Delayed xenon post-conditioning mitigates spinal cord ischemia/reperfusion injury in rabbits by regulating microglial activation and in flammatory factors
Yan-wei Yang, Yun-lu Wang, Jia-kai Lu, Lei Tian, Mu Jin, Wei-ping Cheng
Department of Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart, Lung and Blood Vessel Diseases,Beijing, China
Introduction
Spinal cord ischemia/reperfusion injury (SCIRI) is a major cause of immediate and/or delayed neurological morbidity in people undergoing surgery to treat aortic aneurysm or aortic dissection (Cambria et al., 2002; Tan et al., 2010). Many studies have con firmed that the in flammatory reaction induced by ischemia is a major cause of SCIRI with extensive in flammatory cell in filtration (Savas et al., 2002; Papakostas et al., 2006;Tsuruta et al., 2006; Oz Oyar et al., 2008; Hasturk et al., 2009;Paulson et al., 2013; Temiz et al., 2013). Microglia are important in flammatory cell types that are activated in early stages after SCIRI and are associated with increased in flammatory chemokine release (Tikka et al., 2001; Matsumoto et al., 2003;Olson, 2010). Interleukin-6 (IL-6), a proin flammatory cytokine, is released following SCIRI, and its levels are signi ficantly increased in the days following SCIRI (Smith et al., 2012).Levels of interleukin-10 (IL-10), a potent anti-in flammatory cytokine, are also increased following SCIRI and decrease spinal cord in flammation (Plunkett et al., 2001; Fan et al., 2011).
Studies in many animal models have shown immediate or delayed xenon post-conditioning to be effective at protecting the spinal cord after spinal cord injury (SCI) because it suppresses apoptosis (Yang et al., 2014). Interestingly, the optimal time for administration of 50% xenon to rats was 1 hour after reperfusion and not at the onset of reperfusion. Similarly, Fries et al. (2008, 2009) found that xenon administered 1 hour after resuscitation improved short-term neurocognitive function and histology compared with xenon administered sooner (10 minutes after resuscitation). Furthermore,the protective effects of xenon were most pronounced when administered soon after ischemic insult to the brain, preferably within 8 hours (Ma et al., 2005; David et al., 2008; Peng et al., 2013). Previous studies have focused on the neuroprotective effects of xenon, but have not explored the reasons for its poor neuroprotective effect when used early in the reperfusion period. Therefore, the underlying mechanisms of xenon’s effects remain unknown.
In flammation is an important element in the pathobiology of SCI (David and Kroner, 2011; Karalija et al., 2014; Ren et al., 2016; Shultz and Zhong, 2017). However, whether in flammation plays a harmful or beneficial role following central nervous system injury has yet not to be determined. Different diseases can trigger microglia to become pro- or anti-in flammatory, which can be harmful or protective, respectively(David and Kroner, 2011; Nguyen et al., 2017). Xenon can regulate the in flammatory response and microglial activation in both humans and rats (Clark et al., 2005; Fahlenkamp et al., 2011; Breuer et al., 2015). However, to the best of our knowledge, the effect of xenon on microglial activation and the in flammatory response after spinal cord ischemia reperfusion in rabbits has not been explored.
It is not yet clear whether the length of time between transient ischemia and xenon administration affects microglial activation and/or if microglial polarization influences the pro- or anti-inflammatory effect. This study examined the effects of immediate and delayed xenon post-conditioning on the activation of microglial cells and inflammatory responses after SCI in rabbits.
Materials and Methods
Animals
Twenty-four male New Zealand rabbits aged 8–12 months and weighing 2—2.5 kg were provided by the Experimental Animal Center of Beijing Institute of Heart, Lung and Blood Vessel Diseases, China [license No. SYXK (Jing) 2005-0026].Rabbits were housed under standard conditions in the Animal Research Laboratory at Capital Medical University and received humane care in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996).
Rabbits were randomly assigned into an ischemia/reperfusion group (I/R, n = 6), an ischemia/reperfusion + immediate xenon post-conditioning group (P0, n = 6), an ischemia/reperfusion + delayed xenon post-conditioning group (P2, n= 6), and a sham operation group (S, n = 6).
Spinal cord ischemia model
After fasting for 12 hours, rabbits were anesthetized via intravenous injection of urethane (1,000 mg/kg) via a 22 G catheter (B. Braun Medical Inc., Bethlehem, PA, USA) inserted into the left ear vein. Heparin was injected at a rate of 6 mg/kg per hour during the surgery. A 24-gauge catheter(B. Braun Medical Inc.) was inserted into the right ear artery for continuous monitoring of mean arterial pressure and arterial blood gases. A 24-gauge catheter was inserted into the left femoral artery to measure mean distal aortic pressure.Mean proximal aortic pressure, mean distal arterial pressure, and temperature were recorded using a Powerlab/8SP Polygraph (AD Instruments, Sydney, Australia). Rabbits were placed under heat lamps to maintain body temperature at 38.5°C until recovered from the anesthesia. With rabbits placed in a supine position, spinal cord ischemia was induced by imbedding a 2F balloon catheter (Edwards Life sciences,Irvine, CA, USA), via the right femoral artery (Watanabe et al., 2005). The end of the catheter was located 0.5—1 cm below the renal artery (approximately 11 cm caudal to the insertion point). The aortic occlusion was con firmed by an immediate and sustained loss of detectable pulse pressure and a decrease in mean distal arterial pressure. Each rabbit received an injection of 200 U protamine and then the catheter was removed and blood flow was restored to the spinal cord. The balloon was deflated after the artery was occluded for 22 minutes.The surgical incisions were then sutured and the rabbits were monitored in their cages until full recovery. A clamping time of 22 minutes was chosen based on previous clamping tests(15—25 minutes). Rabbits in the S group underwent the same surgical procedure as described above, but without aortic catheter occlusion. Arterial blood samples were taken from the left femoral artery at pre-ischemia, 5 minutes after aortic clamping and 15 minutes after reperfusion. Arterial blood gases were detected by a blood gas and pH analyzer (Ciba-Corning Diagnostics, East Walpole, MA, USA).
Rabbits of all groups were euthanized 72 hours after reperfusion for histological examination. Neurological as-sessments were performed in all rabbits at 4, 8, 24, 48 and 72 hours after reperfusion.
Experimental protocols
Rabbits were randomly divided into four groups (n = 6 rabbits/group) as follows. The I/R group underwent 22 minutes of ischemia followed by inhalation of 50 vol% nitrogen, 50 vol% O2for 3 hours after onset of reperfusion. The P0 group underwent 22 minutes of ischemia followed by a xenon post-conditioning protocol in which rabbits inhaled 50 vol%xenon, 50 vol% O2for 1 hour at the onset of reperfusion then 50 vol% nitrogen, 50 vol% O2(xenon concentration monitor, Empaer Technology Company, Shenzhen, China)for 2 hours, beginning 1 hour after onset of reperfusion. The P2 group underwent 22 minutes of ischemia followed by a xenon post-conditioning protocol in which they inhaled 50 vol% nitrogen, 50 vol% O2for 2 hours at the beginning of reperfusion and then a 50 vol% xenon, 50 vol% O2for 1 hour beginning 2 hours after the onset of reperfusion. Rabbits in the S group did not undergo aortic occlusion, but inhaled 50 vol% nitrogen and 50 vol% O2for 3 hours.
Assessment of neurological function
Hindlimb locomotor function was scored using the Jacobs locomotor scale (Jacobs et al., 1987), which ranged from 0(no detectable hindlimb movement) to 5 (normal hindlimb locomotion). Jacobs scores were recorded at 4, 8, 24, 48 and 72 hours after reperfusion by two people who were blind to the group identity of the animals.
Sample collection
Rabbits were euthanized via intraperitoneal injection of 3%(30—50 mg/kg) sodium pentobarbital (Sigma-Aldrich Shanghai Trading, Shanghai, China) 72 hours after reperfusion and after a final neurological functional assessment. Lumbar (L3—5)spinal cord segments were rapidly removed after euthanasia.A portion of spinal cord was post- fixed in 4% (w/v) paraformaldehyde at 4°C for 3 days and serial sections (5 μm thick)were then cut for hematoxylin-eosin staining and immunohistochemistry. The remaining spinal cord segments were stored at −80°C for subsequent western blot assays.
Hematoxylin-eosin staining and neuron counts
Spinal cord segments were fixed in 4% paraformaldehyde at 4°C and then transferred to 30% sucrose for 3 days at 4°C.The samples were then frozen and 5-μm sections cut with a microtome (Leica). Sections were then stained with hematoxylin-eosin (Roche Diagnostics, Mannheim, Germany) and neuropathological changes were examined by two pathologists who were blind to the group identity of the animals. The total number of normal motor neurons in half of the anterior horn of each section was counted in five random fields (×200) using a light microscope (Olympus BX60, Tokyo, Japan).
Immunohistochemistry
After deparaffinization, endogenous peroxidase was quenched with 0.3% (v/v) hydrogen peroxide in 60% (v/v) methanol for 30 minutes. Sections were permeabilized with 0.1% (v/v) Triton X-100 in phosphate-buffered saline (PBS) for 20 minutes.Non-specific adsorption was minimized by incubating sections in 2% (v/v) normal goat serum in PBS for 20 minutes.To reduce non-speci fic staining, endogenous biotin-binding or avidin-binding sites were blocked by sequential incubation for 15 minutes with biotin and avidin. Sections were then incubated overnight at 4°C with anti-ionized calcium binding adaptor molecule 1 (Iba1) polyclonal antibody (rabbit McAb, 1:500; Abcam, Cambridge, UK). After washing in PBS(3 × 5 minutes), sections were incubated with a biotinylated sheep anti-rabbit secondary antibody at 37°C for 30 minutes(1:500; Abcam) and then with streptavidin-biotin complex.Reactions were developed with diaminobenzidine and sections counterstained with hematoxylin. Images were analyzed using NIS-ELEMENTS (Nikon, Tokyo, Japan) with ImagePro Plus 3.0 software (ECLIPSE80i 90i; Nikon).
Western blot assay
Seventy-two hours after reperfusion, spinal cord samples were homogenized in lysis buffer [50 mM Tris-HCl (pH 7.4),150 mM NaCl, 1% NP-40, and 0.1% sodium dodecyl sulfate with phenylmethyl sulfonylfluoride]. Forty milligrams of protein per lane were resolved by 10% sodium dodecyl sulfate-polyacrylamide electrophoresis and then transferred to polyvinylidene di fluoride membranes. Membranes were then blocked with 5% nonfat milk and incubated with primary antibodies targeting Iba1, IL6 and IL10 (Rabbit McAb,1:1,000; Abcam) and GAPDH (1:1,000; Beijing Biosynthesis Biotechnology, Beijing, China) at 4°C overnight. Membranes were then washed and incubated with appropriate secondary antibodies (sheep anti-rabbit or sheep anti-mouse 1:10,000; Beijing Biosynthesis Biotechnology) at room temperature for 1 hour. The optical density of each band was quanti fied using Image J software (Scion Corporation, San Francisco, CA, USA) and normalized to that of GAPDH.
Statistical analysis
All data, presented as the mean ± SEM, were analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). All data were tested using one-way analysis of variance followed by Tukey’s post hoc test. A value of P < 0.05 was considered statistically signi ficant.
Results
General condition of rabbits
Blood gases, temperature, and proximal and distal blood pressure were stable and not significantly different among the four groups at any of the three time points (before,during, or after ischemia) (Table 1).
Effects of delayed xenon post-conditioning on neurological function in rabbits with SCIRI
Functional data were collected according to the Jacobs locomotor scale at 4, 8, 24, 48 and 72 hours after reperfusion(Table 2). Rabbits in the I/R, P0 and P2 groups had significant motor dysfunction following SCI at 4, 8, 24, 48 and 72 hours after reperfusion. However, rabbits in the S groupmaintained normal locomotor function. The Jacobs score was signi ficantly higher in the P2 group compared with the P0 and I/R groups 72 hours after reperfusion. However,there was no signi ficant difference in Jacobs score between the P0 and I/R groups at 4, 8, 24, 48 and 72 hours after reperfusion.

Table 1 Physiological parameters before, during and after spinal cord ischemia and reperfusion
Effects of delayed xenon post-conditioning on the number of normal neurons at the injury site of rabbits with SCIRI
At 72 hours after reperfusion, spinal cord tissues and neurons were normal and well maintained in the S group as shown by histological examination and hematoxylin-eosin staining. However, neurons from rabbits in the I/R, P0 and P2 groups were morphologically damaged to different degrees and fewer normal neurons were found in the I/R, P0 and P2 groups compared with the S group. The P2 group had more normal neurons and fewer damaged neurons compared with the I/R and P0 groups (Figure 1).
Effects of delayed xenon post-conditioning on IL-6 and IL-10 protein levels and Iba1 immunoreactivity at the injury site of rabbits with SCIRI
IL-6 and IL-10 levels were detected in all groups by western blot assays. IL-6 and IL-10 levels were lowest in the S group and highest in the P0 group 72 hours after reperfusion (P <0.05;Figure 2). However, IL-6 and IL-10 levels were signi ficantly lower in the P2 group compared with the I/R group after reperfusion (P < 0.05).
Iba1 immunoreactivity was detected in all groups by western blot assay. Iba1 immunoreactivity was lowest in the S group and highest in the P0 group 72 hours after reperfusion

Table 2 Hindlimb locomotor function changes at 4, 8, 24, 48, and 72 hours after reperfusion
Data are expressed as the mean ± SEM (n = 6, one-way analysis of variance followed by Tukey’s post hoc test). *P < 0.05, vs. S group;&P < 0.05, vs. 4 hours after reperfusion in each group; #P < 0.05, vs.I/R group. The Jacobs scale ranged from 0 (no detectable hindlimb movement) to 5 (normal hindlimb locomotion). The I/R group was given 50% N2/50% O2, via inhalation for 3 hours at the onset of blood supply restoration. The P0 and P2 groups were given 50% xenon/50%O2, via inhalation for 1 hour at the onset of reperfusion or 2 hours after reperfusion, respectively. The S group was given 50% N2/50% O2via inhalation for 3 hours, as above, but without aortic occlusion. S:Sham operation group; I/R: ischemia/reperfusion group; P0: ischemia reperfusion + immediate xenon post-conditioning group; P2: ischemia reperfusion + delayed xenon post-conditioning group. I/R: Ischemia/reperfusion.(P < 0.05;Figure 3). However, Iba1 immunoreactivity was signi ficantly lower in the P2 group compared with the I/R group after reperfusion (P < 0.05).
Discussion

Figure 1 Effects of delayed xenon post-conditioning on the number of normal neurons at the injury site.
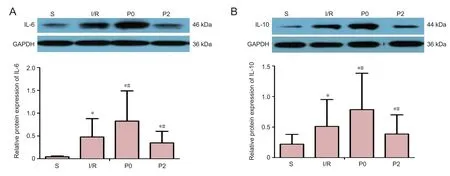
Figure 2 Effect of delayed xenon post-conditioning on IL-6 and IL-10 protein levels in rabbits with spinal cord I/R injury 72 hours after reperfusion.
To the best of our knowledge, this is the first study to examine the effects of immediate and delayed xenon post-conditioning on the release of in flammatory factors and microglial activation in a rabbit model of SCI. The major findings of this study are summarized as follows. (1) Delayed xenon post-conditioning significantly improved neurological function in rabbits and attenuated the microglia-mediated inflammatory response. (2) Immediate xenon post-conditioning amplified the microglia-mediated inflammatory response.
The neuroprotective effects exerted by xenon post-conditioning in animal models of cerebral injury are different according to the timing of administration; the optimal time was delayed rather than immediate administration (David et al., 2003, 2008, 2010; Ma et al., 2005; Dingley et al., 2006;Fries et al., 2008, 2009; Hobbs et al., 2008; Thoresen et al.,2009; Britton et al., 2010; Sheng et al., 2012; Yang et al.,2012; Zhuang et al., 2012; Peng et al., 2013; Metaxa et al.,2014). In the current study, delayed xenon administration following SCI had a neuroprotective effect, which is consistent with our previous study (Yang et al., 2014). Interesting-ly, no neuroprotective effect was observed with immediate xenon post-conditioning and the underlying mechanism for this phenomenon remains unknown.

Figure 3 Effect of delayed xenon post-conditioning on microglial activation in rabbits with spinal cord I/R injury.
The microglia-mediated inflammatory response was stronger in the P0 group than in the other groups and had no neuroprotective effect. Previous studies have shown that pro-in flammatory cytokines, such as tumor necrosis factor and IL6, are the major cause of secondary pathological injury following spinal cord ischemia (Nesic et al., 2001; Pineau and Lacroix, 2007; Jia et al., 2012). In addition, tumor necrosis factor and IL1 are also implicated in microglial activation(Bartholdi and Schwab, 1997; Kwon et al., 2004; Wang et al.,2005). However, exactly how xenon post-conditioning regulates the secondary in flammatory response after reperfusion in SCI is unclear and only a few studies have explored the relationship between xenon and the in flammatory response.de Rossi et al. (2004) demonstrated anti-in flammatory properties of xenon. However, Saravanan et al. (2009) found that xenon aggravated the inflammatory response by activating leucocytes and platelets. It is well known that necrosis and apoptosis can coexist in SCI (Karin and Ben-Neriah, 2000).The inflammatory profiles induced by immediate and delayed xenon post-conditioning were examined in the current study. IL-6 and IL-10 levels were signi ficantly increased in the P0 group compared with the P2 and I/R groups. A previous study had reported a pro-inflammatory effect of xenon in patients receiving cardiac surgery (Breuer et al., 2015); thus,it is possible that the signi ficantly increased in flammatory response in the P0 group may have increased necrosis of spinal cord cells, resulting in the production of large amounts of necrotic cell debris. Therefore, aggravated microglia-mediated in flammatory responses may have resulted in ineffective neuroprotection.
Because there is a relationship between the in flammatory response and microglia, the activation of microglia was also investigated in this study. Levels of Iba1 towards the spinal cord tended to be higher in the P0 group compared to the P2 and I/R groups. Microglia respond within minutes after SCI and activated microglia produce high levels of pro-inflammatory cytokines. However, Yong and Rivest (2009)reasoned that this may be attributed to the bene ficial effect of activated microglia in protecting neurons. Microglia are the first cells to respond to injury in the spinal cord and central nervous system and produce in flammatory cytokines(Probert et al., 2000; Ma et al., 2005). In view of these different in flammatory responses, the number of activated microglia was also examined in the current study. The number of activated microglia tended to be higher in the P0 group compared with the P2 and I/R groups. Therefore, the higher number of activated microglia in the P0 group signi ficantly affected the essential xenon pro-in flammatory mechanisms after spinal cord ischemia injury.
The polarization of microglia may underlie different functional properties of microglia. IL6 is up-regulated by microglia and macrophages during the early stage of SCI (Ma et al., 2005; Longbrake et al., 2007). A recent study characterized macrophage/microglial polarization in a mouse SCI model and reported that most activated microglia were type M1 and not type M2 (Kigerl et al., 2009). It is thought that the low number of anti-in flammatory M2 macrophages may aggravate the pro-in flammatory effect after SCI. In addition, we recently found that M1- and M2-type cells were rapidly up-regulated by immediate xenon post-conditioning in a cultured microglia oxygen-glucose deprivation model,whereas only the M2 markers were up-regulated by 2-hour delayed xenon post-conditioning. Therefore, we speculate that xenon may affect the spinal cord environment after ischemia, which affects the timing of polarization of different subsets of microglia.
This study has several limitations. First, it was performed in a rabbit model of spinal cord ischemia and needs to be validated in other animal models. Second, the analysis only focused on microglia and did not assess astrocytes, which are probably involved in the systemic inflammatory response.Due to the limited number of animals, only a few important cytokines could be detected. Other inflammatory cytokines should be examined in in vivo and in vitro studies. Third, the polarization of microglia lasted for a long time after SCI. The effects of xenon on inflammatory responses and microglial polarization should be evaluated over a longer period.
In summary, delayed xenon post-conditioning provided better protective effects against SCI in the rabbit model in vivo compared with immediate xenon post-conditioning.This was likely due to suppression of microglial activation.Immediate xenon post-conditioning triggered microglial activation and amplified both pro- and anti-inflammatory responses, which could offset neuroprotective effects when used early in the reperfusion period. Microglial polarization may be modulated by xenon depending on the timing of administration after reperfusion. The authors will extend their study to determine which subsets of microglia are affected and whether astrocytes are affected.
Author contributions:WPC, YWY, MJ and JKL participated in study concept, design, data analysis and paper writing. YLW and LT reviewed this paper. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Financial support:This research was funded by the National Natural Science Foundation of China, No. 81271387; the Research Special Fund of Public Welfare and Health Department of China, No. 201402009; the National Key Technology R&D Program in China, No. Z141107002514031.The funders had no role in the study design, data collection and analysis,decision to publish, or preparation of the manuscript.
Research ethics:All procedures were evaluated and approved by the Institutional Animal Care and Use Committee at Capital Medical University, Beijing, China. Rabbits received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” established by NIH Publication No. 85-23, revised 1996.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Bartholdi D, Schwab ME (1997) Expression of pro-in flammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 9:1422-1438.
Breuer T, Emontzpohl C, Coburn M, Benstoem C, Rossaint R, Marx G,Schalte G, Bernhagen J, Bruells CS, Goetzenich A, Stoppe C (2015)Xenon triggers pro-in flammatory effects and suppresses the anti-inflammatory response compared to sevo flurane in patients undergoing cardiac surgery. Crit Care 19:365.
Britton GL, Kim H, Kee PH, Aronowski J, Holland CK, McPherson DD, Huang SL (2010) In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation 122:1578-1587.
Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D(2002) Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 236:471-479.
Clark JA, Ma D, Homi HM, Maze M, Grocott HP (2005) Xenon and the in flammatory response to cardiopulmonary bypass in the rat. J Cardiothorac Vasc Anesth 19:488-493.
David HN, Haelewyn B, Risso JJ, Colloc’h N, Abraini JH (2010) Xenon is an inhibitor of tissue-plasminogen activator: adverse and bene ficial effects in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 30:718-728.
David HN, Haelewyn B, Rouillon C, Lecoq M, Chazalviel L, Apiou G, Risso JJ, Lemaire M, Abraini JH (2008) Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J 22:1275-1286.
David HN, Leveille F, Chazalviel L, MacKenzie ET, Buisson A, Lemaire M, Abraini JH (2003) Reduction of ischemic brain damage by nitrous oxide and xenon. J Cereb Blood Flow Metab 23:1168-1173.
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Neuroscience 12:388-399.
de Rossi LW, Horn NA, Stevanovic A, Buhre W, Hutschenreuter G,Rossaint R (2004) Xenon modulates neutrophil adhesion molecule expression in vitro. Eur J Anaesthesiol 21:139-143.
Dingley J, Tooley J, Porter H, Thoresen M (2006) Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke 37:501-506.
Fahlenkamp AV, Coburn M, Haase H, Kipp M, Ryang YM, Rossaint R,Beyer C (2011) Xenon enhances LPS-induced IL-1beta expression in microglia via the extracellular signal-regulated kinase 1/2 pathway. J Mol Neurosci 45:48-59.
Fan L, Wang K, Shi Z, Die J, Wang C, Dang X (2011) Tetramethylpyrazine protects spinal cord and reduces in flammation in a rat model of spinal cord ischemia-reperfusion injury. J Vasc Surg 54:192-200.
Fries M, Coburn M, Nolte KW, Timper A, Kottmann K, Kuru TH,Weis J, Rossaint R (2009) Early administration of xenon or iso flurane may not improve functional outcome and cerebral alterations in a porcine model of cardiac arrest. Resuscitation 80:584-590.
Fries M, Nolte KW, Coburn M, Rex S, Timper A, Kottmann K,Siepmann K, Hausler M, Weis J, Rossaint R (2008) Xenon reduces neurohistopathological damage and improves the early neurological de ficit after cardiac arrest in pigs. Crit Care Med 36:2420-2426.
Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N (2009) Analysis of serum pro-in flammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turk Neurosurg 19:353-359.
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J (2008) Xenon and hypothermia combine additively, offering longterm functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke 39:1307-1313.
Jacobs TP, Shohami E, Baze W, Burgard E, Gunderson C, Hallenbeck JM, Feuerstein G (1987) Deteriorating stroke model: histopathology,edema, and eicosanoid changes following spinal cord ischemia in rabbits. Stroke 18:741-750.
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50:264-274.
Karalija A, Novikova LN, Kingham PJ, Wiberg M, Novikov LN (2014)The effects of N-acetyl-cysteine and acetyl-L-carnitine on neural survival, neuroin flammation and regeneration following spinal cord injury. Neuroscience 269:143-151.
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination:the control of NF-[kappa]B activity. Annu Rev Immunol 18:621-663.
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435-13444.
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury.Spine J 4:451-464.
Longbrake EE, Lai W, Ankeny DP, Popovich PG (2007) Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem 102:1083-1094.
Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD,Mehmet H, Edwards AD, Franks NP, Maze M (2005) Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol 58:182-193.
Matsumoto S, Matsumoto M, Yamashita A, Ohtake K, Ishida K,Morimoto Y, Sakabe T (2003) The temporal pro file of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth Analg 96:1777-1784.
Metaxa V, Lagoudaki R, Meditskou S, Thomareis O, Oikonomou L,Sakadamis A (2014) Delayed post-ischaemic administration of xenon reduces brain damage in a rat model of global ischaemia. Crit Care 28:364-369.
Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R(2001) IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma 18:947-956.
Nguyen T, Mao Y, Sutherland T, Gorrie CA (2017) Neural progenitor cells but not astrocytes respond distally to thoracic spinal cord injury in rat models. Neural Regen Res 12:1885-1894.
Olson JK (2010) Immune response by microglia in the spinal cord.Ann N Y Acad Sci 1198:271-278.
Oz Oyar E, Korkmaz A, Kardess O, Omeroglu S (2008) Aortic cross-clamping-induced spinal cord oxidative stress in rabbits: the role of a novel antioxidant adrenomedullin. J Surg Res 147:143-147.
Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD,Pappa LS, Gkrepi C, Anagnostopoulos CE, Kappas AM (2006) Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res 133:159-166.
Paulson TA, Goosey-Tolfrey VL, Lenton JP, Leicht CA, Bishop NC(2013) Spinal cord injury level and the circulating cytokine response to strenuous exercise. Med Sci Sports Exerc 45:1649-1655.
Peng T, Britton GL, Kim H, Cattano D, Aronowski, J, Grotta J,McPherson DD, Huang SL (2013) Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neurosci Ther 19:773-784.
Pineau I, Lacroix S (2007) Proin flammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identi fication of the cell types involved. J Comp Neurol 500:267-285.
Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP (2001) Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol 168:144-154.
Probert L, Eugster HP, Akassoglou K, Bauer J, Frei K, Lassmann H,Fontana A (2000) TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain 123:2005-2019.
Ren XB, Wang GH, Lu t, An YB, Liu ZH, Dong YZ (2016) Neuroprotective effect of lipoxin receptor agonist BML-111 on spinal cord ischemia-reperfusion injury in a rat model. Zhongguo Zuzhi Gongcheng Yanjiu 20:2642-2647.
Saravanan P, Exley AR, Valchanov K, Casey ND, Falter F (2009) Impact of xenon anaesthesia in isolated cardiopulmonary bypass on very early leucocyte and platelet activation and clearance: a randomized, controlled study. Br J Anaesth 103:805-810.
Savas S, Delibas N, Savas C, Sutcu R, Cindas A (2002) Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord 40:224-229.
Sheng SP, Lei B, James ML, Lascola CD, Venkatraman TN, Jung JY,Maze M, Franks NP, Pearlstein RD, Sheng H, Warner DS (2012)Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiology 117:1262-1275.
Shultz RB, Zhong Y (2017) Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res 12:702-713.
Smith PD, Puskas F, Meng X, Lee JH, Cleveland JC, Jr Weyant MJ, Fullerton DA, Reece TB (2012) The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation 126:S110-117.
Tan MY, Guo X, Wang YH (2010) Segmental artery ligation for establishment of local spinal cord ischemic injury model in rabbits.Zhongguo Zuzhi Gongcheng Yanjiu 14:4407-4410.
Temiz C, Solmaz I, Tehli O, Kaya S, Onguru O, Arslan E, Izci Y (2013)The effects of splenectomy on lipid peroxidation and neuronal loss in experimental spinal cord ischemia/reperfusion injury. Turk Neurosurg 23:67-74.
Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J (2009)Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 29:707-714.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001)Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580-2588.
Tsuruta S, Matsumoto M, Fukuda S, Yamashita A, Cui YJ, Wakamatsu H, Sakabe T, (2006) The effects of cyclosporin A and insulin on ischemic spinal cord injury in rabbits. Anesth Analg 102:1722-1727.
Wang XJ, Kong KM, Qi WL, Ye WL, Song PS (2005) Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol Sin 26:934-942.
Watanabe K, Kawaguchi M, Kitagawa K (2012) Evaluation of the neuroprotective effect of minocycline in a rabbit spinal cord ischemia model. J Cardiothor Vascan 26:1034-1038.
Yang YW, Cheng WP, Lu JK, Dong XH, Wang CB, Zhang J, Zhao LY,Gao ZF (2014) Timing of xenon-induced delayed postconditioning to protect against spinal cord ischaemia-reperfusion injury in rats.Brit J Anaesth 113:168-176.
Yang YW, Lu JK, Qing EM, Dong XH, Wang CB, Zhang J, Zhao LY,Gao ZF, Cheng WP (2012) Post-conditioning by xenon reduces ischaemia-reperfusion injury of the spinal cord in rats. Acta Anaesth Scand 56:1325-1331.
Yong VW, Rivest S (2009) Taking advantage of the systemic immune system to cure brain diseases. Neuron 64:55-60.
Zhuang L, Yang T, Zhao H, Fidalgo AR, Vizcaychipi MP, Sanders RD,Yu B, Takata M, Johnson MR, Ma D (2012) The protective pro file of argon, helium, and xenon in a model of neonatal asphyxia in rats.Crit Care Med 40:1724-1730.
- 中国神经再生研究(英文版)的其它文章
- The biological clock: future of neurological disorders therapy
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology