Neuroprotective effect of ischemic postconditioning on sciatic nerve transection
Xiao-bin Zhou, Na Liu, Dong Wang De-xin Zou, Chang-wei Wei, Jun-lin Zhou
1 Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
2 Department of Traumatic Orthopedics, the Third Hospital of Shijiazhuang, Shijiazhuang, Hebei Province, China
3 Department of Internal Neurology, the First Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China
4 Department of Spine Surgery, Yan Tai-Shan Hospital, Yantai, Shandong Province, China
5 Department of Anesthesia, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Introduction
Kitagawa et al. (1990) found that the brain has ischemic tolerance, and proposed the concept of ischemic preconditioning.The phenomenon of ischemic tolerance is a perfect example of “what does not kill you makes you stronger”. Ischemic preconditioning or postconditioning is the strongest known procedure to prevent or reverse neurodegeneration. Numerous studies have demonstrated the neuroprotective effects of tolerance to cerebral ischemia. Liu et al. (2014) reported that ischemic preconditioning protected the brain from the effects of focal cerebral infarction by upregulating vascular endothelial growth factor. Other research found that ischemic preconditioning could adjust lower integrin αv levels in the brain following ischemia (Ma et al., 2016). Based on these findings,we wondered whether ischemic tolerance might have protective effects on peripheral nerve injury.
Schwann cells form the important part of the peripheral nerve myelin sheath, and play an essential role in peripheral nerve regeneration. They can release neurotrophic factors to promote the regeneration of peripheral nerves and guide axonal regeneration in the direction of the Bands of Büngner (Terenghi, 1999). Therefore, the production and release of nerve growth factor directly affect the speed and quality of nerve regeneration. We speculate that ischemic tolerance possibly accelerates nerve regeneration by promoting Schwann cells to secrete nerve growth factors. To verify this point, we used ischemic postconditioning that mimics ischemic tolerance, in order to observe the effects of postconditioning on peripheral nerves. We measured the expression of insulin-like growth factor 1 (IGF-1) to examine the mechanism underlying the protective effect of ischemic postconditioning on sciatic nerve regeneration.
Materials and Methods
Animals

Figure 1 Effect of ischemic postconditioning on sensory function in the sciatic nerve transection rats.

Figure 2 Effect of ischemic postconditioning on right limb motor recovery in the sciatic nerve transection rats at eight weeks after surgery detected by electrophysiological measurement.
Seventy-two specific-pathogen-free adult male Sprague-Dawley rats weighing 160—220 g and aged 8 weeks were purchased from Beijing Vital River Laboratory Animal Technology, China (license No. SCXK Jing 2016-0011). Protocols were approved by the Ethics Committee of the Capital Medical University of China (approval No. AEEI-2017-055), and adhered to the guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals(NIH Publication No. 85-23, revised 1996).
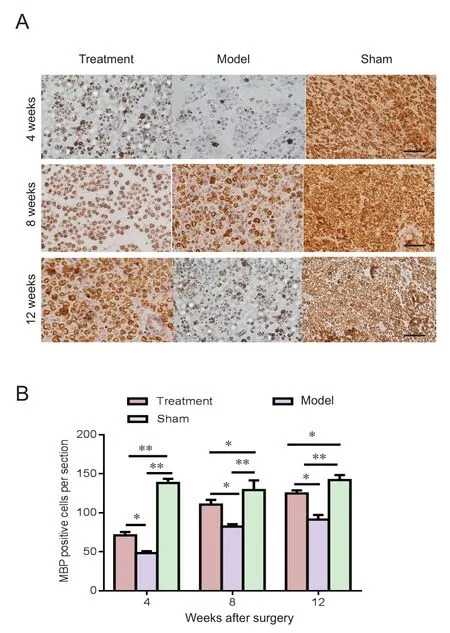
Figure 3 Effect of ischemic postconditioning on MBP expression in the injured sciatic nerve (immunohistochemical analysis).
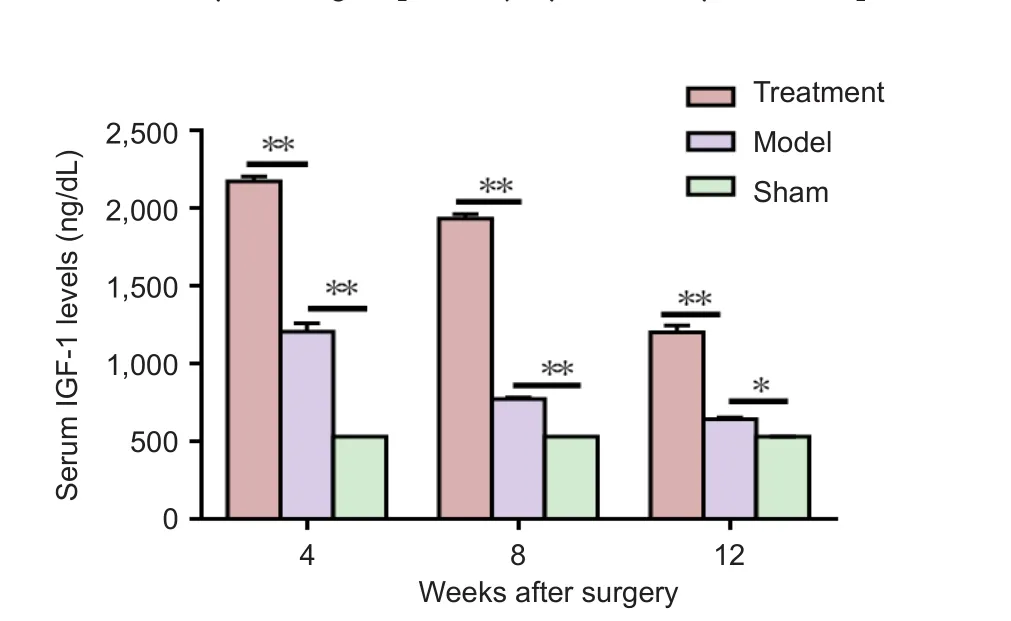
Figure 4 Effect of ischemic postconditioning on serum IGF-1 expression in the sciatic nerve transection rats at 4, 8 and 12 weeks after surgery (enzyme linked immunosorbent assay).
Before the experiment, the rats were adaptively trained for two weeks. The rats were placed at room temperature(22 ± 3°C), with a diurnal cycle of 12:12 hours, and allowed adequate supply of water and feed. The rats were randomly divided into three groups: treatment group (ischemic postconditioning + sciatic nerve injury), model group (sciatic nerve injury) and sham group (n = 24 per group).
Model establishment of sciatic nerve transection
Rats were anesthetized by an intraperitoneal injection of 2%sodium pentobarbital at 40 mg/kg, then fixed in the prone position on the table and maintained at a constant temperature of 37°C. After anesthesia, a 1.5-cm incision was made on the right leg, and the sciatic nerve was transversely cut through under the greater trochanter of the femur 0.5—1 cm from the inferior border of the piriform muscle. Furthermore, it was ensured that the nerve stumps were located in the middle part of the femur, making it convenient for ischemic postconditioning with a tourniquet. Under a 10×microscope (Olympus, Tokyo Japan), an end-to-end suture was performed with 10-0 mono filament nylon sutures; and the incision was sutured layer by layer. Rats were routinely fed after the operation (Hsu et al., 2017).
Ischemic postconditioning
The ischemic postconditioning of the limbs was achieved through three cycles of 10-minute occlusion and 10-minute reperfusion at the proximal end of the femur on the right side by a tourniquet (Chaoyang Hospital, Beijing, China)(~300 mmHg). During hind limb ischemia, rats were anesthetized and maintained at a body temperature of 37°C. After removal of the tourniquet, rats were placed in individual cages to recover from the anesthesia (Zhang et al., 2016).The ischemic postconditioning was carried out once a day,every day after surgery. The model rats did not receive ischemic postconditioning after surgery. The sham group rats were submitted to non-nerve injury, the sciatic nerve was just exposed and then the incision was sutured.
Sensory function recovery assessed by von Frey algesimetry
The mechanical pain threshold was detected by von Frey algesimetry (North coast Medical, Inc., San Jose, CA, USA)for assessing the sensory recovery (Cobianchi et al., 2014).Six rats from each group were placed on a wire net platform located by plastic chambers 15 minutes before starting the experiment for habituation. We stimulated the medial (covered by tibial nerves) regions of the hind paw plantar surface; a non-noxious pointed probe was gently applied to the test site; and pressure was slowly increased. The probe fiber was slightly bent into an S-shape. The threshold, shown in grams (g), was applied to trigger the “ flinch” response. Three measurements per test site were used to determine the mean value, with five minutes intervals between each measurement.A cut-off force was set to 40 g when either no withdrawal or no active response occurred. To minimize variations between rats, the values were stated by the following formula: lesioned side [g]/non-lesioned side [g] (Cobianchi et al., 2014; Meyer et al., 2016a, b). Four weeks after lesioning, rats in the treatment and model groups did not show a flinch response when set at 40 g, indicating no sensory recovery. Hence, this test was performed at 8 and 12 weeks after lesion.
Motor function recovery assessed by electrophysiological measurement
Motor function recovery of sciatic nerves was detected by electrophysiological measurement. Eight weeks after surgery, following the mechanical pain threshold test, six rats from each group were anesthetized and the sciatic nerves were exposed to measure nerve conduction velocity using a Medelec Synergy system (Oxford, UK). The belly of the gastrocnemius muscle was punctured with concentric needle electrodes, which were used as recording electrodes. The sciatic nerve was exposed at the lesion, and single electrical pulses (100 ms durations, 5 mA) were delivered via the bipolar hook electrode placed under the sutured nerve, alternating between 5 mm proximal and 5 mm distal to the nerve lesion site. For each rat, three independent measurements for the latency of the action potential were recorded and averaged. The distance between two stimulating electrodes was measured with a Vernier caliper (AoSen instrument equipment Co., Ltd., Dongguan, China). The motor nerve conduction velocity of the regenerated nerve was calculated as distance/latency of the action potential (m/s).
Immunohistochemical analysis
Myelin basic protein (MBP) was used as a marker for the myelin sheath (Forghani et al., 2001). The remaining six rats in each group were sacrificed at 4, 8 and 12 weeks after surgery and 5 mL tail venous blood was collected for enzyme-linked immunosorbent assay (ELISA). The sciatic nerves were fixed in 4% paraformaldehyde and embedded in paraffin. Subsequently, the tissues were cut into 5-μm thick serial sections, and rehydrated in phosphate buffered saline(PBS). To block endogenous peroxidases, the sections were incubated with 3% hydrogen peroxide for 20 minutes and 2%goat serum in PBS for one hour at room temperature; and incubated at 4°C overnight with rabbit anti-rat MBP (1:1,000;Boster Inc., Beijing, China). Afterwards, the sections were washed three times with PBS and incubated in biotinylated sheep anti-rabbit IgG (1:400; Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) solution for one hour at room temperature. The sections were developed with 3,3′-diaminobenzidine for five minutes. Finally, the sections were counterstained with hematoxylin. Immunoreactive cells were counted under a light microscope (Olympus, Tokyo, Japan) and assessed by two independent investigators.
ELISA
ELISA was adopted to detect the serum IGF-1 concentrations of the different groups at 4, 8, and 12 weeks after surgery. Six rats in each group at each time point were used. The protocol was performed according to manufacturer’s instructions(QuantiCyto Inc., Shenzhen, China). The optical density (OD)of each well was measured at a wavelength of 450 nm, using a full wavelength spectrophotometer (Thermo Fisher, Chicago,IL, USA). A standard curve was drawn with the standard concentrations on the X-axis and OD value on the Y-axis. The concentrations of samples were calculated from the graph.
Statistical analysis
Data were expressed as the mean ± SD, and analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically signi ficant. One-way analysis of variance followed by Dunnett’s test was used for comparison among all experimental groups.
Results
Ischemic postconditioning improved sensory function in the sciatic nerve transection rats
Sensory recovery was measured using the Von-Frey test. Eight weeks after the lesion, the von-Frey measurement values in both the treatment group and model group were higher than that in the sham group (P < 0.05); and the treatment group consistently performed better compared with the model group.This situation did not change over the 12 weeks (Figure 1).
Ischemic postconditioning accelerated motor recovery of sciatic nerves after injury in the sciatic nerve transection rats
Electrophysiology recordings of the action potentials in the right limb motor nerves in the three groups were obtained at eight weeks. These indicate that the latencies are shorter in the ischemia pre-conditioned group than the model group(Figure 2A). Nerve conduction velocity was significantly different across the three groups (P < 0.01), and nerve conduction velocity in the treatment group was higher than in model group at eight weeks (P < 0.01;Figure 2B).
Ischemic postconditioning increased Schwann cell remyelination in the injured sciatic nerve
To investigate the effects of ischemic postconditioning on the myelinization of nerve fibers, the number of neurons expressing MBP was measured in sections of nerves. At 4,8 and 12 weeks post-surgery, the levels of axonal remyelination, assessed by the number of neurons expressing MBP per section, increased following treatment with ischemic postconditioning, when compared with untreated rats (P <0.01) (Figure 3). On the other hand, MBP expression was still lower in the treatment group than in the sham group even at 12 weeks post lesion (P < 0.05).
Ischemic postconditioning increased the expression of serum IGF-1 in the sciatic nerve transection rats
The expression of serum IGF-1 in the treatment group was signi ficantly higher than that in the model and sham groups at 4, 8 and 12 weeks (P < 0.05;Figure 4).
Discussion
The effectors contributing to ischemic tolerance partially resemble the naturally occurring adaptive mechanisms at the cellular level (Obrenovitch, 2008). However, these adaptive responses have not been studied in a model of peripheral nerve transection injury. In the present study, we used a variety of assessments, including electromyograms, immunohistochemistry and von Frey tests, to characterize sciatic nerves subjected to injury and treatment by ischemic postconditioning. The results showed that ischemic postconditioning has a neuroprotective action in accelerating sciatic nerve regeneration.
To assess the degree of recovery of sciatic nerve sensory function we used the von Frey test. This provides a noninvasive and easily quanti fiable method in the rat model when a limb on one side was injured. In the trauma models, data reveal a signi ficant difference at 8 and 12 weeks in sensory functional evaluation among groups. The treatment group has a better sensory function. Hence, ischemic postconditioning promotes the recovery of sensory nerve and its function after sciatic nerve injury.
The conduction velocity of sciatic nerve is an important measure of the functional recovery of the regenerating nerve(Johnson et al., 2005; Wang et al., 2016; Badri et al., 2017). The conduction velocities in the ischemic postconditioning treatment group are higher than those in the model group because the latencies are shorter, reflecting a high number of axons functioning normally.
MBP is expressed by Schwann cells in the peripheral nervous system (Gupta et al., 2005). In this study, there was a higher MBP density in myelinated nerve fibers at 4, 8 and 12 weeks post ischemic injury, which demonstrates better nerve regeneration and axon myelinization after injury.
The neurotrophin factor, IGF-1, has a therapeutic effect on injured neurons. It is a single-chain polypeptide that consists of 70 amino acids with endocrine, autocrine and paracrine functions (Twigg and Baxter, 1998; Rosen and Pollak, 1999; Wang et al., 2017). There is increasing evidence that the expression of IGF-I genes in rat sciatic nerves after a crush promotes the survival (Kostereva et al., 2016), motility (Cheng et al., 2000), proliferation (Ju et al., 2015) and myelination (Liang et al., 2007) of Schwann cells. IGF-1 accelerated glucose uptake in cells, stimulated mitotic activity and cell proliferation, and inhibited apoptosis (Decourtye et al., 2017). The cerebral mRNA expression of IGF-I was increased by ischemic preconditioning (Naylor et al., 2005).In this experiment, ELISA data reveal that IGF-1 increases in the serum of the treatment group. We speculate that the expression of IGF-I is upregulated through ischemic postconditioning.
In this study, MBP immunohistochemistry, nerve conduction velocity and the von Frey test reveal that ischemic postconditioning leads to the recovery of sensory and motor functions in crushed sciatic nerves. Mechanistically, the healing effect of ischemic postconditioning on sciatic nerve regeneration can be partly ascribed to improving IGF-1 expression at the protein level. It was known that hypoxic or ischemic preconditioning activated IGF-I expression, which then increased the hypoxia-inducible factor 1α (HIF-1α)receptor nuclear translocator DNA binding activity (Zelzer et al., 1998). The HIF-1α accumulated in the nucleus, where it trans-activated adaptive genes, such as glucose transporter 1, vascular endothelial growth factor, and erythropoietin(Semenza, 1999), which play a role in neuroprotective activity. These findings indicated that IGF-I improved neuronal survival in hypoxic or ischemic tolerance after hypoxic-ischemic injury in part by activating HIF-1α (Wang et al., 2004).
The strengths of this study are the assessment of three different aspects of nerve regeneration: the sensory function,electrophysiological and morphological evaluations. However, we did not explore the mechanism. In our future research we will study and evaluate changes in HIF-1α expression and their correlation with the results of this study. The mechanism underlying the protective effect of ischemic tolerance remains unknown. The effects of ischemic postconditioning on the expression of other neurotrophic factors, which may mediate the neurotrophic effects on peripheral nerves, also need further research. Further studies should focus on the exact mechanism and key factors that underpin the healing of nerves after ischemic postconditioning.
In summary, this study demonstrates the protective effect of ischemic postconditioning against peripheral nerve injury. We speculate that IGF-1 is key to a potential treatment in ischemic tolerance for the reconstruction of peripheral nerve crush injury. It is hoped that this study would provide a possible basis for the clinical application of ischemic postconditioning to enhance regeneration and recovery, and reduce disability after nerve injury.
Author contributions:JLZ conceived and designed the experiments.DXZ performed the behavioral test. XBZ and NL helped to perform immunohistochemistry. DXZ performed data analysis. XBZ contributed to the writing of the paper. CWW performed enzyme linked immunosorbent assay. DW contributed to electrophysiology. All authors approved the final version of the paper.
Con flicts of interest:The authors declare that they have no con flicts of interest.
Financial support:This work was supported by the 2016 Beijing Municipal Natural Science Foundation (CN), No. 71520061; Beijing Municipal Science and Technology Commission, China, No. Z161100000116080.The conception, design execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of these funding organizations.
Research ethics:The study protocol was approved by the Ethics Committee of the Capital Medical University of China (approval No. AEEI-2017-055). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Badri O, Shahabi P, Abdolalizadeh J, Alipour MR, Veladi H, Farhoudi M, Zak MS (2017) Combination therapy using evening primrose oil and electrical stimulation to improve nerve function following a crush injury of sciatic nerve in male rats. Neural Regen Res 12:458-463.
Cheng HL, Steinway M, Delaney CL, Franke TF, Feldman EL (2000) IGF-I promotes Schwann cell motility and survival via activation of Akt. Mol Cell Endocrinol 170:211-215.
Cobianchi S, de Cruz J, Navarro X (2014) Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp Neurol 255:1-11.
Decourtye L, Mire E, Clemessy M, Heurtier V, Ledent T, Robinson IC, Mollard P, Epelbaum J, Meaney MJ, Garel S, Le Bouc Y, Kappeler L (2017) IGF-1 induces GHRH neuronal axon elongation during early postnatal life in mice.PLoS One 12:e0170083.
Forghani R, Garofalo L, Foran DR, Farhadi HF, Lepage P, Hudson TJ, Tretjakoff I, Valera P, Peterson A (2001) A distal upstream enhancer from the myelin basic protein gene regulates expression in myelin-forming schwann cells. J Neurosci 21:3780-3787.
Gupta R, Gray M, Chao T, Bear D, Modafferi E, Mozaffar T (2005) Schwann cells upregulate vascular endothelial growth factor secondary to chronic nerve compression injury. Muscle Nerve 31:452-460.
Hsu ST, Yao CH, Hsu YM, Lin JH, Chen YH, Chen YS (2017) Effects of taxol on regeneration in a rat sciatic nerve transection model. Sci Rep 7:42280.
Johnson EO, Zoubos AB, Soucacos PN (2005) Regeneration and repair of peripheral nerves. Injury 36 Suppl 4:S24-29.
Ju DT, Liao HE, Shibu MA, Ho TJ, Padma VV, Tsai FJ, Chung LC, Day CH,Lin CC, Huang CY (2015) Nerve regeneration potential of protocatechuic acid in RSC96 Schwann cells by induction of cellular proliferation and migration through IGF-IR-PI3K-Akt signaling. Chin J Physiol 58:412-419.
Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N,Fukunaga R, Kimura K, Mikoshiba K, et al. (1990) ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res 528:21-24.
Kostereva NV, Wang Y, Fletcher DR, Unadkat JV, Schnider JT, Komatsu C, Yang Y, Stolz DB, Davis MR, Plock JA, Gorantla VS (2016) IGF-1 and Chondroitinase ABC augment nerve regeneration after vascularized composite limb allotransplantation. PLoS One 11:e0156149.
Liang G, Cline GW, Macica CM (2007) IGF-1 stimulates de novo fatty acid biosynthesis by Schwann cells during myelination. Glia 55:632-641.
Liu Y, Zhu S, Wang Y, Hu J, Xu L, Ding L, Liu G (2014) Neuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor. Neural Regen Res 9:1117-1121.
Ma XM, Liu M, Liu YY, Ma LL, Jiang Y, Chen XH (2016) Ischemic preconditioning protects against ischemic brain injury. Neural Regen Res 11:765-770.
Meyer C, Wrobel S, Raimondo S, Rochkind S, Heimann C, Shahar A, Ziv-Polat O, Geuna S, Grothe C, Haastert-Talini K (2016a) Peripheral nerve regeneration through hydrogel-enriched chitosan conduits containing engineered schwann cells for drug delivery. Cell Transplant 25:159-182.
Meyer C, Stenberg L, Gonzalez-Perez F, Wrobel S, Ronchi G, Udina E, Suganuma S, Geuna S, Navarro X, Dahlin LB, Grothe C, Haastert-Talini K (2016b)Chitosan- film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 76:33-51.
Naylor M, Bowen KK, Sailor KA, Dempsey RJ, Vemuganti R (2005) Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochem Int 47:565-572.
Obrenovitch TP (2008) Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev 88:211-247.
Rosen CJ, Pollak M (1999) Circulating IGF-I: New perspectives for a new century. Trends Endocrinol Metab 10:136-141.
Semenza GL (1999) Regulation of mammalian O2homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 15:551-578.
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194:1-14.
Twigg SM, Baxter RC (1998) Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem 273:6074-6079.
Wang C, Lu CF, Peng J, Hu CD, Wang Y (2017) Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen Res 12:2106-2112.
Wang H, Li X, Shan L, Zhu J, Chen R, Li Y, Yuan W, Yang L, Huang J (2016)Recombinant hNeuritin promotes structural and functional recovery of sciatic nerve injury in rats. Front Neurosci 10:589.
Wang X, Deng J, Boyle DW, Zhong J, Lee WH (2004) Potential role of IGF-I in hypoxia tolerance using a rat hypoxic-ischemic model: activation of hypoxia-inducible factor 1alpha. Pediatr Res 55:385-394.
Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B (1998) Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17:5085-5094.
Zhang Y, Xu H, Wang T, He J, Wei J, Wang T, Dong J (2016) Remote limb ischemic post-conditioning attenuates ischemia-reperfusion injury in rat skin flapby limiting oxidative stress. Acta Cir Bras 31:15-21.
- 中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology