三氯异氰尿酸对斑马鱼胚胎及幼鱼的发育毒性
闫雪莹,杨淋清,吴德生,刘素纯,袁建辉,刘建军
(1.湖南农业大学食品科学技术学院,湖南长沙 410128;2.深圳市疾病预防控制中心,广东深圳 518055)
三氯异氰尿酸对斑马鱼胚胎及幼鱼的发育毒性
闫雪莹1,杨淋清2,吴德生2,刘素纯1,袁建辉2,刘建军2
(1.湖南农业大学食品科学技术学院,湖南长沙 410128;2.深圳市疾病预防控制中心,广东深圳 518055)
目的探讨新型消毒剂三氯异氰尿酸(TCCA)对斑马鱼胚胎及幼鱼的发育毒性。方法选择受精后2 h的斑马鱼胚胎进行染毒:①将胚胎置于含有TCCA 50,100,150,200,250和300 mg·L-1的培养液中暴露48 h,检测半数致死浓度(LC50)。②将胚胎置于含有TCCA 0,10.4,20.8和41.7 mg·L-1的培养液中暴露96 h,分别于暴露后48,72和96 h检测胚胎死亡率、畸形率和心率,采用羟胺法于暴露后2,4和6 h检测胚胎组织超氧化物歧化酶(SOD)的活性,HE染色观察暴露后7 d幼鱼头部组织病理结构。结果TCCA暴露48 h,斑马鱼胚胎LC50为166.9 mg·L-1。与正常对照组相比,暴露后96 h,TCCA 41.7 mg·L-1组斑马鱼胚胎死亡率和畸形率显著升高(P<0.05),TCCA各剂量组心率显著下降(P<0.05);暴露后72 h,TCCA 41.7 mg·L-1组死亡率显著升高(P<0.05),20.8和41.7 mg·L-1组畸形率显著升高(P<0.05),心率显著下降(P<0.05);暴露后48 h,TCCA 41.7 mg·L-1组畸形率显著升高(P<0.05)、心率显著下降(P<0.05)。TCCA暴露4和6 h,20.8和41.7 mg·L-1组SOD活性较正常对照组显著下降(P<0.05)。TCCA暴露7 d后,与正常对照组相比,各浓度组斑马鱼幼鱼均出现脑和眼部间隙变大及眼部视网膜分层不明显的病理改变。结论TCCA对斑马鱼胚胎发育有毒性作用,能引起胚胎发育早期SOD活性下降,造成幼鱼视网膜组织结构损伤。
三氯异氰尿酸;胚胎发育;视网膜;斑马鱼
氯化消毒剂在生活饮用水、餐具及环境消毒等领域应用广泛,使用便捷,但近年来人们逐渐发现,其分解产物常与水中某些有机物和无机成分产生反应,从而生成氯化消毒副产物,对人体健康构成威胁[1]。三氯异氰尿酸(trichloroisocyanuric acid,TCCA)是第四代氯化消毒剂,其化学名称为三氯-均三嗪2,4,6(1H,3H,5H)三酮,也称强氯精,属有机化合物,白色晶状粉末或粒状固体,具有强烈的氯化刺激味[2]。TCCA在水中分解后的主要产物为次氯酸,可穿透细胞膜进入细菌内,使蛋白质氧化;导致细菌死亡[3]。此外,TCCA还可分解产生尿氰酸,进而转化成三聚氰酸[4-5]。有研究表明,TCCA对剑尾鱼、罗非鱼和藻类具有毒性作用[6-8],但其对胚胎的毒性效应鲜有报道。本研究拟探讨TCCA暴露对斑马鱼胚胎及幼鱼的毒性作用及初步作用机制,为阐明TCCA致胚胎发育毒性提供实验依据。
1 材料与方法
1.1 动物
斑马鱼购自深圳市花鸟鱼市场,体长1.0~3.0 cm,饲养于深圳市疾病预防控制中心生态毒理实验室,水温27~29℃,pH 7.2~7.4,光照/黑暗时间周期为14/10 h,电导率500~550 mS·cm-1,每天投食2次,喂以新鲜的卤虫幼体。
1.2 药品、试剂和仪器
TCCA和HEPES购自美国Sigma公司;NaCl,KCl,CaCl2和MgSO4均购自国药集团化学试剂有限公司,分析纯;超氧化物歧化酶(superoxide dismutase,SOD)测定试剂盒(A001-3)购自南京建成公司。斑马鱼养殖系统(北京爱生有限公司);CKX41倒置显微镜(日本奥林巴斯公司);LRH-250生化培养箱(上海一恒科科技仪器有限公司);石蜡切片机和石蜡包埋机(德国莱卡公司);VCX400超声波破碎仪(Sonics公司)。
1.3 试剂配制和胚胎收集
用 E3培养液(mmol·L-1:NaCl 5,KCl 0.17,CaCl20.33,MgSO40.33,HEPES 0.7;pH 6.8~7.0)配制TCCA母液,超声溶解40 min,水温≤18℃,再用培养液稀释至染毒浓度,4℃保存备用。
将雌、雄斑马鱼以1∶2的比例放入交配盒中,用挡板隔开,适应过夜,第2天早晨给予光照并抽开挡板,1 h后收集胚胎,用E3培养液清洗胚胎2次。
1.4 胚胎染毒方法
在体视显微镜下挑选受精后2 h、发育正常且大小基本一致的胚胎,随机分配至6孔板,每孔30个,分别加不同浓度TCCA染毒液(染毒组)或E3培养液(正常对照组)4.0 mL,置于27~29℃恒温生化培养箱中,每24 h换1/2培养液或染毒液,实验过程中及时清除死亡的胚胎。
1.5 半数致死浓度的测定
按1.4方法,将受精后2 h的胚胎暴露于含TCCA 50,100,150,200,250和300 mg·L-1的E3培养液中持续染毒48 h,每12 h在体视显微镜下观察胚胎发育情况,记录各组胚胎死亡数,计算半数致死浓度(LC50)。以卵凝结作为胚胎死亡学终点。实验重复5次。
1.6 致死率和致畸率的测定
按1.4方法将胚胎暴露于含TCCA 0,10.4,20.8和41.7 mg·L-1的E3培养液中持续染毒96 h,每24 h在体视显微镜下观察记录胚胎或幼鱼死亡数目,同时计数存活胚胎或幼鱼中畸形个体的数目。以卵凝结作为胚胎死亡学终点,以心脏停止跳动作为幼鱼死亡学终点。以脊柱弯曲,眼睛畸形,心包水肿为畸形的判定标准。致死率(%)=死亡数/总胚胎数×100%;致畸率(%)=畸形数/总胚胎数×100%。实验重复5次。
1.7 心率的测定
按1.4方法将胚胎暴露于含TCCA 0,10.4,20.8和41.7 mg·L-1的E3培养液中持续染毒96 h,分别于染毒48,72和96 h在体视显微镜下记录胚胎1 min内心跳次数。实验重复5次。
1.8 羟胺法检测胚胎组织SOD活性
按1.4方法将胚胎暴露于含TCCA 0,10.4,20.8和41.7 mg·L-1的E3培养液中。分别于暴露2,4和6 h后收集各组胚胎,加入生理盐水400 μL,冰上制备匀浆,845×g离心10 min,取上清液,按照试剂盒操作步骤检测SOD活性,以在本体系中SOD抑制率达50%时所对应的酶量为一个SOD活性单位(U)。实验重复3次。
1.9 HE染色观察幼鱼组织病理结构
按1.4方法将胚胎暴露于含TCCA 0,10.4,20.8和41.7 mg·L-1的E3培养液中持续染毒7 d,用4%多聚甲醛将幼鱼组织固定过夜,制成厚度6 μm的石蜡切片,经苏木素-伊红染色,中性树脂封片,置显微镜下观察。
1.10 统计学分析
2 结果
2.1 TCCA暴露48 h斑马鱼胚胎半数致死浓度
TCCA暴露后48 h,胚胎死亡率随暴露浓度的增加而增加,250和300 mg·L-1组胚胎死亡率达99%,LC50为166.9 mg·L-1(图1)。

Fig.1 Mortality of zebrafish embryos exposed to trichloroisocyanuric acid(TCCA)for 48 h.Zebrafish embryos were treated with TCCA during 2-50 h post fertilization(hpf).The TCCA solutions were renewed every 24 h.±s,n=5.
2.2 TCCA对斑马鱼死亡率和畸形率的影响
TCCA暴露后72和96 h,斑马鱼死亡率随TCCA暴露浓度增加而增加,与正常对照组相比,41.7 mg·L-1组死亡率分别为(12.0±3.0)%和(13.3±3.5)%(P<0.05)。暴露后48 h,TCCA各浓度组死亡率与正常对照组无明显差异(图2)。斑马鱼畸形率随TCCA暴露浓度增加而增加。与正常对照组相比,暴露后48 h,TCCA 41.7 mg·L-1组畸形率达(9.3%±2.8)%(P<0.05);暴露后 72 h,TCCA 20.8和41.7 mg·L-1组畸形率分别为(6.7±3.4)%和(13.3±3.4)%(P<0.05);暴露后 96 h,41.7 mg·L-1组畸形率达(14.0%±2.8)%(P<0.05)(图3)。

Fig.2 Mortality of zebrafish embryos exposed to TCCA.Zebrafish embryos were treated with TCCA 10.4,20.8 and 41.7 mg·L-1 during 2-98 hpf.Mortality was measured at 48,72 and 96 h after TCCA exposure,respectively.±s,n=5.*P<0.05,compared with normal control(0.0)group.

Fig.3 Malformation of zebrafish embryos exposed to TCCA.See Fig.2 for the zebrafish embryo treatment.±s,n=5.*P<0.05,compared with normal control(0.0)group.
2.3 TCCA对斑马鱼心率的影响
与正常对照组相比,TCCA暴露后48 h,仅41.7 mg·L-1组心率显著降低(P<0.05);暴露后72 h,TCCA 20.8和41.7 mg·L-1组心率均显著降低(P<0.05);暴露后96 h,TCCA各浓度组心率均显著降低(P<0.05)(图4)。
2.4 TCCA对斑马鱼胚胎组织SOD活力的影响
与正常对照组相比,TCCA暴露后4和6h,TCCA 20.8和41.7 mg·L-1组SOD活力均显著下降(P<0.05),暴露后2 h,TCCA各浓度组SOD活力无显著变化(图5)。
2.5 TCCA对斑马鱼幼鱼视网膜组织病理结构的影响
正常对照组斑马鱼脑部发育正常,视网膜色素上皮层、感光细胞层、外界膜、外核层、外网层、内核层、内网层、神经节细胞层、视神经纤维层和内界膜发育正常,而TCCA 10.4 mg·L-1组眼部与脑部出现间隙;20.8和41.7 mg·L-1组视网膜分层完全消失,眼部与脑部的空隙变大(图6)。
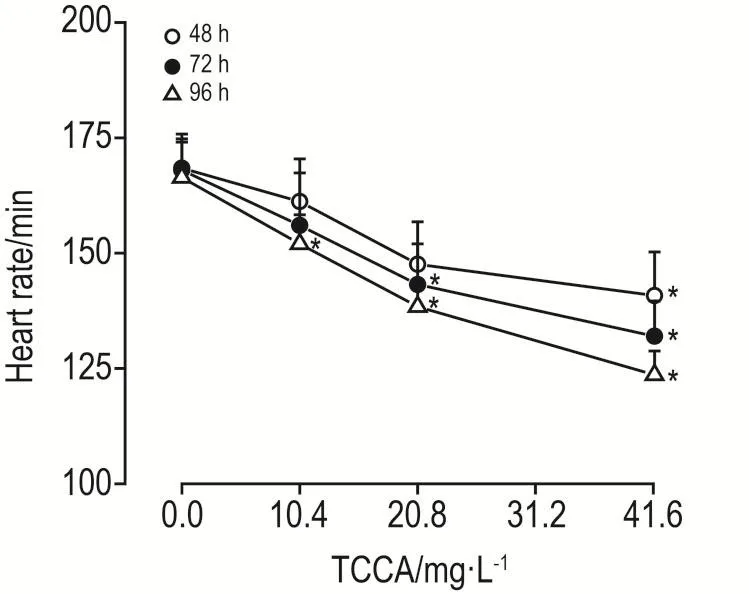
Fig.4 Heart rate of zebrafish embryos exposed to TCCA.See Fig.2 for the zebrafish embryos treatment.±s,n=5.*P<0.05,compared with normal control(0.0)group.

Fig.5 Effect of TCCA on superoxide dismutase(SOD)activity in zebrafish embryos.Zebrafish embryos were treated with TCCA 10.4,20.8 and 41.7 mg·L-1during 2-8 hpf and the SOD activity was detected at 2,4 and 6 h after TCCA exposure,respectively.±s,n=3.*P<0.05,compared with normal control(0.0)group.

Fig.6 Effect of TCCA on histopathology in zebrafish larvae by HE staining(×400).Zebrafish embryos/larvae were treated with TCCA during 2 hpf to 7 d.Yellow arrows indicate the eye and brain tissue gaps and eye lesions.
3 讨论
胚胎时期暴露于毒物中,能对脊椎动物,特别是卵生脊椎动物(如鱼类和鸟类)产生严重的伤害。研究表明,这些物种在生命早期对污染物的生态毒理效应最敏感[9-11]。本研究通过测定TCCA暴露48 h斑马鱼胚胎LC50,选择10.4,20.8和41.7 mg·L-1作为TCCA暴露剂量。研究结果显示,TCCA对斑马鱼胚胎及幼鱼具有致死和致畸的毒性效应,且TCCA对斑马鱼胚胎及幼鱼的致死作用发生在暴露72 h以内,染毒72~96 h幼鱼很少再发生死亡,因此致死率与致畸率很接近,这可能与染毒剂量较低有关,也可能与TCCA在生物体内代谢途径有关,需进一步研究阐明。
氯化消毒剂暴露可导致心功能紊乱,血液流动减慢,使得氧和营养物质不能充分供给机体,从而导致畸形与死亡。经消毒剂氯胺、二氧化氯或次氯酸钠处理的再生水均可引起斑马鱼胚胎心率减缓和畸形等毒性反应[12-13]。本研究发现,TCCA暴露引起斑马鱼胚胎心率下降,表明TCCA对斑马鱼胚胎发育具有毒性。
SOD是机体防御氧化损伤的关键酶之一,普遍存在于水产动物体内。可以通过催化歧化反应清除体内过量的O-2,将其分解为H2O2和O2,保护细胞免受氧化损伤[14-15]。大量研究表明,氯化消毒剂进入生物体内,会产生大量的活性氧。SOD是生物体有效清除活性氧的重要酶类之一,被称为生物体内抗氧化系统的第一道防线[16],其活性可反映机体的氧化应激水平,可作为指示氯化消毒剂对斑马鱼胚胎及幼鱼发育毒性的早期预警[6-7]。研究表明,农药、重金属和纳米材料等暴露导致机体代谢紊乱,自由基增多,H2O2含量相应增加,导致SOD的辅基被还原,活性下降,进一步使其清除自由基的能力降低,自由基积累造成细胞结构和功能的破坏,累积到一定限度会损害机体合成酶的能力,并改变酶本身构象,导致不可逆的毒性损伤[17-18]。本研究结果显示,TCCA暴露后斑马鱼胚胎组织SOD活性下降,提示TCCA对斑马鱼胚胎及幼鱼的发育毒性可能与氧化胁迫有关。
含氯的水溶液对眼及黏膜均有刺激作用[19],消毒剂副产物在动物实验中被证实具有生殖和神经毒性[20-21]。本研究结果显示,TCCA暴露后,斑马鱼幼鱼眼部视网膜组织形态异常。
综上所述,TCCA对斑马鱼胚胎及幼鱼发育具有毒性作用,能引起胚胎发育早期SOD活性下降,并造成幼鱼视网膜组织结构损伤。
[1] Xu XM,Gao KD.Application of by-products from chlorination and chlorine dioxide disinfector[J].Chem Adhes(化学与粘合),2003,(6):319-322.
[2] Wang HB,Zhang H.Safety production and pollution treatment of trichloroisocyanuric acid[J].J Salt Chem Ind(盐业与化工),2013,42(9):48-51,54.
[3] Dodds L,King WD.Relation between trihalomethane compounds and birth defects[J].Occup Environ Med.2001,58(7):443-446.
[4] Lu ML.Screening of toxic target organ and effector molecules of trichloroisocyanuric acid on zebrafish(三氯异氰尿酸对斑马鱼毒性靶器官效应分的筛选)[D].Changsha:Hunan Noamal University(湖南师范大学),2016.
[5] Wang J,E XL.Research progress of chlorinated isocyanurates disinfectants[J].J Environ Health(环境与健康杂志),2010,27(4):370-372.
[6] Nie XP,Wang X,Li KB,Wu SQ.Toxicity and its effects upon the antioxidant enzymes of trichloroisocyanuric acid to swordtail fishXiphophorus helleri[J].Oceanologiat Limnologia Sinica(海洋与湖沼),2008,39(5):494-498.
[7]Ma CG.Toxicity of chlorine dioxide and trichloroisocyanuric acid onTilapia(二氧化氯和三氯异氰尿酸对罗非鱼的毒性试验)[D].Changsha:Hunan Agricultural University(湖南农业大学),2013.
[8] Zhang NL,Xu N,Duan SS,Li AF,Nie XP,Lü SH.Effects of TCCA on growth of phytoplankton in mariculture areas[J].Mar Environ Sci(海洋环境科学),2009,28(1):5-8.
[9] Hoffman DJ,Albers PH,Melancon MJ,Miles AK.Effects of the mosquito larvicide GB-1111 on bird eggs[J].Environ Pollut,2004,127(3):353-358.
[10] Faria M,López MA,Fernández-Sanjuan M,Lacorte S,Barata C.Comparative toxicity of single and combined mixtures of selected pollutants among larval stages of the native freshwater mussels(Unio elongatulus)and the invasive zebra mussel(Dreissena polymorpha)[J].Sci Total Environ,2010,408(12):2452-2458.
[11]Du QP,Peng R,Liu WX,Jia XS,Wei DY.Toxic effects of TBBPA onin vivoandin vitrodevelopments in the zebrafish(Danio rerio)embryos[J].Acta Sci Circum(环境科学学报),2012,32(3):739-744.
[12] Tian WJ.Preliminary study on oxidative stress mechanism of zinc oxide nanoparticles to zebra fish embryos(纳米氧化锌对斑马鱼胚胎氧化应激机制的初步研究)[D].Qingdao:Qingdao University of Science and Technology(青岛科技大学),2010.
[13] Sun SB,Li W,Pan XY,Lin X,He QZ,Zeng HC.Effects of PFOS on zebrafish embryo development and SOD,MDA and GSH levels in zebrafish larvae[J].Pract Prev Med(实用预防医学),2015,22(6):648-651.
[14] Yu FF,Tang TL,Bai JJ,Xiong ZD,Tang WH.Toxicities and hazard classification of reclaimed water after disinfection of different approaches by zebrafish embryos bioassay[J].Asian J Ecotoxicol(生态毒理学报),2015,10(2):313-319.
[15] Tian WJ,Bai W,Zhao CL,Zhang ZY,Cui JA,He X,et al.Effects of ZnO nanoparticles on antioxidant enzyme system of zebrafish embryos[J].China Environ Sci(中国环境科学),2010,30(5):705-709.
[16] Cheng Y,Zhao D,Chen XH,Luo JX,Hao KN,Wang B.Effect of medical ozone on hypoxic brain injury in juvenile zebrafish[J].Matern Child Health Care China(中国妇幼保健),2015,30(15):2376-2380.
[17] Yang LL,Fang ZQ.Effects of estradiol,nonylphenol,polychlorinated biphenyls,cadmium and zinc on the activity of superoxide dismutase inTanichthys albonubes[J].Acta Lab Anim Sci Sin(中国实验动物学报),2012,20(1):38-46.
[18]Zhao XS,Ren X,Duan XY,Luo ZY,Zhu R.The effect of PFOS on developmental toxicity and oxidative stress in zebrafish embryos[J].J Tangshan Univ(唐山学院学报),2016,29(6):12-16.
[19] Colman J,Rice GE,Wright JM,Hunter ES 3rd,Teuschler LK,Lipscomb JC,et al.Identification of developmentally toxic drinking water disinfection byproducts and evaluation of data relevant to modeofaction [J].ToxicolApplPharmacol,2011,254(2):100-126.
[20] Manasfi T,Coulomb B,Boudenne JL.Occurrence,origin,and toxicity of disinfection byproducts in chlorinated swimming pools:an overview[J].Int J Hyg Environ Health,2017,220(3):591-603.
[21] ErdingerL, KirschF, Sonntag HG.Irritating effects of disinfection by-products in swimming pools[J].Zentralbl Hyg Umweltmed,1998,200(5-6):491-503.
(本文编辑:赵 楠)
Developmental toxicity of trichloroisocyanuric acid on zebrafish embryos and larvae
YAN Xue-ying1,YANG Lin-qing2,WU De-sheng2,LIU Su-chun1,YUAN Jian-hui2,LIU Jian-jun2
(1.College of Food Science and Technology,Hunan Agricultural University,Changsha 410128,China;2.Shenzhen Center for Disease Control and Prevention,Shenzhen 518055,China)
OBJECTlVETo investigate the developmental toxicity of a new disinfectant trichloroisocyanuric acid(TCCA)on zebrafish embryos and larvae.METHODSZebrafish embryos of 2 h post fertilization(hpf)were exposed to TCCA:①The embryos were exposed to a culture medium containing TCCA 0,50,100,150,200,250 and 300 mg·L-1for 48 h before 50%lethal concentration(LC50)was calculated.②The embryos were exposed to a culture medium containing TCCA 0,10.4,20.8 and 41.7 mg·L-1for 96 h.The mortality,malformation rate and heart rate of embryos and larvae were measured at 48,72 and 96 h after TCCA exposure.The expression of superoxide dismutase(SOD)was detected at 2,4 and 6 h after TCCA exposure.The pathological changes in the head of zebrafish larvae were observed 7 d after exposure by HE staining.RESULTSLC50of 48 h after TCCA exposure was 166.9 mg·L-1.Compared with normal control group,the mortality and malformation rate of zebrafish embryos were significantly increased(P<0.05)in TCCA 41.7 mg·L-1group,but the heart rate was decreased significantly(P<0.05)in each dose of TCCA group at 96 h after TCCA exposure.At 72 h after TCCA exposure,the mortality rate of zebrafish embryos was significantly increased(P<0.05)in TCCA 41.7 mg·L-1group,the malformation rate of zebrafish embryos was increased(P<0.05)and the heart rate of zebrafish embryos was decreased(P<0.05)in 20.8 and 41.7 mg·L-1groups.At 48 h after TCCA exposure,the heart rate of zebrafish embryos was decreased significantly(P<0.05)in 41.7 mg·L-1group.The SOD activity of zebrafish embryos in 20.8 and 41.7 mg·L-1groups was significantly lower than that of control group(P<0.05).At 7 d after exposure,the brain and ocular space of larvae were enlarged and the ocular retina layers were not obvious in any dose of TCCA groups.CONCLUSlONTCCA has toxic effect on zebrafish embryonic development,which can down-regulate SOD activity in the early developmental stage of embryos and damage the retina tissue of larvae.
trichloroisocyanuric acid;embryonic development;retina;zebrafish
The project supported by Shenzhen Science and Technology R&D Fund Project(CYJ20140410171018510);Shenzhen Municipal Health Commission commissioned the Construction of Key Disciplines to Enhance the Project(201506064);and Shenzhen Science and Technology R&D Funding Disciplines Project(JCYJ20160328144536436)
LIU Su-chun,E-mail:liusuchun@163.com,Tel:(0731)4617007;WU De-sheng,E-mail:dswucn@126.com,Tel:(0755)25601914(
2017-04-10 接受日期:2017-07-17)
A
1000-3002-(2017)07-0736-06
DOl:10.3867/j.issn.1000-3002.2017.07.006
深圳市科技研发资金项目(CYJ2014041017-1018510);深圳市卫计委重点学科建设能力提升项目(201506064);深圳市科技研发资金学科布局项目(JCYJ20160328144536436)
闫雪莹,女,硕士研究生,主要从事营养与食品卫生研究。
刘素纯,E-mail:liusuchun@163.com,Tel:(0731)4617007;吴德生,E-mail:dswucn@126.com,Tel:(0755)25601914

