Synthesis, Structure and Photoelectric Property of a 3D Supramolecular Zinc Coordination Polymer①
LUO Ya-Nan LIU Zhi-Chen JIANG Hui-Ying YU Li-Ying YU Xiao-Yang
Synthesis, Structure and Photoelectric Property of a 3D Supramolecular Zinc Coordination Polymer①
LUO Ya-Nan②LIU Zhi-Chen JIANG Hui-Ying YU Li-Ying YU Xiao-Yang②
(College of Chemical and Pharmaceutical Engineering, Jilin Institute of Chemical Technology, Jilin 132022, China)
Using a rigid azo ligand 4-[(8-hydroxy-5-quinolinyl)azo]-benzoic acid (H2L), a new supramolecular compound [Zn(L)(H2O)2]n(1) has been solvothermally synthesized and structurally characterized by X-ray single-crystal diffraction, infrared spectrum, elemental analysis, power X-ray diffraction and thermal analysis. Compound 1 crystallizes in monoclinic, space group2/with= 30.372(8),= 11.415(3),= 9.248(3) Å,= 106.94(3)º,= 3067.20(15) Å3, C16H13N3O5Zn,M= 392.66,= 8, D= 1.701Mg/m3;(000) = 1600,= 1.636 mm−1, reflections collected: 7290, reflections unique: 2735,int= 0.0282,= 0.0351,(all data) = 0.0919,on2= 1.036. Compound 1 exhibits a one-dimensional (1D)zig-zag chain structure connected into a three-dimensional (3D) supramolecular network through hydrogen bonding interactions. Fluorescent property and electrochemical property were detected on compound 1.
coordination polymer, co-sensitization, solar cell, fluorescent property, electrochemical property;
1 INTRODUCTION
Over the past decade, many peoplefascinated supramolecularcoordination polymer materials con- structed by organic bridging ligands and transition metal ions because they possessed various architec- tures and multiple applications in molecular recog- nition, sensors, photology and electrochemistry[1-5]. During the process of constructing these supramole- cular coordination polymer materials, a valid selec- tion or design of multifunctional organic ligands is crucial to conceivable functions[6-9]. As one of the azo organic ligands, 4-[(8-hydroxy-5-quinolin- yl)azo]-benzenesulfonic acid may be a desired ligand due to its structural characteristic[10-14]: (i) It reveals diversiform coordination abilities because it has multiple coordination sites. (ii) Owing to simultaneous possessing the O and N atoms, it could take on the acceptors or donors of hydrogen bonding to assemble various supramolecular struc- tures. (iii) Quinoline ring has a strong chelating ability to combine with metal ions attributing to the ortho position between hydroxyl and N atom. On the other hand, metal organic coordination polymers with optical performance have been tentatively used in the field of dye-sensitized solar cells (DSSCs) as spectral sensitizers in recent years[15]. Grätzel groups successfully co-sensitized squarylium cya- nine dye and bisthiophene dye based on nanocry- stalline TiO2films. Fortunately, the whole photo- electric conversion efficiency can reach 7.43%. This value reported for co-sensitized DSSC with metal organic coordination polymers is the highest so far[16]. Based on the above, H2L may be a com- mendable ligand to construct supramolecular coor- dination polymer materials, which can own multifarious structures, excellent fluorescent perfor- mance and favorable electrochemical property.
2 EXPERIMENTAL
2. 1 Materials and instruments
IR (KBr pellets) spectra were taken on a Nicolet Impact 410 FT-IR spectrometer in the 4000~400 cm-1range and elemental analyses for C, H and N were performed on a Perkin-Elmer 2400 Elemental Analyzer. PXRD were performed on a Siemens D5005 diffractometer, using Cu-(= 0.15418 nm) with a graphite monochromator. Thermal studies were recorded by thermogravimetric analy- ses (TGA) on a Perkin-Elmer TGA 7 thermogravi- metric analyzer with a heating rate of 10 °C·min-1under N2. The fluorescence property was detected by LS55 luminescence spectrometer. Electrochemi- cal impedance was measured by chi660d elec- trochemical work-station with three-electrode sys- tem in the dark. Frequency range was 0.05~105 Hz, and the applied potential was generally between 0~0.700 V. Under AM 1.5 illumination intensity (100 mW cm–2) by using a solar simulator, thephoto- current-photovoltage () curse of the sealed cells was measured. The fill factor () and the photoelectric conversion efficiency () are defined as(maxmax)/(scoc) and= (scoc)/in, respectively (maxandmaxare the photocurrent density and photovoltage for maxi- mum power output, respectively.scandocare the shortcircuit photocurrent density and open-circuit photovoltage, respectively.in is the power of incident light).
2. 2 Synthesis of [Zn(L)(H2O)2]n
A mixture of ZnCl2(30 mg, 0.22 mmol), H2L (5 mg, 0.015 mmol), ethanol (10 mL), H2O (2 mL) and dimethyl formamide (DMF, 2 mL) was stirred at 25 °C till getting a clarified red solution. After 2 h reaction, the pH value was adjusted with 2 mol/L NaOH to 5.0, and the mixture was finally sealed in a 25 mL Teflon-lined stainless-steel vessel and heated at 100 °C for 3 days. Then, it was cooled to 25 °C for 18 h with a programmed cooling course. Red block crystals were filtered and washed with deionized water, finally dried in air. Yields based on Zn are 50%. Elemental anal. calcd. (%) for C16H13N3O5Zn (392.66): C, 48.89; H, 3.31; N, 10.69%. Found: C, 47.54; H, 3.03; N, 10.12%. IR (KBr pellet,/cm–1, Fig. S1): 3448 (s), 1602 (w), 1540 (m), 1502 (s), 1516 (s), 1466 (s), 1409 (s), 1398 (s), 1386 (s), 1253 (m), 1189 (s), 1131 (s), 1035 (m), 852 (w).
2. 3 Structure determination
A red single crystal with dimensions of 0.30mm × 0.10mm × 0.10mm was measured on a Bruker Apex II CCD area-detector diffractometer (Mo, 0.71070 Å) at 293 K. A total of 7290 reflections were collected byascan mode at room temperature including 2735 independent ones withint= 0.0282, of which 2319 were observed with> 2(). Using the SADABS program,an empirical absorption correction was used to the measured data. The structure of compound 1 was solved by direct methods (SHELXS-97, Sheldrick, 1997)and refined by full-matrix least-squares techniques on2[17]. All non-hydrogen atoms were refined anisotropically. H atoms were placed geometrically by the OLEX 2 program[18]. The= 0.0351 and= 0.0919 (= 1/[2(F2) + (0.0447)2+ 3.2819], where= (F2+ 2F2)/3). (Δ/)max= 0.002,= 1.036, (Δ)max= 0.581 and (Δ)min= –0.443 e/Å3. Selected bond lengths and bond angles are given in Table 1, and the hydrogen bond distances and bond anglesin Table 2.
2. 4 Fabrication of DSSCs
TiO2film was 0.25 cm2. The film electrodes were soaked in compound 1 solution for 3 h at room temperature (solvent is absolute ethanol, the concentration is 5 × 10–4M). And then the film electrodes were soaked in N719 solution for 20 h at room temperature (The solvent mixture is acetone- trile and absolute ethanol in volume ratio of 1:1, with the concentration to be 5 × 10–4 M). Finally, the co-sensitizedfilms were dried in air. A thermally platinized conducting glass (5 mM H2PtCl6in dry isopropanol, heated at 400 °C for 10 min) can act as the counter electrodes. 0.5 M LiI, 0.05 M I2, and 0.1 M 4-tertbutylpyridine in 1:1 (volume ration) acetonitrile-propylene carbonate can compose the electrolyte.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
Symmetry transformation: i: 0.5+,0.5–,0.5+

Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry codes: iii: 1–, –, 1–; iv: 1.5–, –0.5+, 1.5–; v:, –, 0.5+
3 RESULTS AND DISCUSSION
3. 1 Crystal structure
Compound 1 was synthesized by ZnCl2and H2L ligand under solvothermal conditions. Single-crystal X-ray diffractionanalysisreveals that compound 1crystallizes in the monoclinic system, space group2/, which exhibits an infinite 1D zig-zag chainstructure. As shown in Fig. 1, the asymmetrical unit of compound 1 contains one Zinc(II) atom, one L2−ligand and two coordinated water molecules. Zn1 atom is five-coordinated by four oxygen atoms (O(1)i, O(3), O(4), O(5)) and one nitrogen atom (N(3)i), forming a slightly distorted rectangular pyramidal configuration. Among them, (O(1)i, O(3), O(4), N(3)i) form a plane, and (O(5)) looks like the peak of the rectangular pyramid configuration, as shown in Fig. S2. The distances of Zn–O/N (1.988(2)~2.096(2) Å) are comparable with those found in other related zinc(II) complexes[19].

Fig. 1. Coordination environments of Zn1 in compound 1
(Symmetry codes: i: 0.5+, 0.5–, 0.5+; ii: –0.5+, 0.5–, –0.5+)
In compound 1, every L2-gives three donors for coordination, and the coordination mode of the carboxyl group exhibits a monodentate fashion, as shown in scheme S1. L2–ligand shows a2-terden- tate mode, in which the 8-HQ group coordinates to one Zn(II) cation in a bidentate chelating mode, and the carboxylic acid group adopts a monodentate fashion to coordinate with the other Zn(II) cation. As shown in Fig. 2, Zn(II) cations are linkedinto an infinite zig-zag chain along theaxis by L2–ligands with an adjacent sinusoidal ruffling motif Zn···Zn distance of. 29.06 Å.
In the crystal building, each zig-zag chain inte- racts with adjacent chains into a 3D supramolecular network by hydrogen bonds. Crystal structure reveals that the 1D zig-zag chains were connected to each other due to the O–H···O interactions ((O(4)···O(1)iii) = 2.751(3) Å, ∠O(4)–H(4A)···O(1)iii= 153°;(O(5)···O(2)iv) = 2.767(3) Å, ∠O(5)– H(5A)···O(2)iv= 159°), forming a 2D layer structure, as shown in Fig. 3(a). O(5) acting as a hydrogen bond donor bonds to (O(2)v) from the two neighboring supramolecular layers to form a 3D supramolecular network ((O(5)···O(2)v) = 2.581(3) Å, ∠O(5)–H(5B)···O(2)v= 164°), as shown in Fig. 3(b).
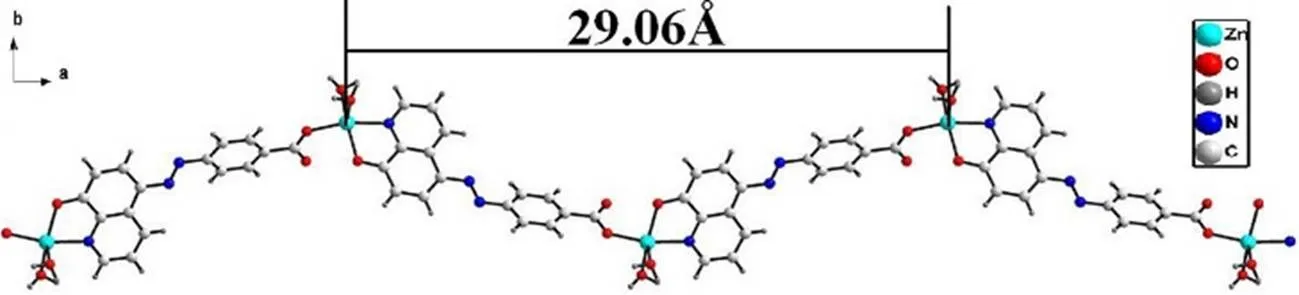
Fig. 2. 1D chain structure in compound 1
Fig. 3. (a) 1D chains are connected into a 2D supramolecular layer structure; (b) 2D supramolecular layer structures are connected into a 3D supramolecular networkhydrogen bonding interactions in compound 1 (Symmetry codes: iii: 1–, –, 1–; iv: 1.5–, –0.5+, 1.5–; v:, –, 0.5+)
3. 2 PXRD
Phase purity of the bulk material was confirmed by comparison of its PXRD patterns with the simu- lated pattern from the single-crystal X-ray diffrac- tion study (Fig. S3).
3. 3 TGA
TGA result of compound 1 is provided in the Supporting Information (Fig. S4). Experimental result indicates that 1 lost its two coordinated water molecules from 110 to 210 °C (expt. 8.56%, calcd. 9.19%). A total mass loss of 52.96% from 370 to 990 °C can be attributed to the release of L2–ligand (calcd. 53.60%).
3. 4 Fluorescent property
Under the same situation the fluorescent pro- perties of compound 1 and the ligand H2L have been measured in the solid state at room tempe- rature. As shown in Fig. 4, the free ligand exhibits emission maxima at 381 nm upon 220 nm excitation, which can be attributed to the-* or n-* intrali- gand electronic transitions including the -N=N- based-* transition[20]. Compound 1 displays the emission maxima at 384 nm when excited at 222 nm. Compound 1 displays similar emission, which can be mainly assigned to the intraligand electronic transfer.
3. 5 Electrochemical property
We performed photoelectric conversion efficiency measurements for compound 1. Fig. 5 shows photo- current-photovoltaic curves of dye-sensitized solar cell (DSSC) based on compound 1/N719/TiO2and N719/TiO2photoanodes. As shown in Fig. 5, the photovoltaic performances of DSSC based on com- pound 1/N719/TiO2photoanode are improved rela- tively to that of DSSC based on N719/TiO2photo- anode. The short-circuit photocurrent (sc), open- circuit photovoltage (oc), fill factor (), and photoelectric conversion efficiency () of DSSC for compound 1 and N719 co-sensitization improved from 10.58 mA∙cm–2, 648 mV, 0.49, 3.45% to 11.71 mA∙cm–2, 678 mV, 0.57, 4.5%, respectively. As we know, H2L is a rigid ligand including simultaneously benzene and quinoline rings. In compound 1, each 1D zig-zag chain is connected with adjacent chains to form a 3D supramolecular network by hydrogen bonds. All of these can make compound 1 possess a relatively larger-conjugated system and a highly rigid framework structure with excellent aggre- gation-induced emission (AIE) properties. The enhancement of photovoltaic performances of DSSC indicates that the co-sensitization of compound 1 and N719 are positive for the transfer and collection of electrons. An increasingscis due to the increase of light harvesting in DSSC.
It is widely known that the absorption peak of N719 is 550 nm in visible light region. The com- bination of compound 1 and N719 enhances the absorption intensity in the 300~400 nm range of DSSC, resulting in a higher light harvesting. The increase ofhints that the electron transfer resistance at the interface of compound 1/N719/TiO2is lower than that for N719/TiO2. Above results imply the HOMO and LUMO levels of compound 1 matched the energy level of TiO2conduction band. In a whole, the co-sensitization of compound 1 and N719 is beneficial toof DSSC by enhancing the absorption of 300~400 nm range.

Fig. 4. Solid-state fluorescent spectrum of the H2L (a) and compound 1 (b) at room temperature
Fig. 5. Photocurrent-photovoltaic curves of dye-sensitized solar cell based on compound 1/N719/TiO2and N719/TiO2photoanodes
4 CONCLUSION
In summary, a new supramolecule zinc coordi- nation polymer has been successfully synthesized under solvothermal conditions. Compared with co-sensitization with dye N719/TiO2, photoelectric conversion efficiency has a definite increase based on compound 1/N719/TiO2photoanodes, because the modification of TiO2electrode with compound 1 and N719 not only extends the photoresponse of DSSC to the UV region of the solar spectrum, but also intensifies the optical spectrum absorption. The fluorescent properties and electrochemicalpro- perties of compound 1 demonstrate that it might provide a potential application in the region of photology and electrochemistry.
(1) Long, J. R.; Bloch, E. D.; Britt, D.; Lee, C.; Donna, C. J.; Uribe-Romo, F. J.; Furukawa, H.; Yaghi, O. M. Metal insertion in a micro porous metal-organic framework lined with 2,2΄-bipyridine.2010, 132, 14382–14384.
(2) Zhang, D. D.; Zhou, X. B.; Jian, L. J.; Lin, M. J.; Li, H. P.; Chen, J. X.; Zhang, Z. C. Synthesis, structure and norbornene polymerization catalyzed by bis{(2-benzhydryl-4,6-dimethyl-phenyl)-(3,5-di-tert-butyl-2-methyl-benzylidene)-amine-N,O}nickel(II).. 2017, 36, 1479–1485.
(3) Tan, X. W.; Li, C. H.; Li, H. F.; Yang, Y. Q. Synthesis, electrochemical and fluorescent properties of a new zinc(II) complex through self-assembly reaction of 2,2΄-bipyridine-3,3΄-dicarboxylic acid.. 2017, 36, 310–315.
(4) Wu, L.; Guo, X. L.; Wang, Z. J.; Chen, J. X. Syntheses, crystal structure, and fluorescent property of a new (4,10)-connected 3D Cd(II) coordination polymer.2015, 44, 451–453.
(5) Zhang, C. L.; Zheng, H. G. Synthesis, crystal structure and fluorescent property of one Zn(II) complex with 1,3-bis(imidazol-1-yl)benzene and 1,3-benzenedicarboxylate.. 2016, 35, 1070–1076.
(6) Luo, Y. N.; Xu, X. Z.; Sun, F. X.; Yu, X. Y.; Zhang, X.; Zhang, T.; Yu, L. Y. Synthesis, structure and properties of two new coordination polymers based on 4-[(8-hydroxy-5-quinolinyl)azo]-benzenesulfonic acid.2014, 30, 27–31.
(7) Zhu, X. F.; Zhang, H.; Zhou, Y. H.; Guan, L. Hydrothermal synthesis, crystal structure, and magnetic properties of two new coordination polymers [Ni(IHQS)(4,4΄-bipy)0.5·(H2O)2]nand [Cu(IHQS)(4,4΄-bipy)0.5·H2O]n.2010, 636, 457–461.
(8) Wu, T.; Xu, H. Y.; Kong, F. Z.; Yu, Z. Y.; Wang, R. H. Synthesis and structure of a Mn(II)-triazolyl coordination polymer consisting of dinuclear units.2012, 31, 1557–1562.
(9) Wang, J. J.; Gou, L.;Hu, H.M.;Han, Z. X.;Li, D.S.;Xue, G.L.; Yang, M.L.;Shi,Q.Z. Ligand and pH-controlled ZnIIbilayer coordination polymers based on biphenyl-3,3΄,4,4΄-tetracarboxylate.2007, 7, 1514–1521.
(10) Feng, X.; Wang, L. Y.; Wang, J. G.; Xie, C. Z.; Zhao, J. S.; Sun, Q. A unique example of a 3D framework based on the binuclear dysprosium(III) azobenzene-3,5,4΄-tricarboxylate with 3,6-connected topology showing ferromagnetic properties.2010, 12, 3476–3482.
(11) Gu, X. J.; Lu, Z. H.; Xu, Q. High-connected malodorous metal-organic framework.2010, 46, 7400–7402.
(12) Li, L.; Yang, Y. L.; Fan, R. Q.; Wang, X.; Zhang, Q. M.; Zhang, L. Y.; Yang, B.; Cao, W. W.; Zhang, W. Z.; Wang, Y. Z.; Ma, L. Q.Photocurrent enhanced dye-sensitized solar cells based on TiO2loaded K6SiW11O39Co(II)(H2O)·H2O photoanode materials.2014, 43, 1577–1582.
(13) Zhang, L. L.; Lu, C. Y.; Chen, S. P.; Yu, F. S.; Li, X.; Tan, J. T.; Yang, X. W. Synthesis, structure and properties of novel 3-D porous lanthanide-3,4΄,5-azobenzenetricarboxylate frameworks.2011, 14, 143–145.
(14) Yu, X. Y.; Cui, X. B.; Zhang, X.; Jin, L.; Luo, Y. N.; Yang, J. J.; Zhang, H.; Zhao, X. A novel 3D cadmium coordination polymer constructed from hydrazine and benzene-1,2,4,5-tetracarboxylic acid: synthesis, structure and fluorescent property.2011, 14, 848–851.
(15) Zhang, L. Y.; Yang, Y. L.; Fan, R. Q.; Wang, P.; Li, L. Enhance the performances of dye-sensitized solar cell by a new type of sensitizer to co-sensitize zinc oxide photoelectrode with ruthenium complex.2012, 92, 1314–1319.
(16) Yum, J. H.; Jang, S. R.; Walter, P.; Geiger, T.; Nüesch, F.; Kim, S.; Ko, J.; Grätzela, M.; Nazeeruddin, M. K. Efficient cosensitization of nanocrystalline TiO2films by organic sensitizers.2007, 44, 4680–4682.
(17) Sheldrick, G. M.. Bruker AXS. Madison 1998.
(18) Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysisprogram.2009, 42, 339–341.
(19) Qi, Y.; Che, Y. X.; Zheng, J. M. A zinc(II) coordination polymer constructed from mixed-ligand 1,2-bis(2-(1H-imidazol-1-yl)ethoxy)ethane and 1,4-benzenedicarboxylic acid.2008, 10, 1137–1139.
(20) Yu, X. Y.; Ye, L.; Zhang, X.; Cui, X. B.; Zhang, J. P.; Xu, J. Q.; Hou,Q.; Wang, T. G. Fluorescent metal-organic polymers of zinc and cadmium from hydrothermalacylation reaction.2010, 39, 10617–10625.
19 June 2017;
14 September 2017 (CCDC 1031063)
the National Science Foundation of China (No.20831002 and 21531003) and Project of Science and Technology Development of Jilin City (No.20166024)
Luo Ya-Nan, 1979, doctor, majoring in coordination chemistry. E-mail: yanan_meimei@163.com.Yu Xiao-Yang, 1980, doctor, majoring in coordination chemistry. E-mail: 107850005@qq.com
10.14102/j.cnki.0254-5861.2011-1755
- 结构化学的其它文章
- Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

