移植途径对人脐带间充质干细胞治疗小鼠糖尿病效果的影响*
蒋豆蔻, 李 青, 杨晓菲, 李 阳, 李富荣△
(1暨南大学第二临床医学院,深圳市人民医院,干细胞与细胞治疗重点实验室,广东 深圳 518020; 2暨南大学医学院病理生理学系,广东 广州 510632)
移植途径对人脐带间充质干细胞治疗小鼠糖尿病效果的影响*
蒋豆蔻1,2, 李 青1, 杨晓菲1,2, 李 阳1, 李富荣1△
(1暨南大学第二临床医学院,深圳市人民医院,干细胞与细胞治疗重点实验室,广东 深圳 518020;2暨南大学医学院病理生理学系,广东 广州 510632)
目的: 观察不同途径移植人脐带间充质干细胞(hUCMSCs)对小鼠糖尿病的治疗效果。方法: 利用增强绿色荧光蛋白和萤光素酶报告系统(EGFP/Luc)标记hUCMSCs,通过胰腺包膜下途径或尾静脉途径将携带萤光标记的hUCMSCs移植到链脲霉素诱导的糖尿病模型小鼠体内。移植后利用萤光素酶报告基因追踪hUCMSCs在活体内的迁移和定位;组织学检测小鼠胰岛形态变化;功能学实验动态检测小鼠血糖、血清胰岛素水平和糖耐量。结果: 活体生物发光成像显示胰腺包膜下途径移植的hUCMSCs主要定位于胰腺,尾静脉途径移植的hUCMSCs主要定位于肺,仅少量细胞向胰腺部位迁移。组织学检测发现,胰腺包膜下途径移植的小鼠胰岛边界清晰,无炎症细胞浸润;而尾静脉途径移植的小鼠胰腺组织有少量炎症细胞浸润和纤维化形成。功能学检测发现胰腺包膜下移植较尾静脉移植降低小鼠血糖作用显著,血糖可降至接近正常水平,且血清胰岛素水平明显升高,葡萄糖的调节能力显著增强。结论: 移植途径对 hUCMSCs治疗糖尿病的效果有影响。胰腺包膜下移植在降低小鼠血糖、升高胰岛素水平及改善胰岛功能方面均优于尾静脉移植。
人脐带间充质干细胞; 移植; 1型糖尿病
1型糖尿病是一种以胰岛细胞损伤为特征的自身免疫性疾病。胰岛细胞移植是目前临床上治疗该疾病最有效的方案[1-2],然而供体的不足和免疫排斥反应等限制了该方案的实施[3-4]。近年来,干细胞治疗为糖尿病患者带来了新的曙光[5-7],大量研究发现,间充质干细胞(mesenchymal stem cells, MSCs)治疗可有效地缓解糖尿病小鼠的症状[8-11]。间充质干细胞是一种具有分化为成体细胞能力的干性细胞,可通过免疫抑制、旁分泌等作用修复损伤细胞[12-15]。研究表明,MSCs通过尾静脉移植糖尿病小鼠后血糖显著下降,糖尿病症状得到有效缓解[16-19]。但直接移植到糖尿病小鼠的胰腺组织是否较尾静脉移植治疗作用更好尚不知晓,且缺乏对移植的MSCs 在体内迁移和定位的动态监测。
为此,本研究观察了萤光素酶报告系统标记的人脐带间充质干细胞(human umbilical cord mesenchymal stem cells, hUCMSCs)通过胰腺包膜下移植和尾静脉移植2种移植途径治疗小鼠糖尿病的效果。发现2种移植途径均能降低糖尿病小鼠血糖,但尾静脉移植的hUCMSCs主要定位在肺部,而胰腺包膜下移植的hUCMSCs主要定位在胰腺,发挥抑制炎症反应、促进损伤胰岛细胞修复及再生等治疗糖尿病的作用。
材 料 和 方 法
1 实验动物
6周龄雄性BALB/c小鼠,体质量 15~20 g,购于广东省医学实验动物中心。
2 主要试剂与仪器
DMEM-F12培养基和胎牛血清(Gibco);α-MEM培养基和HG-DMEM培养基(HyClone);胰蛋白酶、抗坏血酸、β-磷酸甘油、地塞米松、胰岛素、吲哚美辛、3-异丁基-1-甲基黄嘌呤和油红染料(Sigma);茜素红S染色液(森贝伽生物科技有限公司);Alexa488-CD44、APC-CD90、APC-CD29、FITC-CD105、Alexa488-CD34、APC-CD45和FITC-HLA-DR抗体(BD);pLEX-GFP-Luc2病毒液(北京中科汇文遗传公司);萤光素酶底物D-luciferin (Cellcyto); 链脲霉素(Sigma);小鼠胰岛素ELISA试剂盒(Millipore);流式细胞分选仪 (Beckman Coulter);血糖仪 (Accu-Chek);荧光显微镜(Nikon);活体生物发光成像系统(Caliper Life Science)。
3 实验方法
3.1 hUCMSCs的分离、培养和鉴定 人脐带来自暨南大学第二临床医学院产科,均获得产妇的知情同意和医院伦理委员会的审核批准。脐带采集后需在12 h内进行处理,用含双抗的磷酸盐缓冲液(phosphate-buffered saline, PBS)冲洗净脐带表面的血液,无菌手术刀横向将其切成3~5 cm的小块,再纵向切开,暴露并移除脐带内的血管后放入消化液中(消化液成分如下:3×105U/L的胶原酶Ⅰ,1 g/L透明质酸酶,含3 mmol/L 氯化钙的 PBS溶液),37 ℃消化1 h。PBS缓冲液冲洗后加入0.1%胰酶,37 ℃孵育30 min,终止消化后1 000 r/min离心5min,用含体积分数10%胎牛血清的DMEM/F12完全培养基重悬细胞,按1×106/cm2密度接种于T25培养瓶中,放置于37 ℃、5%CO2、饱和湿度培养箱内培养[20]。胰蛋白酶消化第3代hUCMSCs后收集至EP管内,加入Alexa488-CD44、APC-CD90、APC-CD29、FITC-CD105、Alexa488-CD34、APC-CD45和FITC-HLA-DR,单克隆抗体染色,工作浓度1∶100,室温下避光孵育30 min,PBS洗涤3次后使用流式细胞仪分析表面标志物。成脂试验和成骨试验方法如文献所述[21]。
3.2 萤光素酶和增强绿色萤光蛋白(enhanced green fluorescent protein, EGFP)标记hUCMSCs 将分离纯化的hUCMSCs 按 2×108/L 的密度接种于75 cm2培养瓶,48 h后使用pLEX-GFP-Luc2慢病毒进行感染[22],感染复数(multiplicity of infection, MOI)为25,感染48 h后通过荧光显微镜观察感染效率,使用0.8 mg/L嘌呤霉素连续进行药物筛选7~10 d后,PBS洗涤细胞3次,0.25%胰蛋白酶消化细胞,400×g离心5 min,PBS重悬细胞,流式细胞术检测稳定表达EGFP 细胞(hUCMSCs-EGFP/Luc)的比例。
3.3 糖尿病小鼠模型的建立 6周龄雄性BALB/c 小鼠,体重约15~20 g,禁食12 h后予75 mg/kg 链脲霉素(使用前溶于0.1 mol/L 柠 檬 酸 缓 冲 液,pH 4.5)腹腔注射,连续3 d[23]。连续2次测得随机血糖>300 mg/dL,且伴有多饮、 多食、多尿症状即为建模成功[24]。
3.4 hUCMSCs-EGFP/Luc细胞移植 用生理盐水将第5代hUCMSCs-EGFP/Luc细胞制备成密度为5×109/L的细胞悬液。将30只6周龄雄性BALB/c糖尿病小鼠随机分为胰腺包膜下移植组、尾静脉移植组、糖尿病组,每组10只,另取10只未造模小鼠作为正常对照组。以0.004 mL/g剂量腹腔注射水合氯醛对胰腺包膜下移植组小鼠进行麻醉,剔除小鼠左侧腰部毛发,消毒备皮。无菌眼科手术剪剪开小鼠肾脏附近的表皮、腹膜,开口方向与脊柱呈60°的角,切口长约1 cm。手指轻轻按压切口两侧挤出肾脏,吸取0.2 mL细胞悬液肾包膜下多点注射,电热灼合包膜小口,缝合腹膜、表皮。尾静脉移植组取0.2 mL细胞悬液尾静脉注射,糖尿病组和正常对照组不予处理。所有操作均按外科手术操作要求在无菌条件下进行。
3.5 活体生物发光成像 胰腺包膜下移植组和尾静脉移植组小鼠予以吸入异氟烷麻醉,活体生物发光成像前15 min每只小鼠经腹腔注射萤光素底物200 μL。经Lumina II IVIS成像系统检测萤光素酶发光信号(曝光时间为1 min),于移植后 1、 3、 5、 7、14、21和28 d观察活体生物发光成像。移植后第7天各组取2只小鼠,分离其心脏、肺、脾、肾、肝及胰腺,置入含400 mg/L萤光素底物的6孔板中,检测萤光素酶发光信号(曝光时间为1 s)。
3.6 组织病理学观察 hUCMSCs-EGFP/Luc细胞移植28 d后,水合氯醛麻醉胰腺包膜下移植组、尾静脉移植组、糖尿病模型组和正常对照组小鼠,心脏灌注法固定后取胰腺组织,置于10%多聚甲醛4 ℃固定4 h,常规石蜡包埋、切片,苏木精-伊红染色法染色后显微镜下观察。
3.7 移植后功能学检测 随机血糖检测:移植后各组小鼠每3 d尾静脉采血检测血糖至第28天。血清胰岛素含量检测:各组取5只小鼠于移植后3、7、14、21及28 d眼眶取血,分离血清后按照小鼠胰岛素 ELISA试剂盒说明稀释标准品、加样、显色,酶标仪测定吸光度,通过标准曲线计算样品中小鼠胰岛素浓度。葡萄糖耐量实验:移植后21 d,各组取5只小鼠禁食8 h后腹腔注射葡萄糖生理盐水2 g/kg,于注射后0、15、 30、 60、 120和180 min尾静脉采血检测血糖水平。
4 统计学处理
采用 SPSS 13.0 软件进行统计学处理,计量资料以均数 ±标准差(mean±SD) 表示。均数间的比较采用单因素方差分析,多重比较采用LSD-t检验。以P<0.05为差异有统计学意义。
结 果
1 hUCMSCs和hUCMSCs-EGFP/Luc的鉴定结果
分离的人脐带间充质干细胞形态均一,呈长梭形或鼓锤状生长。流式细胞术分析显示间充质干细胞表面标志物CD44、CD90、CD29和CD105的表达率分别为99.3%、99.6%、99.8%和96.4%,造血细胞标志物CD34和CD45的表达率仅为1.41%和1.27%,1.02%表达白细胞表面抗原HLA-DR。经过4周的成骨实验,茜素红染色证实骨结节形成;成脂实验发现,hUCMSCs诱导4周后,细胞增大、形态不规则、富含脂肪颗粒囊泡,脂肪小滴可被油红O红染。成骨成脂实验证明所分离的细胞具有分化潜能,符合hUCMSCs的特性。hUCMSCs感染病毒48 h后,免疫荧光显微镜观察细胞成功标记EGFP。经过7~10 d嘌呤霉素筛选,流式细胞术分析95.4%的hUCMSCs稳定表达EGFP,见图1。
2 hUCMSCs-EGFP/Luc细胞移植后的体内分布
活体生物发光成像系统动态监测显示,胰腺包膜下移植组的hUCMSCs-EGFP/Luc主要定位于胰腺部位,移植后24 h发光强度为(3.15±0.58)×106p·s-1·cm-2·sr-1,相当于5×105个细胞;移植后7 d监测离体胰腺、心脏、肺、脾、肾及肝组织的光信号进一步证实,仅胰腺组织发出光信号;发光可持续至少28 d,随时间推移进行性减弱,28 d时为(8.48±0.78)×104p·s-1·cm-2·sr-1。尾静脉移植组的细胞主要定位于肺部,胰腺及肝脏仅发出少量光信号,移植后24 h胰腺部位的发光强度为(2.93±0.36)×104p·s-1·cm-2·sr-1,相当于5×103个细胞,并且于移植后5 d细胞光信号完全衰减,见图2。
3 组织病理学变化
胰腺组织HE染色显示正常小鼠胰岛大小正常、细胞形态清晰,富含β细胞;糖尿病模型组胰岛萎缩、边界模糊,炎症细胞浸润伴纤维化形成;胰腺包膜下移植组较糖尿病模型组胰岛边界清晰,细胞形态恢复,无炎症细胞浸润;尾静脉移植组胰岛较糖尿病模型组清晰,但仍有炎细胞浸润和纤维化形成,见图3。

Figure 1.Identification of purified hUCMSCs and hUCMSCs-EGFP/Luc. A: flow cytometry confirmed the surface markers of purified hUCMSCs; B: morphology of hUCMSCs (×100); C: osteogenesis test confirmed the differentiation potential of hUCMSCs (alizarin red staining, scale bar=50 μm); D: adipogenesis test confirmed the differentiation potential of hUCMSCs (oil red O staining, scale bar=50 μm); E: fluorescence image of hUCMSCs-EGFP/Luc (scale bar=50 μm); F: flow cytometry confirmed the expression rate of EGFP in hUCMSCs-EGFP/Luc.
图1 hUCMSCs和hUCMSCs-EGFP/Luc的鉴定
4 移植后功能学改变
4.1 血糖 移植前除正常对照组外各组血糖值均大于成模标准300 mg/dL,组间差异无统计学意义。移植后第3天,胰腺包膜下移植组随机血糖开始逐渐下降,第15天降至(200.0±54.5)mg/dL,并维持到观察结束,显著逆转高血糖(P<0.05)。尾静脉移植组于移植后第3天血糖开始下降,第6天降至(300.0±36.7)mg/dL,之后血糖缓慢上升,血糖明显高于胰腺包膜下移植组(P<0.05),见图4。
4.2 血清胰岛素水平 ELISA检测小鼠外周血胰岛素含量显示,胰腺包膜下移植组胰岛素水平在各时点均明显高于糖尿病模型组和尾静脉移植组(P<0.05),且随时间推移胰岛素分泌能力有增高趋势,但仍低于正常对照组(P<0.05)。尾静脉移植组胰岛素水平与糖尿病组相比差异无统计学意义,见图5、表1。
4.3 糖耐量 腹腔注射葡萄糖后,胰腺包膜下移植组血糖值在30 min达峰值,60 min恢复至空腹水平,葡萄糖调控能力明显高于糖尿病模型组(P<0.05)。静脉移植组血糖值在60 min达峰值,之后缓慢下降,葡萄糖调控能力明显低于胰腺包膜下移植组(P<0.05),见图6、表2。

Figure 2.Invivoandinvitrobioluminescence imaging of hUCMSCs-GFP/Luc. A: location and intensity of luminescence signal at day 1, day 3, day 5, day 7, day 14, day 21 and day 28 after hUCMSCs-GFP/Luc transplanted by pancreatic capsule method; B: location and intensity of luminescence signal at 4 h, day 1, day 3, day 5 and day 7 after hUCMSCs-GFP/Luc transplanted by tail vein method; C: quantification of bioluminescence intensity at day 1, day 3, day 5, day 7, day 14, day 21 and day 28 after hUCMSCs-GFP/Luc transplanted by pancreatic capsule method; D:exvivobioluminescence imaging of various tissues and organs after hUCMSCs-GFP/Luc transplanted by pancreatic capsule method. The color bar represents photons per second per square centimeter per steradian (p·s-1·cm-2·sr-1).
图2 移植后hUCMSCs的体内分布

Figure 3.Morphological changes of islets (HE staining, ×200). A: untreated nondiabetic group; B: untreated diabetic group; C: pancreatic capsule transplantation group; D: tail vein injection group.
图3 小鼠胰岛形态变化
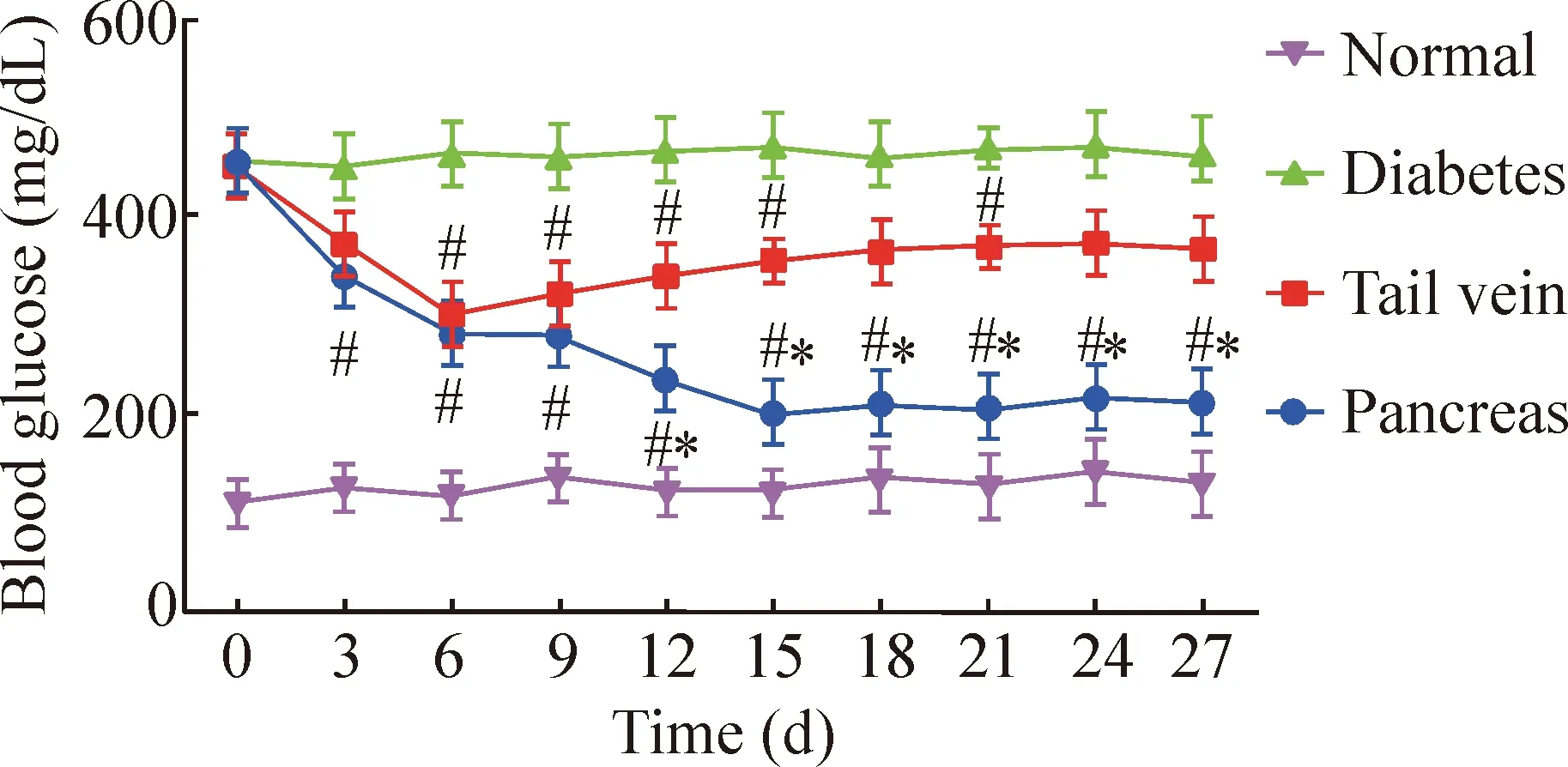
Figure 4.Blood glucose levels of diabetic mice after hUCMSCs transplantation.Mean±SD.n=10.#P<0.05vsnormal group;*P<0.05vstail vein group.
图4 移植后各组血糖变化
讨 论
本研究使用酶消化法分离纯化hUCMSCs,利用流式细胞术分析细胞表面标志物的表达,通过成脂成骨实验证实分离纯化的人脐带来源细胞符合间充质干细胞特性[25]。利用EGFP和萤光素酶报告系统标记hUCMSCs,直观监测移植的hUCMSCs在体内的迁移和定位。通过胰腺包膜下移植和尾静脉移植2种途径移植hUCMSCs治疗糖尿病小鼠,体内生物发光信号追踪发现胰腺包膜下移植的hUCMSCs主要定位于胰腺组织,光信号可持续至少28 d,而尾静脉移植的hUCMSCs主要定位于肺部,光信号在移植后5 d完全衰减。组织病理学观察发现胰腺包膜下移植途径对胰岛细胞的修复和组织形态的改善作用更良好。功能学检测发现,胰腺包膜下移植途径较尾静脉移植途径降糖作用显著,在观察期内可将血糖降至接近正常水平,胰岛素分泌能力和β细胞对葡萄糖的调控能力强于尾静脉移植组,表明胰腺包膜下移植途径在损伤胰岛细胞修复、胰岛细胞再生以及改善胰岛功能方面优于尾静脉移植途径。

Figure 5.Serum insulin levels of diabetic mice after transplantation. Mean±SD.n=5.#P<0.05vstail vein group;&P<0.05vsnormal group.
图5 移植后各组血清胰岛素水平变化
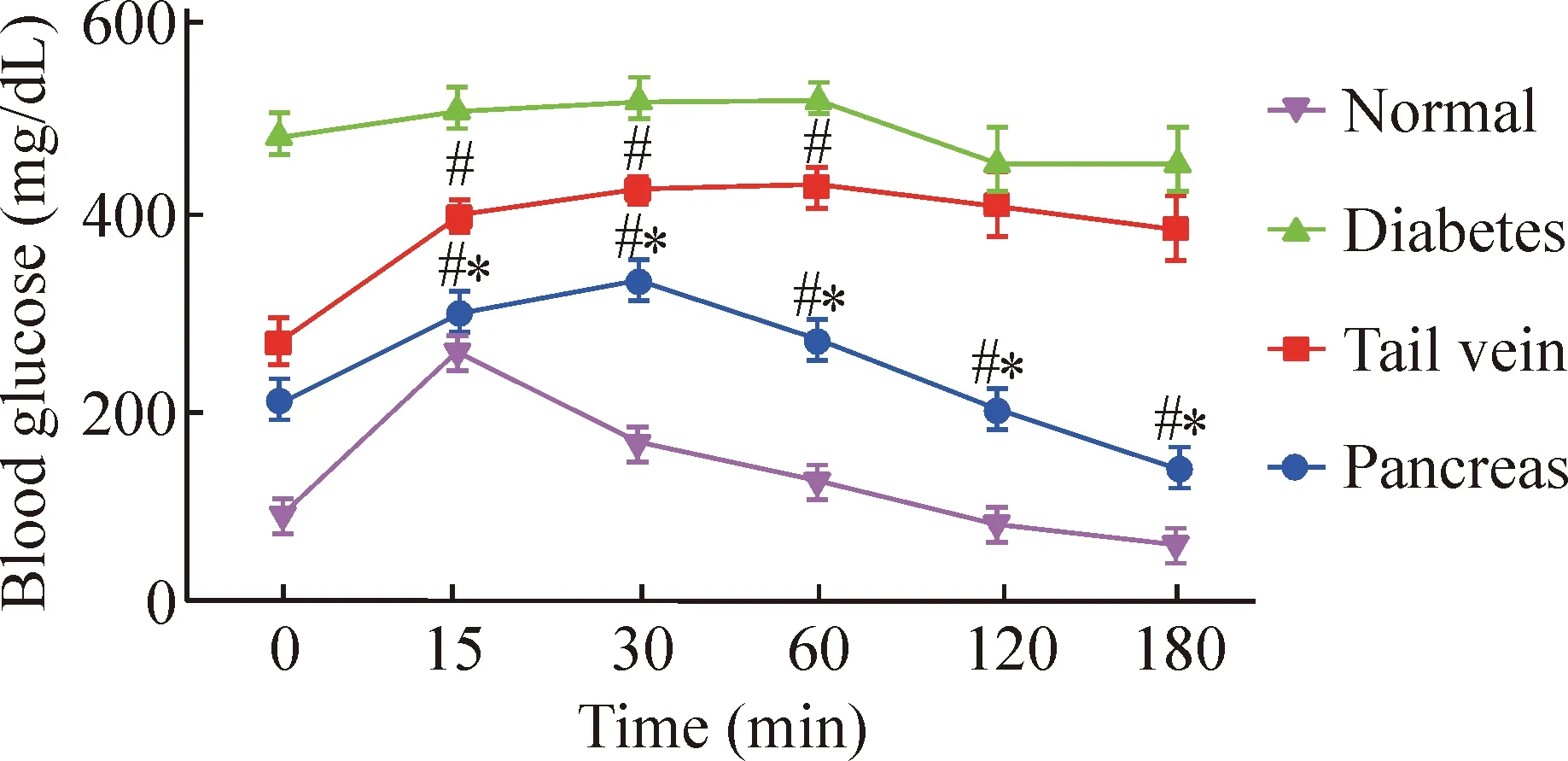
Figure 6.The blood glucose level by glucose tolerance test at day 21 after transplantation.Mean±SD.n=5.#P<0.05vsnormal group;*P<0.05vstail vein group.
图6 移植后第21天糖耐量试验血糖水平
对细胞的动态追踪可监测细胞在体内的生物活性,萤光素酶在三磷酸腺苷(ATP)和体内活细胞的氧气环境下,可催化D-luciferin使其发出能穿透5~10 mm组织的光。该光信号具有低背景和高信噪比的优势,稳定性好,发光强度与细胞数量呈线性相关,且不影响细胞生长动力学和干细胞的分化能力[26-27],因此我们利用萤光素酶生物发光成像追踪活细胞在生物体内的迁移、定位和细胞数量,实现对小鼠体内移植的hUCMSCs的动态监测。本研究中,我们将携带EGFP和萤光素酶报告基因的慢病毒载体感染hUCMSCs,移植到小鼠体内后经活体生物发光成像技术分析发现,胰腺包膜下途径移植的细胞约有5×105个细胞定位在胰腺部位,而尾静脉途径移植的细胞仅有5×103个细胞,2种移植途径最终定位于胰腺的细胞数量存在明显差异。此前有研究发现[28],静脉移植间充质干细胞,细胞主要定位于肺部,仅有不到0.04%的细胞迁移到心脏、肝、脾和肾,尾静脉移植的细胞同样到达肺部后被滞留,仅有不到0.1%细胞到达胰腺,说明MSCs更倾向于迁移至血流丰富且流速相对缓慢的组织器官。我们认为,hUCMSCs细胞较大,直径为20~24 μm,而肺毛细血管直径为10~15 μm,当通过尾静脉途径移植的hUCMSCs经血液循环到达肺毛细血管后,大部分形成栓子发生凋亡,造成局部炎症反应,受损的肺部组织会释放间质金属蛋白酶、血小板源性生长因子等细胞因子,促使hUCMSCs向受损组织迁移,因此通过尾静脉途径移植的hUCMSCs大部分定位在肺部。当hUCMSCs到达胰腺组织后,可分泌生长因子抑制胰腺细胞的凋亡[29],分泌的促血管生长因子可促进血管生长,改善微循环,利于胰岛损伤的修复[30],hUCMSCs可优先迁移到胰腺的淋巴结,发挥免疫调节功能,抑制致糖尿病的T细胞介导的免疫反应,减少T细胞对胰岛的破坏[31],除此之外,hUCMSCs细胞自身可发生自发性细胞融合以适应胰岛细胞表型[32],可能有少量hUCMSCs在胰腺微环境下分化为胰岛细胞,直接代偿胰岛功能[33]。由此我们猜测到达胰腺组织的细胞数量将影响干细胞改善糖尿病作用的发挥。直接移植到胰腺包膜下的干细胞,无需经血液循环,不受肺部组织的截留影响,因此能更加直接地发挥其治疗效果,改善糖尿病作用更加显著。
综上所述,我们利用萤光素酶报告系统标记人脐带间充质干细胞,追踪干细胞移植糖尿病小鼠后在体内的迁移和定位情况,结合组织病理和功能学检测比较胰腺包膜下移植途径和尾静脉移植途径对糖尿病的治疗效果。研究结果表明,2种移植途径均可改善糖尿病症状,但胰腺包膜下移植治疗效果更好,干细胞移植途径可影响糖尿病的治疗疗效。
[1] Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation[J]. Diabetes, 2005, 54(7):2060-2069.
[2] Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen[J]. N Engl J Med, 2000, 343(4):230-238.
[3] Matsumoto S.Clinical allogeneic and autologous islet cell transplantation: update[J]. Diabetes Metab J, 2011, 35(3):199-206.
[4] Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin indepen-dence after islet transplantation in type 1 diabetes[J]. Am J Transplant, 2012, 12(6):1576-1583.
[5] Stanekzai J, Isenovic ER, Mousa SA. Treatment options for diabetes: potential role of stem cells[J]. Diabetes Res Clin Pract, 2012, 98(3):361-368.
[6] Davey GC, Patil SB, O’Loughlin A, et al.Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus[J]. Front Endocrinol (Lausanne), 2014, 5:86.
[7] Wallner C, Abraham S, Wagner JM, et al. Local application of isogenic adipose-derived stem cells restores bone healing capacity in a type 2 diabetes model[J]. Stem Cells Transl Med, 2016, 5(6):836-844.
[8] Hashemian SJ, Kouhnavard M, Nasli-Esfahani E, et al. Mesenchymal stem cells: rising concerns over their application in treatment of type one diabetes mellitus[J]. J Diabetes Res, 2015, 2015:675103.
[9] Rackham CL, Dhadda PK, Chagastelles PC, et al. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice[J]. Cytotherapy, 2013, 15(4):449-459.
[10]Park JH, Park J, Hwang SH, et al. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury[J].Transplant Proc, 2012, 44(4):1123-1126.
[11]Borg DJ, Weigelt M, Wilhelm C, et al. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model[J]. Diabetologia, 2014, 57(3):522-531.
[12]Tsai PJ, Wang HS,Shyr YM, et al. Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats[J]. J Biomed Sci, 2012, 19:47.
[13]Jiao F, Wang J, Dong ZL, et al. Human mesenchymal stem cells derived from limb bud can differentiate into all three embryonic germ layers lineages[J]. Cell Reprogram, 2012, 14(4):324-333.
[14]Katikireddy KR, Dana R, Jurkunas UV. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells[J]. Stem Cells, 2014, 32(3):717-729.
[15]Amiri B, Ghollasi M, Shahrousvand M, et al. Osteoblast differentiation of mesenchymal stem cells on modified PES-PEG electrospun fibrous composites loaded with Zn2SiO4bioceramic nanoparticles[J]. Differentiation, 2016,92(4):148-158.
[16]Hao H, Liu J, Shen J, et al. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats[J]. Biochem Biophys Res Commun, 2013, 436(3):418-423.
[17]Thakkar UG, Trivedi HL, Vanikar AV, et al. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus [J].Cytotherapy, 2015, 17(7):940-947.
[18] Xiao N, Zhao X, Luo P, et al. Co-transplantation of mesenchymal stromal cells and cord blood cells in treatment of diabetes [J].Cytotherapy, 2013, 15(11):1374-1384.
[19]Kerby A, Jones ES, Jones PM, et al. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice[J]. Cytotherapy, 2013, 15(2):192-200.
[20] Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord[J]. Methods Cell Biol, 2008, 86:101-119.
[21] Anter J, Quesada-Gomez JM, Dorado G, et al. Effect of hydroxytyrosol on human mesenchymal stromal/Stem cell differentiation into adipocytes and osteoblasts[J]. Arch Med Res, 2016, 47(3):162-171.
[22] Bai X, Pinkernell K, Song YH, et al. Genetically selected stem cells from human adipose tissue express cardiac markers[J]. Biochem Biophys Res Commun, 2007, 353(3):665-671.
[23]Chen H, Carlson EC, Pellet L, et al. Over expression of metallothionein in pancreatic β-cells reduces streptozotocin-induced DNA damage and diabetes[J]. Diabetes, 2001, 50(9):2040-2046.
[24]Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets[J].Science, 2001, 292(5520):1389-1394.
[25]Oh SH, Muzzonigro TM, Bae SH, et al. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes[J]. Lab Invest, 2004, 84(5):607-617.
[26]Bai X, Yan Y, Song YH, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction[J]. Eur Heart J, 2010, 31(4):489-501.
[27]Bai X, Yan Y, Coleman M, et al. Tracking long-term survival of intramyocardially delivered human adipose tissue-derived stem cells using bioluminescence imaging[J]. Mol Imaging Biol, 2011, 13(4):633-645.
[28]Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6[J]. Cell Stem Cell, 2009, 5(1):54-63.
[29]Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration[J]. Nat Biotechnol, 2003, 21(7):763-770.
[30]Mathews V, Hanson PT, Ford E, et al. Recruitment of bone marrow-derived endothelial cells to sites of pancreatic beta-cell injury[J]. Diabetes, 2004, 53(1):91-98.
[31]Madec AM, Mallone R, Afonso G, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells[J]. Diabetologia, 2009, 52(7):1391-1399.
[32]Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion[J]. Nature, 2002, 416(6880):542-545.
[33]Ezquer F, Ezquer M, Contador D, et al. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment[J]. Stem Cells, 2012, 30(8):1664-1674.
(责任编辑: 陈妙玲, 罗 森)
Transplantation of human umbilical cord mesenchymal stem cells through different approaches for treatment of diabetic mice
JIANG Dou-kou1,2, LI Qing1, YANG Xiao-fei1, 2, LI Yang1, LI Fu-rong1
(1TheKeyLaboratoryofStemCellandCellularTherapy,TheSecondClinicalMedicalCollegeofJinanUniversity,ShenzhenPeople’sHospital,Shenzhen518020,China;2DepartmentofPathophysiology,SchoolofMedicine,JinanUniversity,Guangzhou510632,China.E-mail:frli62@163.com)
AIM: To compare the therapeutic effects of transplantation of human umbilical cord mesenchymal stem cells (hUCMSC) through different ways on diabetic mice. METHODS: hUCMSCs were labeled with enhanced green fluorescent protein (EGFP) and luciferase (Luc) reporter gene, and then the cells were transplanted into the diabetic mice through pancreas or tail vein to monitor the migration of the hUCMSCsinvivo. The pathological changes of pancreas tissue sections were determined by HE staining. Weight and blood glucose of the mice were measured dynamically. To compare the therapeutic effects, serum insulin levels were analyzed and glucose tolerance test were also performed. RESULTS:Invivobioluminescence imaging results showed that the hUCMSCs transplanted into pancreatic capsule was mainly located in the pancreas while the hUCMSCs transplanted through vein tail injection was mainly located in the lung. HE staining illustrated that islet cells presented distinctive boundary and no infiltration of inflammatory cells in pancreatic capsule transplantation group was observed, but a little inflammatory cell infiltration and fibrosis formation in tail vein injection group were seen. A significant decrease in blood glucose level and a significant increase in serum insulin level in pancreas transplantation group were showed as compared with vein tail injection group. CONCLUSION: Transplantation of hUCMSCs through different approaches demonstrates different effects.The transplantation of hUCMSCs into pancreatic capsule is more effective on hyperglycemia reversion, insulin secretion and improvement of beta-cell function than that through tail vein.
Human umbilical cord mesenchymal stem cells; Transplantation; Type 1 diabetes mellitus
1000- 4718(2017)04- 0612- 08
2016- 10- 24
2016- 12- 22
国家自然科学基金资助项目(No.81270857; No.81670702); 广东省自然科学基金资助项目(No.2015A030313762); 深圳市科技计划(No.JCYJ2016031115823245; No.CXZZ20140903103747568; No.JCYJ20140416122811921);博士后科学基金资助项目(No.2016M592603)
R587.1; R363
A
10.3969/j.issn.1000- 4718.2017.04.007
△通讯作者 Tel: 0755-25533018; E-mail: frli62@163.com

