新生儿坏死性肺炎2例报告并文献复习
张 可 周建国 胡 兰 邓英平 陈 超
复旦大学附属儿科医院(上海 201102)
新生儿坏死性肺炎2例报告并文献复习
张 可 周建国 胡 兰 邓英平 陈 超
复旦大学附属儿科医院(上海 201102)
目的分析新生儿坏死性肺炎的临床特点及诊治。方法回顾分析2例新生儿坏死性肺炎患儿的临床资料,并总结文献报道。结果2例新生儿均确诊为社区获得性金黄色葡萄球菌致坏死性肺炎,以发热起病,胸片示渗出性改变伴囊性影,CT示为多发空洞改变,痰/血培养均为金黄色葡萄球菌阳性,万古霉素治疗有效,影像学检查随访示好转。通过数据库检索,共有4篇病例报道,加上本组2例共7例新生儿坏死性肺炎病例。致病菌均有金黄色葡萄球菌,其中1例合并有铜绿假单胞菌,6例为社区获得性感染,均发生于无免疫缺陷新生儿,6例为原发性坏死性肺炎,6例为单侧肺受累;5例有发热表现,5例合并败血症,分别有3例、2例、1例并发胸腔积液、气胸或支气管胸膜瘘,2例有肺外感染;C反应蛋白均明显升高;3例需要机械通气,6例预后良好。结论新生儿坏死性肺炎以金黄色葡萄球菌为主要致病菌,诊断主要依靠典型影像学和病原学检查,治疗主要选择针对革兰阳性球菌的抗生素。
坏死性肺炎; 临床特点; 新生儿
坏死性肺炎(necrotizing pneumonia)为继发于感染的一种肺实质炎性坏死损伤,同时伴有多个含气或液体的薄壁空洞形成[1],其病理特点为病变肺组织坏死、坏疽[1,2]。近十年报道儿童坏死性肺炎发病率有所增加[3],但是新生儿报道较为少见。本研究报道复旦大学附属儿科医院收治的2例新生儿坏死性肺炎的临床资料,并复习相关文献,以加强儿科医师对本病的认识,早期诊断、及时治疗,降低死亡发生率。
1 临床资料
例1,男,19天,因纳差4天、发热1天入院。患儿足月顺产,出生体质量3 400 g,出生时无异常。Apgar评分正常,生后早期无特殊表现,配方奶喂养。出生15天时无明显诱因下出现纳差,伴呕吐。出生18天时出现发热,热峰为38.5℃,精神反应变差,以新生儿肺炎收住入院。入院体格检查:反应萎,上颚根部可见0.5 cm×0.5 cm溃疡,呼吸平稳,两肺呼吸音粗,未及啰音。实验室检查:血气分析正常,肝肾功能正常,血钠121 mmol/L,余电解质正常;血常规白细胞计数8.7×109/L,中性粒细胞 57%,血红蛋白158.1 g/L,血小板计数161×109/L;C反应蛋白133 mg/L,降钙素原15.7 ng/mL。血培养、脓液培养均为金黄色葡萄球菌(甲氧西林敏感),痰培养、支原体、衣原体检查均阴性。免疫球蛋白、CD系列及中性粒细胞功能检查无异常。胸片提示两肺渗出,右下肺、左上肺囊状影。CT提示双肺多发大小不等薄壁空洞(图1)。入院后予氨苄西林舒巴坦钠、头孢他定抗感染。病原学检查明确为金黄色葡萄球菌感染后改用万古霉素抗感染治疗。期间因左面颊及牙龈脓肿予切开引流治疗。入院后2周C反应蛋白恢复正常,胸片、CT较前好转,治疗4周后出院。
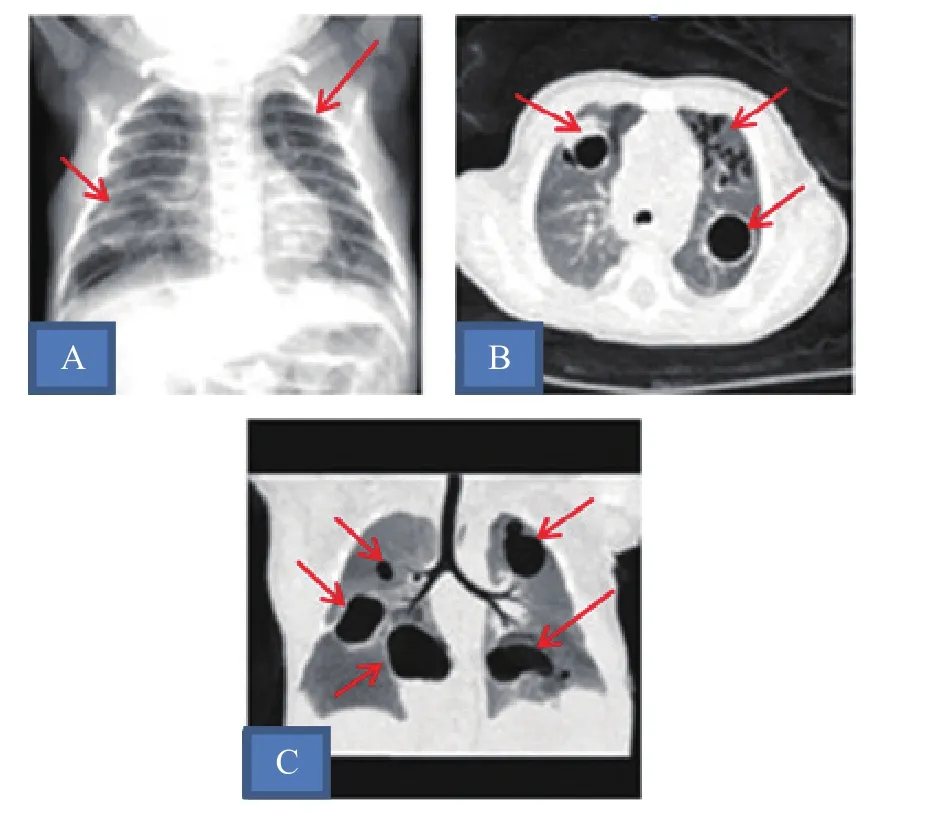
图1 病例1肺部影像学检查
例2,女,28天,因发热2天入院。患儿34+4周,因双胎剖宫产娩出,出生体质量1 970 g,Apgar评分10分,混合喂养。于出生26天无明显诱因出现发热,体温波动在36.8℃~38.5℃,伴有哭闹不安。以新生儿肺炎收住入院。入院体格检查:精神萎靡,反应差,贫血貌,呼吸平稳,两肺呼吸音粗,未及啰音。实验室检查:血常规白细胞计数34.5×109/L,中性粒细胞78.4%,血小板计数579×109/L;血红蛋白101.0 g/L;C反应蛋白>160 mg/L,红细胞沉降率102 mm/h,降钙素原1.23 ng/mL。血培养、痰培养均为金黄色葡萄球菌(甲氧西林敏感),痰支原体、衣原体检查均阴性。免疫球蛋白、CD系列及中性粒细胞功能检查无异常。肝功能、肾功能、电解质检查正常。胸片示左上肺渗出伴囊状影。CT示左上肺部分实变伴多发空洞形成(图2)。左肩关节增强磁共振成像(MRI)示左侧肱骨近端骨骺斑片状异常强化灶,左肩关节软组织肿胀;双下肢长骨平片示骨质改变伴干骺端骨质破坏,考虑骨髓炎。入院后予氨苄西林舒巴坦钠联合美罗培南抗感染治疗。病原学检查明确为金黄色葡萄球菌感染后改万古霉素抗感染治疗,治疗47天好转出院。
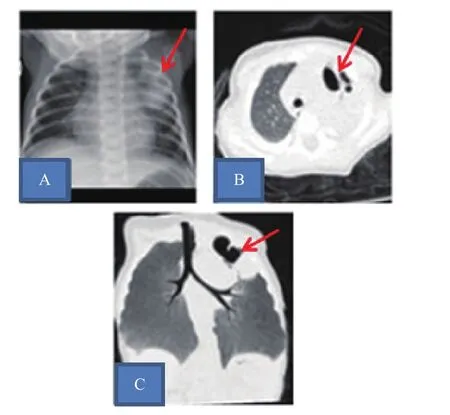
图2 病例2肺部影像学检查
2 讨论
以“坏死性肺炎、新生儿”为关键词或主题词,在万方、维普网、中国知网全文数据库中检索国内文献,未检索到相关病例报道。以“necrotizing pneumonia”作为主题,限定研究对象年龄为新生儿(检索式:necrotizing pneumonia [Title] AND "infant, newborn" [MeSH Terms]),在PubMed检索至2016年8月的文献,共检索到4篇文献,含5例新生儿坏死性肺炎报道[4-7]。加上本组2例,共7例新生儿坏死性肺炎。
7例新生儿坏死性肺炎的主要特点:致病菌均为金黄色葡萄球菌,其中1例合并有铜绿假单胞菌;6例为社区获得性感染;7列均无免疫缺陷;6例为原发性坏死性肺炎;6例为单侧肺受累;5例有发热表现,仅1例有咳嗽症状,5例合并败血症,分别有3例、2例、1例并发胸腔积液、气胸或支气管胸膜瘘;2例有肺外感染。C反应蛋白均明显增高。3例需要机械通气,1例予激素治疗,1例予引流和肺叶切除手术治疗,6例预后良好。
坏死性肺炎是肺炎的一种严重疾病形式,诊断主要依赖CT等影像学检查[8]。CT早期主要表现为肺实变,可伴有胸腔积液,48小时内即可从液化坏死发展致空洞形成,故典型影像学表现为在肺实变的基础上出现肺实质缺损及多发薄壁空洞。坏死性肺炎是介于肺脓肿与肺梗死之间的一种疾病,早期临床表现相似,但是疾病进程及影像学表现相差较大[1]。肺脓肿病情相对略轻,一般为单一较大的脓腔,伴有气液平面,脓腔壁较厚,多与最近的支气管相通排出脓液[9,10]。肺梗死病情较重,大血管血栓是其发病的主要因素,表现为梗死肺段邻近血管充盈缺损和阻塞[11,12]。
新生儿坏死性肺炎多发生于免疫功能正常新生儿,一般为侵袭性细菌感染所致。 Sawicki等[8]认为感染触发了强烈的炎症反应导致坏死性肺炎发生。Hsieh等[11]则认为感染菌分泌毒素递质引起血管炎和血管内血栓形成,影响支气管和肺的血供,进一步发展为坏死性肺炎。
坏死性肺炎的致病病原主要为金黄色葡萄球菌和肺炎链球菌[3,8]。本组2例致病菌均为金黄色葡萄球菌。社区获得性金黄色葡萄球菌致新生儿感染也呈增多趋势[13],故新生儿坏死性肺炎仍需重视。临床上多有发热表现[8],持续发热并非反映细菌清除不佳,而是与炎症反应、组织坏死后持续释放致热源相关。部分患儿有呼吸困难表现,多累及单侧多个肺叶[14]。
总结本组2例及文献报道5例患儿,6例为社区获得性坏死性肺炎,且均为金黄色葡萄球菌感染。金黄色葡萄球菌需结合受损的上皮细胞致病,有报道其感染与呼吸道的病毒感染相关[6],致病机制则与杀白细胞素相关性更强[15,16],可直接导致肺部发生炎症和损伤[17],同时也可通过影响免疫相关基因表达增加金黄色葡萄球菌的致病危害[18,19]。
新生儿坏死性肺炎多合并败血症[1],且易合并有胸腔积液、气胸、支气管胸膜瘘等并发症[2]。或许炎症损伤之后的肺泡、胸膜易脆性改变与气胸、支气管胸膜瘘发生相关。本组2例新生儿均有肺外感染病灶,例1为皮肤、牙龈脓肿,例2为多发骨髓炎。金黄色葡萄球菌可导致多部位感染,尤其是骨关节部位感染[16]。有报道认为这与其毒力强度有关[16],且与USA300(ST8)基因表达相关[20,21]。
新生儿坏死性肺炎多伴有白细胞增高,以中性粒细胞比例增高为主,可引发炎症因子风暴损伤致病[15], C反应蛋白明显增高[16]。
新生儿坏死性肺炎感染症状较重,早期发现、诊断和治疗对疾病的转归非常重要。新生儿坏死性肺炎需采用综合支持措施,药物推荐首先经验性使用万古霉素治疗,获得药敏结果后可据药敏结果选药[16]。若效果不佳,也可选择试用利奈唑胺治疗[6]。其他治疗如激素、肺泡灌洗等在新生儿应用不多,基于保守治疗后病情普遍好转,不推荐手术治疗[8]。
坏死性肺炎的成人病例预后较差,儿童和新生儿治愈率较成人高[8]。本组2例患儿预后良好;检索到的5例中,1例死亡,为出生胎龄25周、出生体质量仅700 g的早产儿,生后19天发病,最终因感染性休克和弥漫性血管内凝血而死亡,其余均痊愈。
总之,新生儿坏死性肺炎常以发热为主要表现,以金黄色葡萄球菌为主要致病菌,诊断主要依靠典型影像学和病原学检查,经敏感抗生素治疗后效果好。对于有发热表现,X线有囊性影伴渗出改变者,需警惕金黄色葡萄球菌感染致新生儿坏死性肺炎可能。
[1] Chatha N, Fortin D, Bosma KJ. Management of necrotizing pneumonia and pulmonary gangrene: a case series and review of the literature [J]. Can Respir J, 2014, 21(4): 239-245.
[2] Krenke K, Sanocki M, Urbankowska E, et al. Necrotizing pneumonia and its complications in children [J]. Adv Exp Med Biol, 2015, 857: 9-17.
[3] Lemaitre C, Angoulvant F, Gabor F, et al. Necrotizing pneumonia in children: report of 41 cases between 2006 and 2011 in a French tertiary care center [J]. Pediatr Infect Dis J, 2013, 32(10):1146-1149.
[4] Catho G, Gillet Y, Dumitrescu O, et al. Interfamilial transmission of Staphylococcus aureus Panton-Valentine leukocidin responsible for two cases of neonatal necrotizing pneumonia [J]. Arch Pediatr, 2011, 18(10): 1090-1094.
[5] Al TR, Al AS, Al SH, et al. Necrotizing pneumonia following cardiac surgery in a neonate [J]. J Infect Public Health, 2013, 6(3): 154-157.
[6] Lim W H, Lien R, Huang YC, et al. Community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia in a healthy neonate [J]. J Microbiol Immunol Infect, 2014, 47(6): 555-557.
[7] Mcadams RM, Mazuchowski E, Ellis MW, et al. Necrotizing staphylococcal pneumonia in a neonate [J]. J Perinatol, 2005, 25(10): 677-679.
[8] Sawicki GS, Lu FL, Valim C, et al. Necrotising pneumonia is an increasingly detected complication of pneumonia inchildren [J]. Eur Respir J, 2008, 31(6): 1285-1291.
[9] Pages PB, Bernard A. Lung abscess and necrotizing pneumonia: chest tube insertion or surgery? [J]. Rev Pneumol Clin, 2012, 68(2): 84-90.
[10] Seo H, Cha SI, Shin KM, et al. Focal necrotizing pneumonia is a distinct entity from lung abscess [J]. Respirology, 2013, 18(7): 1095-1100.
[11] Hsieh YC, Hsiao CH, Tsao PN, et al. Necrotizing pneumococcal pneumonia in children: the role of pulmonary gangrene [J]. Pediatr Pulmonol, 2006, 41(7): 623-629.
[12] Schweigert M, Dubecz A, Beron M, et al. Surgical therapy for necrotizing pneumonia and lung gangrene [J]. Thorac Cardiovasc Surg, 2013, 61(7): 636-641.
[13] Fortunov RM, Hulten KG, Hammerman WA, et al. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates [J]. Pediatrics, 2006, 118(3): 874-881.
[14] Hacimustafaoglu M, Celebi S, Sarimehmet H, et al. Necrotizing pneumonia in children [J]. Acta Paediatr, 2004, 93(9): 1172-1177.
[15] Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young immunocompetent patients [J]. Lancet, 2002, 359(9308): 753-759.
[16] Chen CJ, Su LH, Chiu CH, et al. Clinical features and molecular characteristics of invasive community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children [J]. Diagn Microbiol Infect Dis, 2007, 59(3):287-293.
[17] Diep BA, Chan L, Tattevin P, et al. Polymorph nuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury [J]. Proc Natl Acad Sci U S A, 2010, 107(12):5587-5592.
[18] Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection [J]. J Immunol, 2000, 165(10): 5392-5396.
[19] Vu CH, Kolata J, Stentzel S, et al. Adaptive immune response to lipoproteins of Staphylococcus aureus in healthy subjects [J]. Proteomics, 2016, 16(20):2667-2677.
[20] Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health careassociated blood stream infections [J]. Clin Infect Dis, 2006, 42(5):647-656.
[21] Jung J, Song EH, Park SY, et al. Emergence of Panton-Valentine leucocidin-positive ST8-methicillin-resistant Staphylococcus aureus (USA300 clone) in Korea causing healthcare-associated and hospital-acquired bacteraemia [J]. Eur J Clin Microbiol Infect Dis, 2016, 35(8):1323-1329.
Neonatal necrotizing pneumonia: two case report and literature review
ZHANG Ke, ZHOU Jianguo, HU Lan, DENG Yingping, CHEN Chao
(Department of Neonatology, Children’s Hospital of Fudan University, Shanghai 201102, China)
ObjectiveTo explore the clinical features, diagnosis, and treatment of neonatal necrotizing pneumonia.MethodsThe clinical data of two cases of neonatal necrotizing pneumonia were retrospectively analyzed. The clinical features, diagnosis, and treatment of neonatal necrotizing pneumonia in literatures were summarized.ResultsTwo cases were diagnosed of community-acquired Staphylococcus aureus necrotizing pneumonia and had the onset with fever. The chest X-ray showed exudative change with cystic shadow. The chest CT showed multiple cavity changes. The sputum and blood cultures were positive for Staphylococcus aureus. Both of them were effectively treated by vancomycin. The imaging was improved during the follow-up. Searching the database, 4 related literatures were being found, and there were totally 7 cases of neonatal necrotizing pneumonia including current 2 cases. The main features were as follows: The pathogenic bacteria in all cases include Staphylococcus aureus. One case was combined with pseudomonas aeruginosa. Six cases were community-acquired infections. All of them were non-immune deficiency newborn. Six cases were primary necrotizing pneumonia. Six cases were unilateral lung involvement. Five cases got fever, 5 cases had septicemia, 3 cases had pleural effusion, 2 cases had aerothorax, one case had bronchial chest and 2 cases had extrapulmonary infection. The C-reactive protein was increased in all cases. Three cases need mechanical ventilation. Six cases had a good prognosis.ConclusionsThe main pathogenic bacterium in neonatal necrotizing pneumonia was Staphylococcus aureus. The diagnosis was mainly depends on the typical imaging and pathogenic examination. The treatment is mainly the use of antibiotic for gram positive cocci.
necrotizing pneumonia; clinical feature; neonate
10.3969/j.issn.1000-3606.2017.03.002
2016-12-06)
(本文编辑:邹 强)
陈超 电子信箱:chen6010@163.com

