Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
Georgia Gwinnett College, 1000 University Center Ln, School of Science and Technology, Lawrenceville, GA, USA
Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
Robert L. Haining*, Cindy Achat-Mendes
Georgia Gwinnett College, 1000 University Center Ln, School of Science and Technology, Lawrenceville, GA, USA
How to cite this article:Haining RL, Achat-Mendes C (2017) Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res 12(3):372-375.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
The loss of pigmented neurons from the human brain has long been the hallmark of Parkinson’s disease (PD). Neuromelanin (NM) in the pre-synaptic terminal of dopamine neurons is emerging as a primary player in the etiology of neurodegenerative disorders including PD. This mini-review discusses the interactions between neuromelanin and different molecules in the synaptic terminal and describes how these interactions might affect neurodegenerative disorders including PD. Neuromelanin can reversibly bind and interact with amine containing neurotoxins,e.g., MPTP, to augment their actions in the terminal, eventually leading to the instability and degeneration of melanin-containing neurons due to oxidative stress and mitochondrial dysfunction. In particular, neuromelanin appears to confer susceptibility to chemical toxicity by providing a large sink of iron-bound, heme-like structures in a pi-conjugated system, a system seemingly purposed to allow for stabilizing interactions including pi-stacking as well as ligand binding to iron. Given the progressive accumulation of NM with age corresponding with an apparent decrease in dopamine synthetic pathways, the immediate question of whether NM is also capable of binding dopamine, the primary functional monoamine utilized in this cell, should be raised. Despite the rather glaring implications of this finding, this idea appears not to have been adequately addressed. As such, we postulate on potential mechanisms by which dopamine might dissociate from neuromelanin and the implications of such a reversible relationship. Intriguingly, if neuromelanin is able to sequester and release dopamine in membrane bound vesicles, this intracellular pre-synaptic mechanism could be the basis for a form of chemical memory in dopamine neurons.
dopamine; dopamine storage; Parkinson’s disease; neurodegeneration; neuromelanin; neurotoxicity
Neuromelanin: What Is It?
For over 300 years, Parkinsonism has been associated with a loss of pigmentation from the human brain. Termed neuromelanin (NM) to distinguish it from the numerous melanin sources found throughout the body, it is absent at birth and naturally increases throughout a person’s lifetime, accelerating in synthesis and accumulation in adolescence. This pigmentation is most pronounced in catecholaminergic neurons of the substantia nigra pars compacta (SNpc) and locus coeruleus, leading to a blackened appearance in these regions of aged brains. Loss of NM and subsequent depigmentation of these brain regions is a hallmark feature of Parkinson’s disease (PD). This is primarily due to immune-mediated death of pigmented cells. There is evidence to suggest that DA neurons with high amounts of NM are more susceptible to degeneration. For example, NM-rich SNpc neurons undergo cell death compared to non-pigmented ventral tegmental area neurons in PD (Zecca et al., 2003; Zucca et al., 2015).
Dopamine is normally thought to be protected from auto-oxidation in secretory vesicles through co-localization of ascorbic acid and other antioxidants. Sulzer et al. (2000) showed that neuromelanin forms in cultured dopaminergic neurons when cytoplasmic concentrations of dopamine are artificially raised. In their experiment, PC-12 cells were exposed to high levels of dopamine through addition of L-3,4-dihydroxyphenylalanine (L-DOPA) to the cell culture media, which is rapidly taken up and subsequently converted to dopamine. Cells which had been artificially enhanced in their ability to sequester dopamine into vesicles showed significantly less accumulation of pigment. This critical result suggests that under the normal range of living conditions, times exist when a firing neuron becomes over-burdened and is unable to sequester dopamine into vesicles before overloading the neuron’s antioxidant defenses. Such antioxidants often take the form of thiols including cysteine and glutathione; as such, it is perhaps not surprising that natural NM has cysteine in its pheomelanin core, while being surrounded by a eumelanin component lacking cysteine.
NM is eventually found sequestered into double membrane granules inside neurons and contains lipids and peptides bound to its structurein vivo(Zecca et al., 2000). The finding that this structure is carefully packaged and retained is evidence in favor of an important function for this molecule, yet we still know little about its implications for the cell. Tat this pigmentation is not an enzymatically driven process in dopaminergic neurons may be highly relevant given the lack of a central genetic basis for most cases of PD.
Structure of Endogenous NM
NM has been historically poorly characterized largely due to the intrinsic nature of this spontaneously occurring polymer. It is structurally similar to non-neural melanins, with a predicted 3-D lattice structure and the ability to chelate and neutralize transition metals, ions and lipids. However, due to the spontaneous nature of the free radical reaction that occurs in neurons, this process is likely to be characterized by a certain degree of lack of control. In contrast, other natural melanins found in humans are enzymatically driven, not spontaneous. As such, the assortment of chemical precursors of ill-defined proportion which can incorporate in addition to dopamine and cysteine during the polymerization cascade could also result in the lack of a consistent, regularly ordered structure, particularly in the case of toxic chemical insult such as that imposed by 6-hydroxydopamine. In addition, catechol polymers are not easily de-polymerized, unlike other macromolecules utilized in biological systems such as polysaccharides, proteins, and nucleic acids which can by analyzed by relatively simple hydrolysis. Nevertheless, progress has been made in understanding the subunit makeup of natural NM. In one study, the ratio of hydrogen peroxide (H2O2) degradation products indicated that NM is derived mostly from dopamine, with 25% incorporation of cysteine in the form of a benzothiazine structure. In the same study, hydriodic acid reductive hydrolysis of NM revealed a 21% incorporation of Cys-DA-derived units into NM, while DOPA was found incorporated at a level near 6% for that observed with dopamine (Wakamatsu et al., 2003).
It is now known that NM isolated from human brain is comprised of granules with a diameter of 30 nm, similar to that observed for Sepia cuttlefish, bovine eye, and human eye and hair melanosomes. NM is a mixture of pheomelanin and eumelanin, the two major forms corresponding to the widely known light-haired and dark-haired phenotypes, respectively. Kinetic studies suggest that in such mixed pigments, pheomelanin formation occurs first with eumelanin formation occurring only after cysteine levels have been depleted. This leads to a predicted structural motif with pheomelanin at the core and eumelanin at the surface (Bush et al., 2006). The major lipid component of neuromelanin pigment derived from human SN was found to be the polyisoprenoid dolichol, accounting for 14% of the mass of the isolated pigment (Fedorow et al., 2005).
Chemical Binding to NM as a Causative Factor in PD
It has also been known for several decades that a curious feature of natural melanins is their ability to bind and retain organic compounds for long periods of times. Not surprisingly, abundant evidence suggests that the massive accumulation of pigment within the aged dopaminergic neuron also corresponds with the accumulation of numerous potentially toxic chemical entities. In particular, organic amines and metal ions such as manganese have been shown to exhibit high binding affinities with melanins (Karlsson and Lindquist, 2013). MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) for example is believed to induce its Parkinsonian-inducing effects after first being converted to methyl-phenyl-pyridinium ion (MPP+) by the enzyme monoamine oxidase B in nearby glial cells (Singer et al., 2006). MPP+appears to accumulate in pigmented neurons due to the chelating nature of the pigmentation itself (D’Amato et al., 1986). However, it is also thought to eventually kill the cell by disrupting mitochondrial electron transport. Sequestration of MPP+then appears to induce cellular apoptosis leading to cell death and a downstream cascading immune response due to the release of previously unseen antigens (Sulzer et al., 2008). Blocking the uptake of MPP+blocks the toxic effects of MPTP administration, yet it is unclear whether MPTP is concentrated into pigmented tissue before or after conversion to MPP+. MPP+, apparently binds to NM in a reversible equilibria in order to exert its known toxic effects on mitochondria.
Possible Beneficial Functions for Chemical Binding to NM: Is There a Natural Substrate?
Many investigators have assumed that the accumulation of NM pigment is simply an unavoidable by-product of aging. Merely a form of molecular garbage that the cell is unable to dispose of, they argue, thus dismissing its presence as uninteresting and non-essential in the process. In this view, the binding of toxic chemicals to NM may simply be a by-product of the existence of the garbage itself, eventually leading to the instability and death of melanin-containing neurons due to oxidative stress. Another prevailing hypothesis for NM’s protective role in dopaminergic neurons lies in the prevention of neurotoxic pathways of quinones that are formed during dopamine oxidation. When dopamine is oxidized to dopamine o-quinone, aminochrome and 5,6-indolequinone are formed and typically undergo polymerization to form the dark pigment, neuromelanin (Munoz et al., 2012). In the absence of polymerization and neuromelanin, aminochrome can form adducts with alpha synuclein generating neurotoxic oligomers that can trigger mitochondrial dysfunction (Wang et al., 2012), and could induce oxidative and endoplasmic reticulum stress thereby affecting protein synthesis and degradation dysfunction. Alternatively, it has been proposed that NM serves a dual function, protecting the cell from oxidative stress through iron chelation in the early stages, yet contributing to the autoimmune aggravation of PD through release of novel antigens upon eventual cell death (Zecca et al., 2006). Ultrafast laser spectroscopy studies suggest that the integrity of the pigmented granules in tissue may play an important role in the balance between the photo-protective and photo-damaging behaviors attributed to melanins (Liu and Simon, 2003).
What cannot be ignored in this discussion however is the oxidative stress necessitated by the handling of high concentrations of catecholamines. In other words, in order to function as an active dopaminergic or adrenergic cell, the cell is necessarily at risk for toxicity from the catechols themselves, molecules known primarily for their high reactivity and redox potential in forming quinones. As has been pointed out, sequestration into secretory vesicles apparently alleviates thistoxicity and stress on the cell. What few seem to have suggested to date however is that the accumulation of NM itself could serves as a storage, protection, and re-release mechanism for dopamine (in the substantia nigra) or epinephrine (in the locus coeruleus), possibly acting as a very real molecular memory loop. This seems at first glance counter-intuitive, that the product of oxidative stress (NM) could then function to alleviate the very thing that created itself (excess dopamine), yet in reality would be quite an elegant solution to a problem.
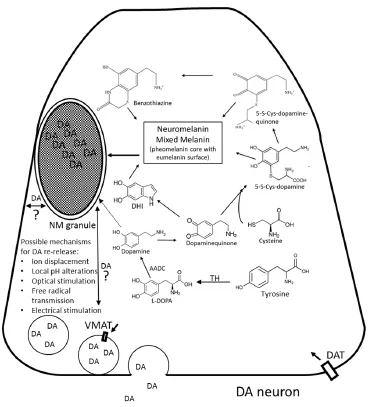
Figure 1 Hypothetical role of Neuromelanin (NM) in the storage and release of dopamine.
Since the structure of NM granules has not been established at sub-μm resolutions, it is not known exactly how the polymer is arranged. However, modelling studies suggest the granules adopt a layered structure containing a semi-regularly ordered array highly suitable for trapping monoamines (Charkoudian and Franz, 2006). In the case of such naturally occurring, aromatic organic catecholamines, the coordination of a lone-pair of electrons from the de-protonated amine nitrogen to NM-chelated iron combined with potential pi-stacking interactions through NM indole subunits provides a plausible mechanism for observed binding interactions. The putative ability of pigmented granules to concentrate catecholamines such as dopamine from surrounding tissue could supplant some of the function of vesicular monoamine transporters, which serve to sequester potentially toxic dopamine into vesicles for synaptic transmission. Such an extraordinary ability could even supplant the function of tyrosine hydroxylase (TH), the enzyme primarily used to locally synthesize dopamine and which is normally used as a marker for dopaminergic neurons. In one intriguing study, the authors compared the number of dopaminergic neurons in young, middle aged, and older squirrel monkeys. The absolute number of these neurons appeared to decrease over the lifespan of the animals when anti-TH antibodies were used alone to detect their presence, yet remained constant when pigmented neurons were counted along with TH-positive neurons (McCormack et al., 2004). This suggests that as pigmentation accumulates in a given neuron, TH expression is down regulated and dopamine synthesis presumably slowed.
Possible Role of NM in Neurons: Sequestration and Release of Dopamine as a Chemical Memory System?
Though highly speculative in nature at the current time, such a system (illustrated inFigure 1) presents an elegant mechanism whereby the very subunits that need protection from oxidation result in a polymer that is capable of protecting and furthering an identical signal. If dopamine that becomes NM is packaged into double-membrane bound vesicles in a reducing environment (as illustrated for example by its susceptibility to Fontana-Masson silver staining) has the ability to bind, store, protect, and release free dopamine, the chemical loop is complete. It could explain in part the reinforcement of addictive memories and associated behavior that comes with dopamine-releasing drugs, since excess cytoplasmic dopamine creates oxidative stress which leads to self-polymerization up to a certain point.
One of the strongest proposals for memory in human brains involves synaptic plasticity, that is, the extensive pattern of connections between the neurons can be altered through strengthening (by repeated stimulation) or weakening (by lack of stimulation) of particular pathways. Dopamine-binding NM structures would seem to retain a semi-permanence despite being made of components that are themselves susceptible to turnover. Thus it’s possible that pigmentation in neurons could play a significant and as yet unappreciated role in the strengthening of neuronal connections through localized catecholamine sequestration. Concentration of neurotransmitter into pigmented granules would localize a pool of transmissible signal in locations that had previously seen high dopamine release and uptake activity. If there is then a mechanism for re-release of bound dopamine to synaptic vesicles or to the synapse itself, the pigmented granule could replace or assist in neurotransmis-sion. Such a mechanism would appear to constitute a form of molecular memory. Terefore, we believe NM represents an obvious, if overlooked, mechanism for implicit (nondeclarative) memory which should be on the radar of many more investigators than it is today.
Disruption of NM Function as a Predictor of PD Inducers
If membrane bound, granular NM indeed proves to bind dopamine in a reversible manner, we can predict that any subsequent disruption of this function would result in symptoms of PD, analogous to what is seen in cases including MPP+, haloperidol (HPP+), and quite possibly, 6-OH dopamine, all potent chemical inducers of Parkinson’s disease. Given its structure, it is not surprising for example that the 6-hydroxy analogue of dopamine is taken up through dopaminergic transport mechanisms. Owing to the extra hydroxyl group on the aromatic ring, it is also much more susceptible to quinone formation than is dopamine itself, a process believed underlying its toxic effects. To our knowledge, the possible interaction between NM and 6-hydroxy dopamine has not been previously explored. Manganism, which exhibits symptoms very similar to Parkinson’s disease (Lucchini et al., 2009), also displays a clearly plausible mechanism in this scenario through replacement of the iron normally found bound to NM. Utilizing such a mechanism appears to have the unfortunate consequence of binding toxic agents that resemble neurotransmitter monoamines. Such sequestration could lead to several possible outcomes including abnormal polymerization of NM, abnormal packaging of the resulting NM polymer, or abnormal functioning of NM granules following successful formation. Given the commonalities between these agents and the unique environment afforded by pigmented neurons, we believe that chemical binding to and/or incorporation into the NM polymer itself may represent a general mechanism for early stage chemical induction of PD.
Conclusions
Though most often associated with toxin binding, the uniquely reducing environment of the pigmented granule may instead provide safe harbor for endogenous catecholamines such as dopamine itself which are otherwise highly susceptible to the oxidative stress of the cytoplasm in an actively firing neuron. Such stabilization could supplant or assist in the role of synaptic vesicles to form a semi-permanent connection in an elegant form of molecular memory. Dopamine and adrenaline should be thoroughly investigated as candidates for an endogenous NM substrate with profound implications. Preliminary evidence from our laboratory suggests that dopamine does in fact bind to a synthetic NM polymer in a saturable manner at micromolar concentrations (Haining et al., 2016). At a minimum, the possible influence of catecholamine binding and release by NM must be ruled out as a contributory mechanism to dopamine storage and release if we are to understand this phenomenon fully. Following the discovery of the role of DNA in heredity, scientific focus has inevitably shifted toward genes and gene products as being the most important players in the process of life. What cannot be forgotten during this transition however is that life is driven by biochemistry at its core, not by genes and gene products. In any case, it is becoming abundantly clear that neuromelanin is not a mere spectator in the brain but in fact serves a very important active function.
Conflicts of interest:None declared.
Bush WD, Garguilo J, Zucca FA, Albertini A, Zecca L, Edwards GS, Nemanich RJ, Simon JD (2006) The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc Natl Acad Sci U S A 103:14785-14789.
Charkoudian LK, Franz KJ (2006) Fe(III)-coordination properties of neuromelanin components: 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid Inorganic. Inorg Chem 45:3657-3664.
D’Amato RJ, Lipman ZP, Snyder, SH (1986) Selectivity of the parkinsonian neurotoxin MPTP: toxic metabolite MPP+ binds to neuromelanin. Science 231:987-989.
Federow H, Pickford R, Hook JM, Double KL, Halliday GM, Gerlach M, Riederer P, Garner B (2005) Dolichol is the major lipid component of human substantia nigra neuromelanin. J Neurochem 92:990-995.
Haining RL, Jones TM, Hernandez A (2016) Saturation Binding of Nicotine to Synthetic Neuromelanin Demonstrated by Fluorescence Spectroscopy. Neurochem Res 41:3356.
Karlsson O, Lindquist N (2013) Melanin affinity and its possible role in neurodegeneration. J Neural Transm 120:1623-1630.
Liu Y, Simon JD (2003) Isolation and biophysical studies of natural eumelanins: applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res 16:606-618.
Lucchini RG, Martin CJ, Doney BC (2009) From manganism to manganese-induced Parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med 11:311.
McCormack AL, Di Monte DA, Delfani K, Irwin I, DeLanney LE, Langston WJ, Janson AM (2004) Aging of the nigrostriatal system in the squirrel monkey. J Comp Neurol 471:387-395.
Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J (2012) Dopamine oxidation and autophagy Parkinson’s disease. Article ID 920953.
Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A 97:11869-11874.
Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L (2008) Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J Neurochem 106:24-36.
Wakamatsu K, Fujikawa K, Zucca FA, Zecca L, Ito S (2003) The structure of neuromelanin as studied by chemical degradative methods. J Neurochem 86:1015-1023.
Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X (2012) Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem 121:830-839.
Zecca L, Costi P, Mecacci C, Ito S, Terreni M, Sonnino S (2000) Interaction of human substantia nigra neuromelanin with lipids and peptides. J Neurochem 74:1758-1765.
Zecca L, Zucca FA, Wilms H, Sulzer D (2003) Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci 26:578-580.
Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG (2006) A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology 67:S8-11.
Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2015) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol doi:10.1016/j.pneurobio.2015.09.012.
*Correspondence to: Robert L. Haining, Ph.D., rhaining@ggc.edu.
orcid: 0000-0002-3944-056X (Robert L. Haining)
10.4103/1673-5374.202928
Accepted: 2017-03-10
- 中国神经再生研究(英文版)的其它文章
- Telomerase and mTOR in the brain: the mitochondria connection
- The emerging role of autophagic-lysosomal dysfunction in Gaucher disease and Parkinson’s disease
- Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- Novel insights into the role of NF-κB p50 in astrocytemediated fate specification of adult neural progenitor cells
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Impacts of the retinal environment and photoreceptor type on functional regeneration

