A New Cd(II) Coordination Compound Based on 4-(1,2,4-Triazol-4-yl)phenylacetic Acid: Synthesis, Structure and Photoluminescence Property①
ZHU Mei-An GUO Xing-Zhe XIAO Li CHEN Shui-Sheng
A New Cd(II) Coordination Compound Based on 4-(1,2,4-Triazol-4-yl)phenylacetic Acid: Synthesis, Structure and Photoluminescence Property①
ZHU Mei-An GUO Xing-Zhe XIAO Li CHEN Shui-Sheng②
(236041)
A new complex [Cd2(L)2(Cl)2(H2O)]n(1) was synthesized by reacting CdCl2·2.5H2O with 4-(1,2,4-triazol-4-yl)phenylacetic acid (HL) ligand. The structure of the complex was charac- terized by single-crystal X-ray diffraction, IR spectroscopy, elemental analysis and PXRD. Complex 1 crystallizes in triclinic, space group21/with= 11.4303(8),= 14.1792(10),= 14.6857(10) Å,= 96.3780(10)º,= 2365.4(3) Å3,= 4, C20H16Cl2N6O5Cd2,M= 716.09,D= 2.011 g/cm3,=mm-1,= 1.051,(000) = 1392, the final= 0.0458 and= 0.0949 for 5402 observed reflections (> 2()). Complex 1 is a two-dimensional (2D) layer structure and non-covalent bonding interactions such as C–H···and···extend the 2D to form a three-dimensional supramolecular polymer.
synthesis, supramolecular polymer, photoluminescent property;
1 INTRODUCTION
The rational design and fabrication of metal- organic frameworks (MOFs) have flourished as an emerging area of research because of their promising applications in guest molecule inclusion, gas and vapor storage, chemical sensing, magnetism, hetero- geneous catalysis, etc[1-5]. Although synthetic con- ditions have great influence on the structures of the resulted complexes such as reaction temperature, solvent, pH of the medium, ligand-to-metal ratios and choice of metal precursors, the most important factor for the construction of desired MOFs is the judicious choice of appropriate linkers[6, 7]. In addition, the typical non-covalent interactions (such as H-bonding and/stacking C−H···π)[8-10]also play important roles in the assembly of supramo- lecular polymers. As for ligands linkers, the N- and/or O-donors have been extensively to build novel MOFs with interesting structures and pro- perties[11, 12]. Among the N-donors, the imidazole, triazole and tetrazole analogues are widely employed in the construction of MOFs. Our groups have engaged in the design of N-donor 4-imidazole ligands, such as 1,4-di(1H-imidazol-4-yl)benzene and 1,3,5-tri(1H-imidazol-4-yl)benzene, which exhibit diverse coordination modes[13-15]. Further- more, we have constructed diverse frameworks with favorable gas adsorption or selective gas adsorption properties based on the metal-imidazolate units. Similarly, the O-donors of carboxylate groups have versatile coordination modes as well, and thus the designable ligands incorporating multi-N and carboxylate groups may be a useful strategy for the construction of novel MOFs[16, 17]. Therefore, our groups have furthermore designed difunctional organic linker combining carboxyl and 4-imidazolyl donors such as 4-(1H-imidazol-4-yl)benzoic acid which are employed as good candidates for the construction of MOFs in our previous study[18]. Taking favorable coordination ability of the multi-N and O-donor into account, we synthesized the ligand of 4-(1,2,4-triazol-4-yl)phenylacetic acid (HL) con- taining the triazolyl and carboxyl groups, which may exhibit rich coordination modes in the assembly of MOFs. Herein, we report the synthesis and crystal structure of a new coordination polymer [Cd2(L)2(Cl)2(H2O)]n(1) obtained by the reaction of HL and CdCl2·2.5H2O as an extension of our previous work.
2 EXPERIMENTAL
2. 1 Materials and measurements
All the commercially available chemicals and solvents were of reagent grade and used as received without further purification. Elemental analyses were performed on a Perkin-Elmer 240C Elemental Ana- lyzer. IR spectra were recorded on a Bruker Vector 22 FT-IR spectrophotometer using KBr pellets. Thermogravimetric analyses (TGA) were performed on a simultaneous SDT 2960 thermal analyzer under nitrogen at a heating rate of 10 ℃/min. Power X-ray diffraction (PXRD) patterns were measured on a Shimadzu XRD-6000 X-ray diffractometer with Cu(= 1.5418 Å) radiation at room temperature.
2. 2 Synthesis of complex[Cd2(L)2(Cl)2(H2O)]n (1)
A mixture of HL (0.020 g, 0.1 mmol), CdCl2·2.5H2O (0.022 g, 0.1 mmol) and 10 mL H2O was adjusted to pH = 7 with 0.5 mol·L-1solution. The mixture was then sealed into a 20 mL Teflon-lined stainless-steel container and heated at 160 ℃ for 48 h. Colorless block crystals of 1 were collected with a yield of 42% by filtration and washed with water and ethanol for several times. Anal. Calcd. (%) for C20H16Cl2N6O5Cd2: C, 33.54; H, 2.25; N, 11.74. Found (%): C, 33.33; H, 2.41; N, 11.61.IR(KBr): 3215 (w), 1591 (s), 1575 (vs), 1543 (m), 1529 (vs), 1395 (vs), 1243 (m), 1093 (s), 1037 (m), 1019 (m), 821 (m), 777 (s), 670 (w), 638 (w), 588 (w), 515 (w) cm-1.
2. 3 Crystal structure determination
The colorless crystals of complex 1 were selected for diffraction data collection at 296(2) K on a Bruker Smart Apex II CCD diffractometer equipped with a graphite-monochromatic Mo-radiation (= 0.71073 Å). A total of 14109 reflections were collected for 1,of which 5402 (int= 0.0574) were independent in the range of 1.79≤≤27.62º by using a-scan mode. The structure was solved by direct methods with SHELXS-97[19]program and refined by full-matrix least-squares techniques on2with SHELXL-97[20]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in their calculated positions and refined following the riding model. The final= 0.0458,= 0.0949 (= 1/[2(F2) + (0.0572)2+ 0.4516], where= (F2+ 2F2)/3),int= 0.0574, (Δ/)max= 0.000,= 1.051, (Δ)max= 1.230 and (Δ)min= –1.193 e/Å3for 1. The selected bond distances and bond angles for complex 1 are listed in Tables 1 and 2, respectively.
3 RESULTS AND DISCUSSION
3. 1 Crystal structure of 1
Single-crystal X-ray diffraction analysis revealed that complex 1 crystallizes in the monoclinic system with space group21/. The asymmetric unit of 1 contains two crystallographically independent Cd(II) atoms, two Cl−anions, two L−ligands and one coordinated water molecule. As exhibited in Fig. 1, the Cd(1) atom has octahedral coordination geometry with N2O3Cl binding set coordinated by two nitrogen (N(1), N(4)) atoms from two L−ligands, two oxygen atoms (O(1D), O(4B) and O(7)) from other two L−ligands, one oxygen (O(7))from coordinated water molecule and one Cl-anion, while Cd(2) is also octahedral coordination geometry with NO3Cl2coordination sphere. The Cd–O distances are in the range of 2.229(5)~2.420(4)(19) Å while the Cd–N distances are 2.316(5) 2.382(5) Å, and the coor- dination angles around Cd(II) are in the range of 73.27(6)~180.0º (Table 1). A notable feature of the structure of 1 is that the HL ligands exhibit two coordination modes existing together in one com- pound (Fig. 2): (i) one acts as a μ3-bridging linker, using one N atom to bridge one Cd(II) atom and two oxygen atoms from carboxyl group in the2-1:1- bridging mode to ligate two Cd(II) atoms; (ii) the other one adopts4-bridging modes, using two N atoms to bridge two Cd(II) atoms and the carboxyl group in3-2:1-bridging mode to chelate two Cd(II) atoms. Two adjacent Cd(II) ions are alternately linked together by one triazolate group, one carboxylate group, and one bridged Cl−ion with the distances of 3.994(7) and 4.056(7) Å to give a one-dimensional (1D) chain (Fig. 3).It is noteworthy that both of two different L−ligands connect Cd(II) atoms to form 1D chains respectively, and these two 1D chains intersect each other, forming a 1D helix chain structure (Fig. 4a). Similarly, there are two different Cl−anions, one acting as a terminal coordination atom to balance the positive charges while another employing as2-bridging to connect the Cd(II) atom. Because of the bridging interaction of trizaole and carboxyl group together with Cl−, the 1D chains are further connected into a two-dimensional (2D) layer structure (Fig. 4b). And the hydrogen bonding interactions (C(1)···Cl(2) 3.56Å, C(1)–H(1)···Cl(2) 148°;C(5)···N(5)a3.48Å, C(5)–H(5)···N(5) 160°; O(2)···O(7) 3.36 Å, O(2)–H(5)···O(7) 156°; C(8)···Cl(2) 3.55Å, C(8)–H(8)···Cl(2) 165°) exist in the 2D structure (Table 2), further reinforcing the stability of this complex. For the overall framework of 1, it can be seen clearly that the 2D layers repeat in the unit ···AAA··· stacking sequence along theaxis, and numerous intramolecular-and C–H···interactions exist among the aromatic rings, as shown in Fig. 5. The benzene rings of the L−ligands between the adjacent 2D layers are parallel and are separated by a centroid-centroid distance of 3.68 Å and C–H···distance of 2.96 Å[21, 22]. The non- classic weak-and C–H···interactions further link the 2D layers into a three-dimensional (3D) supramolecular polymer (Fig. 5).

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) of [Cd2(L)2(Cl)2(H2O)]n
Symmetry transformation: A:–1–,0.5+, 1.5–; B:1–, –0.5+, 1.5–;C:–, –0.5+, 1.5–; D:1+,,

Table 2. Hydrogen Bonding Interactions for Complex 1 (Å, °)
Symmetry code: (a) –, 0.5+, 1.5–

Fig. 1. Coordination environment of Mn(II) in complex 1 with ellipsoids drawn at 30% probability level.
Hydrogen atoms were omitted for clarity (Symmetry codes: (A) –1, –0.5+, 1.5–; (B) 1–, −0.5+, 1.5−; (C) –, −0.5+, 1.5−; (D) 1+,,)

Fig. 2. Coordination environment of 4-tba ligands in complex 1
Fig. 3. Cl−and carboxyl group-bridged 1D Cd(II) chains for 1
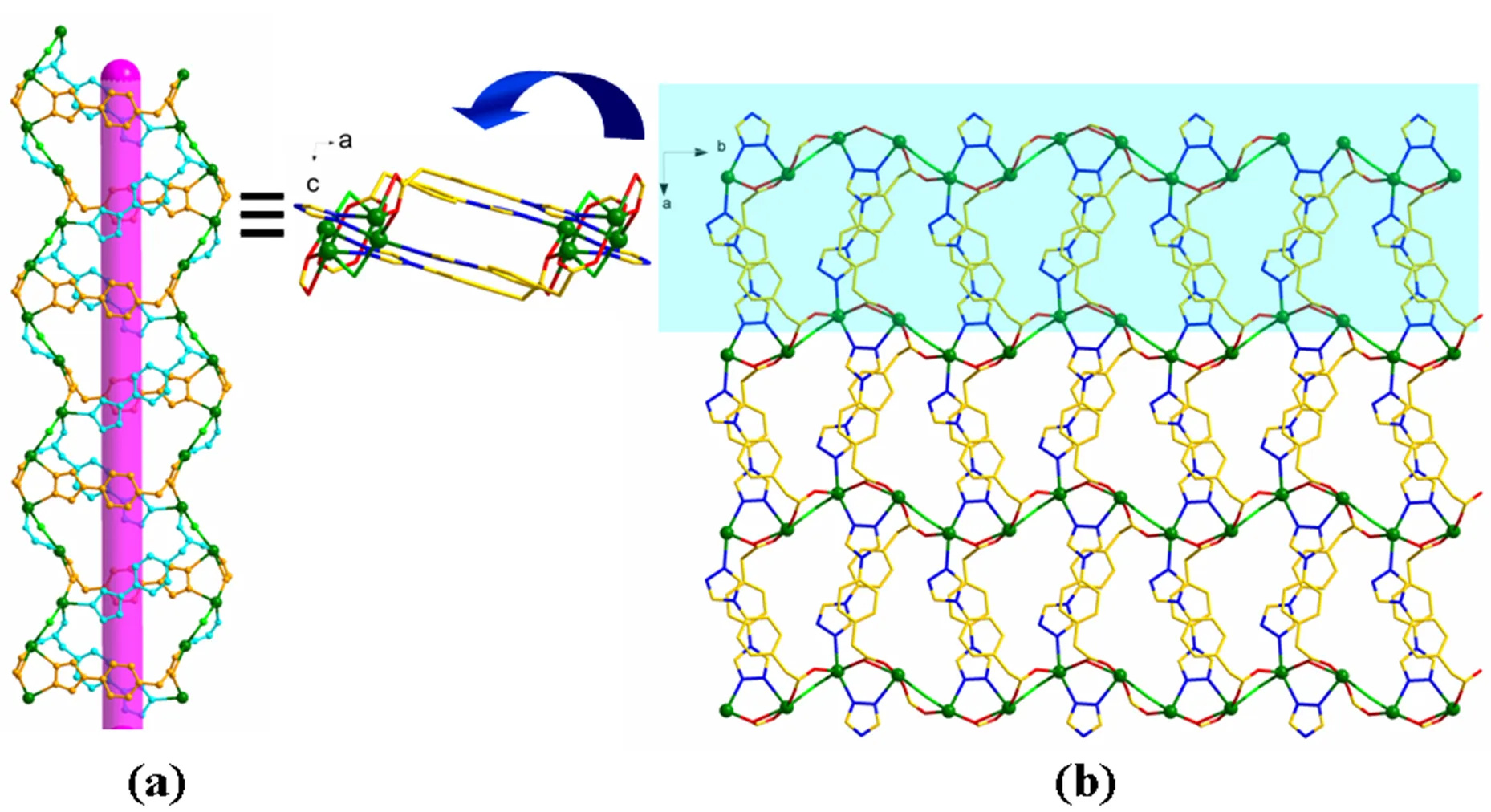
Fig. 4. 1D chain (a) and 2D network (b) of 1
Fig. 5. 3D supramolecular structure constructed from 2D stacking units in sequence of AAA along theaxis, linked by···and C-H···stacking interactions
3. 2 IR spectrum, thermal stabilities and powder X-ray diffraction of 1
The infrared spectrum of the complex has been recorded between 4000 and 450 cm-1and some important assignments are shown in the experimental section (Fig. 6). Strong characteristic bands of car- boxylic group are observed in the range of 1590~1529 cm–1for asymmetric vibrations. The carboxylic acid of HL is deprotonated to form L-ligand, and the strong absorption band around 1590 cm-1is attributed to the asymmetric stretching vibration of C=O group, which is significantly smaller than that of the protonated carboxylate group monomer (1695 cm-1), indicating the delocalization of C=O double bond[23]. Complex 1 was subjected to thermogravi- metric analysis (TGA) to ascertain the stability of supramolecular architecture, and the result is shown in Fig. 7.A total weight loss of 2.36% was observed for 1around 90 ℃, which is attributed to the loss of coordinated water molecule (calcd. 2.51%), and the residue is stable up to about 380 ℃.Powder XRD experiment was carried out to confirm the phase purity of bulk sample (Fig. 8), and the experimental pattern of the as-synthesized sample is comparable to the corresponding simulated one, indicating the phase purity of the sample.

Fig. 6. IR spectra of HL and complex 1
Fig. 7. TG curves of complex1

Fig. 8. Simulated and experimenta XRPD patterns of complex 1l
Fig. 9. Solid-state photoluminescent spectra of HL and complex 1 at room temperature
3. 3 Photoluminescent property
It is known that coordination polymers with10closed-shell metal center with the rational selection and design of conjugated organic spacers may exhibit excellent fluorescence properties and serve as good candidates for potential applications as photoactive materials[24, 25]. We investigate the solid-state fluorescence property of complex 1 as well as the HL1ligand at room temperature. The free HL1ligand shows intense emission band at 440 nm upon excitation at 365 nm, which may be attributed to*→transition of the intraligands[26, 27], while complex 1 exhibits light blue emission with maximum at 433 nm upon excitation at 355nm as depicted in Fig.9. By contrast with the free ligand, the emission bands ofcomplex1are 7 nm blue-shifted.Such board emission bands may be tentatively assigned toligand-to-metal charge transfer (LMCT)[28, 29].In addition,Fig. 9 shows that the luminescence intensity of 1 has increased compared with the free ligand underthe same conditions, which may mainly originate from thecoordination interactions between the metal Cd(II) atom andthe ligand, thus enhancing its conformational rigidity and thendecreasing the non-radiative energy loss[30].Therefore, complex 1 may appear to be potential hybrid inorganic-organic photoactive material.
(1) Zhao,T.; Heering, C.; Boldog, I.; Domasevitch, K. V.; Janiak, C. A view on systematic truncation of tetrahedral ligands for coordination polymers.. 2017, 19, 776–780.
(2) Ahmad, N.; Younus, H. A.; Chughtaia, A. H.; Verpoort, F. Metal-organic molecular cages: applications of biochemical implications.. 2015, 44, 9–25.
(3) Yuan, S.; Qin, J. S.; Zou, L.; Chen, Y. P.; Wang,X.; Zhang, Q.; Zhou, H. C. Thermodynamically guided synthesis of mixed-linker Zr-MOFs with enhanced tenability.. 2016, 138, 6636−6642.
(4) Xiao, J.; Oktawiec,J.; Milner, P. J.; Long, J. R. Pore environment effects on catalytic cyclohexane oxidation in expanded Fe2(dobdc) analogues Dianne.. 2016, 138, 14371−14379.
(5) Xiao, L.; Li, W. D.; Fang, X.; Jiang, L. Y.; Chen, S. S. Two three-dimensional supramolecular polymer built from mixed N-donor and carboxylate ligands.. 2016, 35, 781–788.
(6) Zhang, W. Q.; Zhang, W. Y.; Wang, R. D.; Ren, C. Y.; Li, Q. Q.; Fan, Y. P.; Liu, B.; Liu, P.; Wang, Y. Y. Effect of coordinated solvent molecules on metal coordination sphere and solvent-induced transformations..2017, 17, 517–526.
(7) Yao, P. F.; Liu, H. F.; Huang, F. P.; Feng, F. L.; Qin, X. H.; Huang, M. L.; Yu, Q.; Bian, H. D. A family of Zn(II)/Cd(II) halide systems incorporating 5,5΄-di(pyridin-2-yl)-3,3΄-bi(1,2,4-triazole).2016, 18, 938–947.
(8) Qiao, R.; Chen, S. S.; Sheng, L. Q.; Yang, S.; Li, W. D. Syntheses, crystal structures, and properties of four complexes based on polycarboxylate and imidazole ligands.. 2015, 228, 199–207.
(9) Zhu, M. A.; Guo, X. Z.; Chen, S. S. Synthesis, crystal structure and luminescent property of a Zn(II) complex based on 4-imidazole-carboxylate ligand,2017, 36, 1348─1354.
(10) Li, W. D.; Fang, X.; Qiao, R.; Chen, S. S. Non-covalent bonded 3D supramolecular architectures based on acid-base adducts.2016, 35, 46–54.
(11) Zhang, Z. Y.; Xiao, L.; Chen, S. S.; Qiao, R.; Yang, S. A novel Zn(II) complex with 4-connected umc topology: synthesis, crystal structure and luminescent property.2017, 36, 819─824.
(12) Wang, D.; Sun, L.; Hao, C.; Yan, Y.; Liang, Z. Lanthanide metal-organic frameworks based on a 1,2,3-triazole-containing tricarboxylic acid ligand for luminescence sensing of metal ions and nitroaromatic compounds..2016, 6, 57828–57834.
(13) Chen, S. S.; Chen, M.; Takamizawa, S.; Chen, M. S.; Su, Z.; Sun, W. Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] (H2L = 1,4-di(1H-imidazol-4-yl)benzene).. 2011, 47, 752–754.
(14) Chen, S. S.; Qiao, R.; Sheng, L. Q.; Zhao, Y.; Yang, S.; Chen, M. M.; Liu, Z. D.; Wang, D. H. Cadmium(II) and zinc(II) complexes with rigid 1-(1H-imidazol-4-yl)-3-(4H-tetrazol-5-yl)benzene and varied carboxylate ligands.. 2013, 15, 5713–5725.
(15) Chen, S. S.; Wang, P.; Takamizawa, S.; Okamura, T. A.; Chen, M.; Sun, W. Y. Zinc(II) and cadmium(II) metal-organic frameworks with 4-imidazole containing tripodal ligand: sorption and anion exchange properties.. 2014, 43, 6012–6020.
(16) Shao, Y. L.; Cui, Y. H.; Gu, J. Z.; Wu, J.; Wang, Y. W. Kirillov, A. M. Exploring biphenyl-2,4,4΄-tricarboxylic acid as a flexible building block for the hydrothermal selfassembly of diverse metal–organic and supramolecular networks.2016, 18, 765–778.
(17) Pachfule, P.; Garai, B.; Banerjee, R. Functionalization and isoreticulation in a series of metal-organic frameworks derived from pyridinecarboxylates.. 2016, 55, 7200−7205.
(18) Chen, S. S.; Liu, Q.; Zhao, Y.; Qiao, R.; Sheng, L. Q.; Liu, Z. D.; Yang, S.; Song, C. F.New metal-organic frameworks constructed from the 4-imidazole-carboxylate ligand: structural diversities, luminescence, and gasadsorption properties.. 2014, 14, 3727−3741.
(19) Sheldrick, G. M.. University of Göttingen 1997.
(20) Sheldrick, G. M.. University of Göttingen 1997.
(21) Ye, R. P.; Zhang, X.; Zhai, J. Q.; Qin, Y. Y.; Zhang, L.; Yao, Y. G.; Zhang, J. N-donor ligands enhancing luminescenceproperties of seven Zn/CdIJII) MOFs based on alarge rigid π-conjugated carboxylate ligand.2015, 17, 9155–9166.
(22) Surov, A. O.; Solanko, K. A.; Bond, A. D.;Brandl, A. B.; Perlovich, G. L. Cocrystals of the antiandrogenic drug bicalutamide: screening, crystal structures, formation thermodynamics and lattice energies.2016, 18, 4818–4829.
(23) Nakamoto, K.. 5th ed.,Wiley&Sons, New York 1997.
(24) Wang,D. Z.; Fan, J. Z.; Jia, D.; Du, C. C. Zinc and cadmium complexes based on bis-(1Htetrazol-5-ylmethyl/ylethyl)-amine ligands: structures and photoluminescence properties.2016, 18, 6708-6723.
(25) Xing, K.; Fan, R.; Gao, S.; Wang, X.; Du, X.; Wang, P.; Fang, R.; Yang, Y. Controllable synthesis of Zn/Cd(II) coordination polymers: dual-emissive luminescent properties, and tailoring emission tendency under varying excitation energies..2016, 45, 4863–4878.
(26) Sun, Y. X.; Sun, W. Y. Zinc(II)- and cadmium(II)-organic frameworks with 1-imidazole-containing and 1-imidazolecarboxylate ligands.2015, 17, 4045–4063.
(27) Singh,D.; Nagaraja, C. M.Auxiliary ligand-assisted structural variation of Cd(II) metal-organic frameworks showing 2D → 3D polycatenation andinterpenetration: synthesis, structure, luminescence properties, andselective sensing of trinitrophenol.. 2015, 15, 3356−3365.
(28) Yang,L. B.; Wang, H. C.; Fang, X. D.; Chen, S. J.; Xu, Q. Q.; Zhu, A. X.; Yang, Z. A series of Zn(II) and Cd(II) coordination compounds based on 4-(4H-1,2,4-triazol-4-yl)benzoic acid: synthesis, structure and photoluminescence properties.2016, 18, 130–142.
(29) Xue, Z.; Sheng, T.; Wang, Y.; Hu, S.; Wen,Y.; Wang, Y.; Li, H.; Fu, R.; Wu, X. A series of10coordination polymers constructed with a rigid tripodal imidazole ligand and varied polycarboxylates: syntheses, structures and luminescence properties.2015, 17, 2004–2012.
(30) Xu, J.; Bai, Z. S.; Chen, M. S.; Su, Z.; Chen, S. S.; Sun, W. Y. Metal-organic frameworks with six- and four-fold interpenetration and their photoluminescence and adsorption property.. 2009, 11, 2728–2733.
19 June 2017;
16 October 2017 (CCDC 1556044)
① This work was supported by the Natural Science Foundation of Colleges of Anhui
Province (KJ2017ZD29) and National Undergraduates Innovation Project (201510371010)
. Chen Shui-Sheng, doctor, majoring in coordination chemistry. E-mail: sscfync@163.com
10.14102/j.cnki.0254-5861.2011-1756
- 结构化学的其它文章
- Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

