, Shyu A, Lavrov I, Zhong H, Roy RR, Edgerton VR (2012) Variability in step training enhances locomotor recovery aer a spinal cord injury. Eur J Neurosci 36:2054‐2062.
10.4103/1673‐5374.199010
- 中国神经再生研究(英文版)的其它文章
- Restoring axonal localization and transport of transmembrane receptors to promote repair within the injured CNS: a critical step in CNS regeneration
- Information for Authors -Neural Regeneration Research
- A new computational approach for modeling diffusion tractography in the brain
- Celebration of the 10thAnniversary of Neural Regeneration Research
- Terapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury
- Blood microRNAs as potential diagnostic markers for hemorrhagic stroke

