Synthesis, Crystal Structure and Fluorescent Properties of Silver and Cadmium Complexes①
WANG Qing-Wei LIU Bo SUI Wei
Synthesis, Crystal Structure and Fluorescent Properties of Silver and Cadmium Complexes①
WANG Qing-Wei②LIU Bo SUI Wei
(()136000)
Two new complexes [Ag(bix)]n·nNAA·nH2O (1) and [Cd(NAA)(phen)2(H2O)]2·2CH3COO-·H2O (2)(bix = 1,4-bis(imidazol-1-ylmethyl)benzene, HNAA =-naphthylacetic acid, phen = 1,10-phenanthroline) have been successfully synthesized under hydrothermal conditions. Their structures have been determined by elemental analyses, IR spectroscopy, TG and single-crystal X-ray diffraction analysis. The intermolecular hydrogen bonding orstacking interactions extend the complexesinto a 3D supramolecular structure. Moreover, the luminescent properties of complex 2 have been investigated in the solid state.
hydrothermalsynthesis, crystal structure, Ag(I) complex, Cd(II) complex;
1 INTRODUCTION
Metallosupramolecular species assembled from transition metals and organic bridging ligands with novel structures and properties have been rapidly developed because of their intriguing structural diversity and potential applications as functional materials[1-5]. However, the rational design of new materials for special applications is still at an early evolutionary stage with the current focus mainly on understanding the factors to determine the crystal packing. During the last few years, several types of forces, such as coordination bonding[6-8], hydrogen bonding[9-11],stacking[12,13]and electrostatic interactions[14],have been well used in constructing extended supramolecular networks. Up to now, the most important driving forces in crystal engineering are coordination, hydrogen-bonding andstacking interactions, and many networksassembled from mono- or polynuclear metal complexes via hydrogen bonding andstackinginteractions have been reported recently[15]. The uniquestrength, direc- tionality and complementary of such non-covalent interactions play key roles in the construction of various architectures for molecular self-assembly and recognition[16, 17].
Considering all the aspects stated above, here we report the syntheses and crystal structures of two coordination supramolecules, [Ag(bix)]n·nNAA·nH2O (1) and [Cd(NAA)(phen)2(H2O)]2·2CH3COO-·H2O (2). In the solid state, complexes1and 2 both form three-dimensional (3D) networks resulted from intermolecular hydrogen-bonding orstacking interactions.
2 EXPERIMENTAL
2. 1 Materials and instruments
All the chemicals for synthesiswere commercially purchased and used without further purification. Elemental analyses of C, H and N were performed on an Elementar Vario III Elemental Analyzer. IR spectrum was recorded in the range of 4000~400 cm-1on a Nicolet 6700 spectrometer using a KBr pellet. Thermal stability (TG-DTA) studies were carried out on a Dupont thermal analyzer from room temperature to 800 ℃.Powder X-ray diffraction (PXRD) patterns were collected in the 2range of 5~50º with a scan speed of 0.1 º·S-1on a Bruker D8 Advance instrument using a Curadiation (= 1.54056 Å) at room temperature. The fluorescent studies were carried out on a computer-controlled JY Fluoro-Max-3 spectrometer at room temperature.
2. 2 Synthesis
[Ag(bix)]n·nNAA·nH2O (1) Complex 1 was pre- pared by the reaction of Ag(OAc) with-naphthyl- acetic acid and 1,4-bis(imidazol-1-ylmethyl)benzene in an equimolar ratio in the mixed solvents of CH3OH and H2O. Suitable amount of NaOH was added to this solution to adjust the pH value to 7 and it was stirred at room temperature for 0.5 h until a homogeneous solution was obtained. Then it was sealed in an acid digestion bomb (30 mL) at 140 ℃ for 5 days. After the reaction system was slowly cooled to room temperature, colorless block crystals were obtained by filtration and dried in air. The yield of the target complex is ca. 21% (based on Ag salt). Anal. Calcd. for C26H23AgN4O3: C, 57.05; H, 4.24; N, 10.24%. Found: C, 56.93; H, 4.05; N, 9.97%. IR (KBr pellet, cm-1) spectra: 3385w, 3103w, 1565s, 1512w, 1379s, 1230w, 1074w, 792w, 710w, 650w, 539w.
[Cd(NAA)(phen)2(H2O)]2·2CH3COO-·H2O (2)
Complex 2 was prepared from a mixture of Cd(OAc)2·2H2O (0.026 g, 0.1 mmol), HNNA (0.019 g, 0.1 mmol), phen (0.04 g, 0.2 mmol) and H2O (18 mL) in a 30 mL Teflon-lined stainless steel vessel, and then the vessel was sealed and heated at 130 ℃ for 5 days. After the reaction mixture was slowly cooled down to room temperature at the rate of 5 ℃/h, colorless block crystals of complex 2 were obtained. Yield: 27% (based on Cd salt). Anal. Calcd. (%) for C76H52Cd2N8O11: C, 61.76; H, 3.55; N, 7.58. Found (%): C, 61.08; H, 3.07; N, 7.01. IR (cm–1): 3058w, 1584s, 1570w, 1378s, 1324w, 1144w, 848w, 722m, 632w.
2. 3 X-ray crystallography
All diffraction data of complexes1and 2were collectedon a Bruker/Siemens Smart Apex II CCD diffractometer with graphite-monochromated Moradiation (= 0.71073 Å) at 293(2) K. Data reduc- tions and absorption corrections were performed using the SAINT and SADABS programs, respec- tively. The structures were solved by direct methods, and all of the non-hydrogen atoms were refined anisotropically on2by full-matrix least-squares techniques using the SHELXL-97 crystallographic software package[18]. All the hydrogen atoms were generated geometrically and refined isotropically using the riding model. For 1, a total of 4557 reflections were collected in the range of 2.03≤≤26.03°, of which 3601 were independent (int= 0.0194). The final= 0.0455 and= 0.1137 for observed reflections with> 2(), and= 0.0587 and= 0.1217 for all data with (Δ)max= 0.900 and (Δ)min= –0.802 e·Å-3. For 2, a total of 12668 reflections were collected in the range of 1.07≤≤26.15°, of which 7090 were independent (int= 0.0710). The final= 0.0551 and= 0.1112 for observed reflections with> 2(), and= 0.0950 and= 0.1220 for all data with (Δ)max= 0.789 and (Δ)min= –0.396 e·Å-3.Selected bond lengths and bond angles of complexes1and 2are shown in Table 1.
3 RESULTS AND DISCUSSION
3. 1 Structural description
Single-crystal X-ray diffraction analysis indicates that complex 1 crystallizes in21/space group and comprises of a one-dimensional chain-like structure. There are one Ag(I) ion, one bix ligand, one HNAA ligand and one crystal water molecule in the asymmetric unit (Fig. 1). Please note, the HNAA ligand does not coordinate with the Ag(I) ion, and it’s just a balanced charge. The Ag(1) ion is coordinated by two nitrogen atoms from two bix ligands (Ag(1)– N(1) = 2.127(3), Ag(1)–N(4A) = 2.136(3) Å). The coordination angle round the Ag ion is 168.51(12)°.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1 and 2
Symmetry transformations used to generate the equivalent atoms: 1: A:–1,,+1

Fig. 1. View of the asymmetric unit of complex 1. All hydrogen atoms are omitted for clarity
In the crystal structure of complex 1, the bix ligands adopt a-conformation bridging mode with a dihedral angle between two imidazole rings of 24.07º and link the Ag(I) ions to form a one- dimensionalchain-like structure with the Ag···Ag distance of 14.301 Å. The Ag(1) ion shows a slightly distorted linear coordination construction. It is worthwhile to note that free-naphthylacetic acid and neighboring bix ligand contribute to the formation of the chain by C–H···O and O–H···O hydrogen bonding interactions (Table 2). In addition, the one-dimensional trapezoidal banded structure is formed between the adjacent one-dimensional chains and-naphthylacetic acid through C–H···O and O–H···O hydrogen bonding interactions. It is noteworthy that there exist important C–H···inter- actions between C(4) and benzene ring C(5)~C(9) (symmetry code: 1–, 1–, –), C(11) and benzene ring C(17)~C(22) (symmetry code: 1/2+, 1/2–, –1/2+), C(13) and benzene ring C(21)~C(26) (symmetry code: 1/2+, 1/2–, –1/2+), C(24) and benzene ring C(5)~C(9) (symmetry code : 1/2+, 1/2–, 1/2+), from symmetry-related bix and NAA ligands with the C–H···distances of 3.377(4), 3.829(5), 3.825(5), 3.580(10) Å, respectively. In this mode, the two-dimensional layered structure comes into being (Fig. 2).

Fig. 2. View of the two-dimensional layered structure by C–H···interactions in complex 1
A single-crystal X-ray diffraction study reveals that complex 2 crystallizes in monoclinic21/space group. The coordination environment of Cd(II) in 2 is shown in Fig. 3. There are two coordination centers, Cd(1) and Cd(2), in the crystal with the same coordination modes, four phen ligands, two HNAA ligands, two coordinated water molecules, one crystal water molecule and two free acetates. The Cd(1) ion is six-coordinated by four nitrogen atoms from two different phen ligands (Cd(1)–N(1) = 2.429(4), Cd(1)–N(2) = 2.371(4), Cd(1)–N(3) =2.381(4), Cd(1)–N(4) = 2.385(4) Å), one carboxylate oxygen atom (Cd(1)–O(1) = 2.183(4) Å) from the HNAA ligand and one coordinated water molecule (Cd(1)–O(2W) = 2.291(3) Å) to furnish a distorted octahedral coordination architecture. The coordina- tion angles around the Cd atom are in the range of 69.07(15)~161.00(15)º. In the coordination environ- ment, one carboxylate oxygen atom (O(1)) and three nitrogen atoms (N(2), N(3) and N(4)) are located in the basal plane, whereas the other nitrogen atom (N(1)) and coordinated water molecule (O(2W)) occupy the axial positions from the opposite direc- tions. It is noteworthy that the acetate does not coordinate with Cd(I) ion, and it’s just a balanced charge.
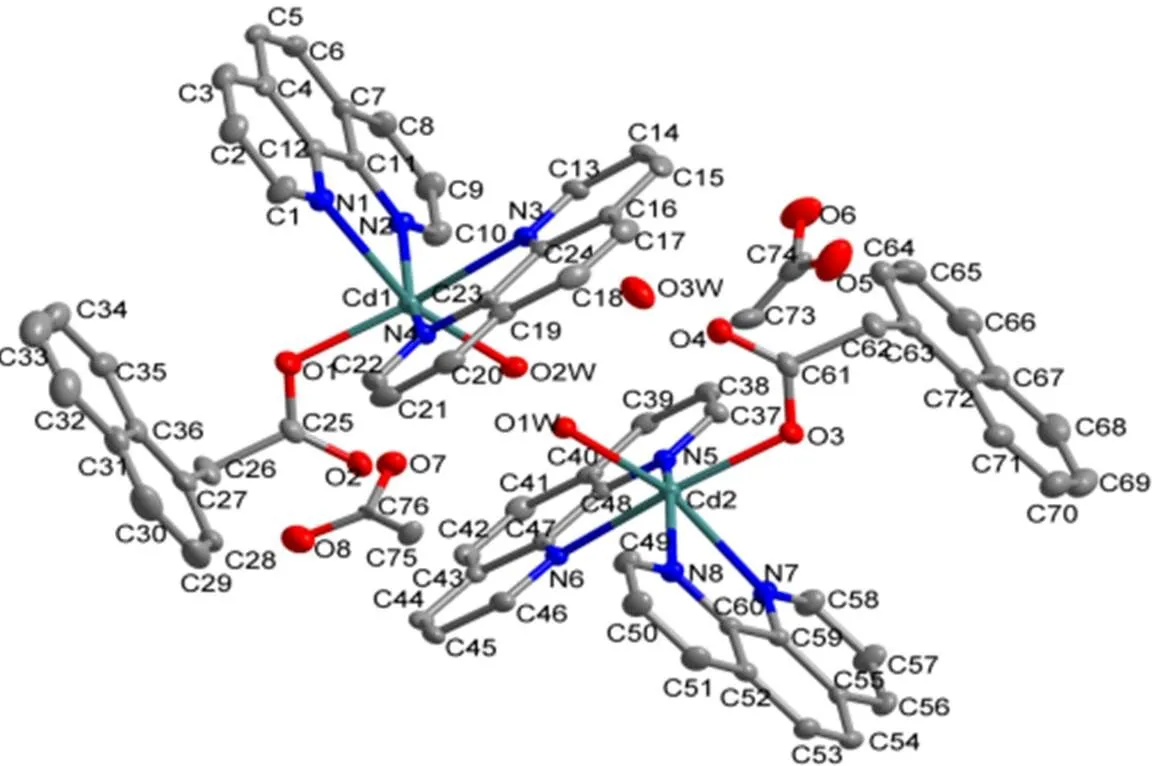
Fig. 3. View of the asymmetric unit of complex 2. All hydrogen atoms are omitted for clarity
In the crystal structure of complex 2, phen ligands adopt a classic bidentate chelating mode, and HNAA ligand displays one kind of coordination modes, namely a monodentate bridging mode. In the structure unit, the up and down independent structure is connected to a stable structure through O–H···O, C–H···O hydrogen bonding (Table 2)andstacking (Table 3) between pyridine ring of phen ligands and benzene ring of phen and NAA ligands. Moreover, each structure unit is linked into a double-chain by O–H···O and C–H···O hydrogen bonding interactions. Moreover, the two-dimensional layer architecture is formed between the double- chain by means of O–H···O, C–H···O hydrogen bonding andstacking interactions, which are connected with free acetate to form a three- dimensional supramolecular structure by O–H···O, C–H···O hydrogen bonding (Fig. 4).
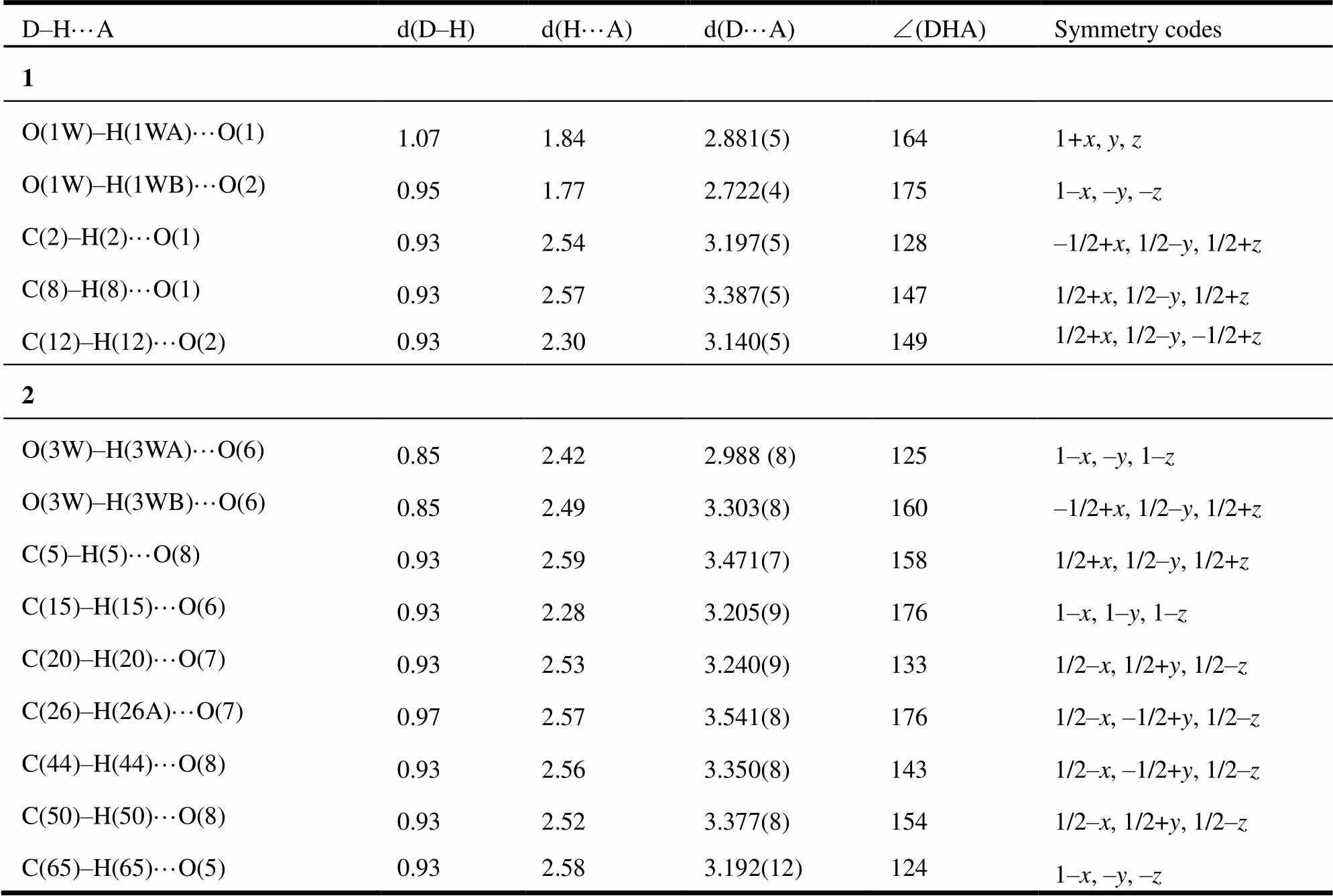
Table 2. Hydrogen Bonds for Complexes 1 and 2

Table 3. Parameters between the Planes in 2
Symmetry codes: i = –, –1–, –; ii = 1–, 1–, –

Fig. 4. View of the 3D supramolecularstructure of 2
3. 2 IR analysis of complex 1
IR spectrum of 1 shows a broad absorption band at 3385 cm-1corresponding to the H···O stretching of crystal water molecules in the complex. Asymmetric and symmetric COO–stretching modes of the lattice NAA-anion were evidenced by very strong, slightly broadened bands at 1565 and 1379 cm-1[19], which is consistent with the results of X-ray analysis.
IR spectrum of 2 shows a broad absorption band at 3058 cm-1corresponding to the H···O stretching of water molecule in the complex. Asymmetric and symmetric COO–stretching modes of the lattice NAA-anion were evidenced by very strong, slightly broadened bands at 1584 and 1378 cm-1[19], which is consistent with the results of X-ray analysis.
3. 3 Thermal stability and powder X-ray diffraction (PXRD)
To confirm the phase purity of complexes 1 and 2, powder X-ray diffraction (PXRD) patterns were recorded for 1 and 2, and it was comparable to the corresponding simulated patterns calculated from the single-crystal diffraction data (Fig. 5), indicating a pure phase of bulky sample.

Fig. 5. PXRD analysis of the title complex 1: bottom-simulated, top-experimental
In order to better understand the thermal stability of complexes 1 and 2, its thermal decomposition behaviors were investigated at 50~800 ℃ under nitrogen atmosphere (Fig. 6). The TG curve of 1 indicates a weight loss of 3.10% from 50 to 70 ℃ corresponding to the departure of crystal water molecule (calcd. 3.28%). The TG curve presents a platform and the framework starts to decompose at 170 ℃. The TG curve of 2 represents a weight loss of 10.00% from 50 to 252 ℃ due to the departure of water molecule and acetate (calcd. 10.65%). The TG curve presents a platform and the framework starts to decompose at 255 ℃.
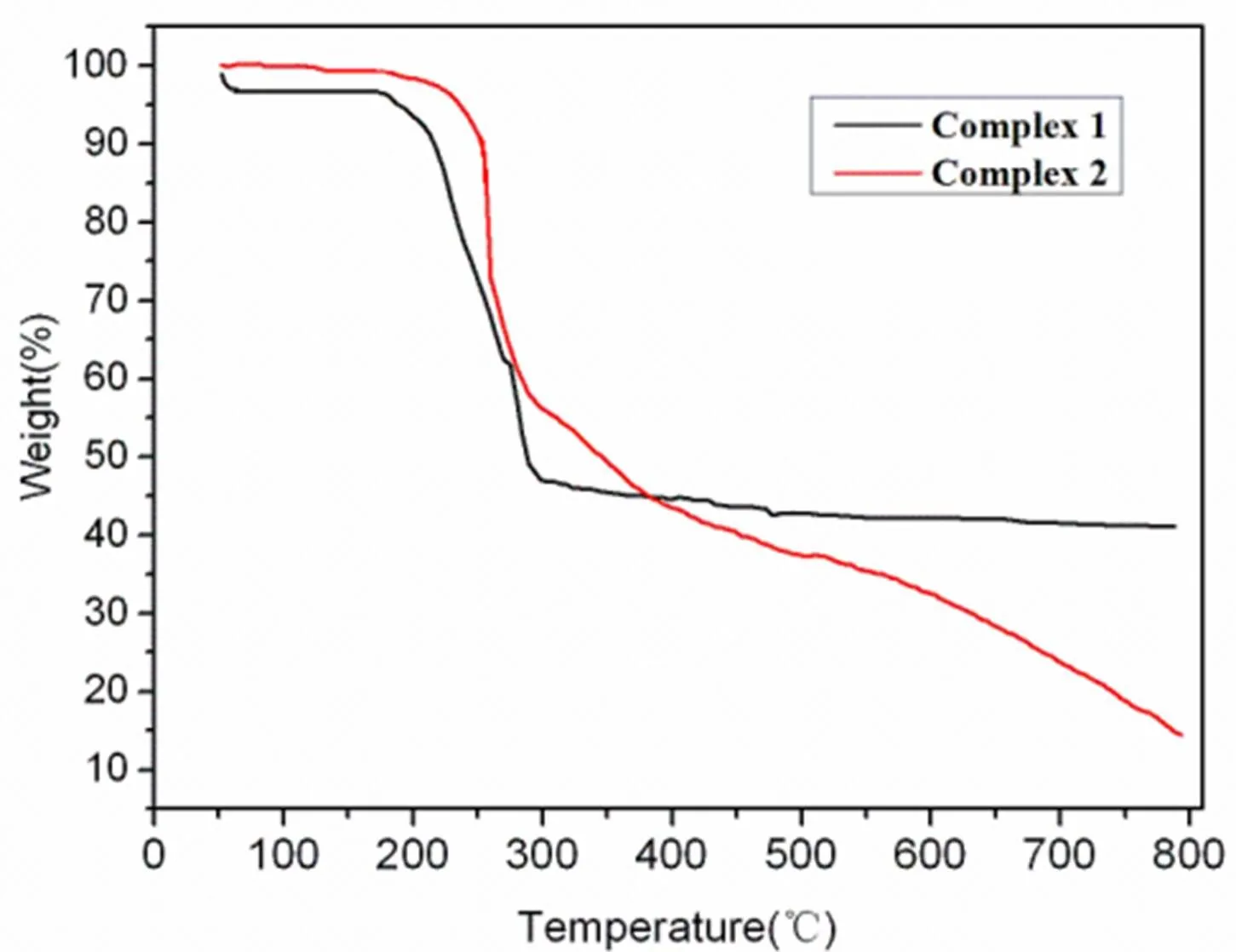
Fig. 6. TG curves of complexes 1 and 2
3. 4 Photoluminescent properties
The emission spectrum of complex 2 in the solid state at room temperature is exhibited in Fig. 7. It can be observed that 2 exhibits blue photoluminescence with an emission maximum at465 nm upon excitation at 325 nm. In order to realize the nature of these emission bands, we first studied the photolu- minescence properties of free HNAA (em= 275 nm) ligand, and proved that it does not emit any lumine- scence in the range of 400~800 nm. And then we researched the emission spectrum of phen (em= 285 nm) itself and the result revealed that it does not emit any luminescence in the range of 400~800 nm, which has also been proved previously. Thus, on the basis of the earlier literature[20], the emission band could be assigned to the emission of ligand-to-metal charge transfer (LMCT).

Fig. 7. Solid-state emission spectrum of 2 at room temperature
(1) Moulton, B.; Zaworotko, M. J.From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids.. 2001,101, 1629–1658.
(2) Tabares, L.C.; Navarro,J.A.R.; Salas,J.M.Cooperative guest inclusion by a zeolite analogue coordination polymer. Sorption behavior with gases and amine and group 1 metal salts.. 2001, 123, 383–387.
(3) Moulton, B.; Zaworotko, M. J.From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids.. 2001,101, 1629–1658.
(4) Rao, C. N. R.; Natarajan, S.; Vaidhyanathan, R.Metal carboxylates with open architectures.2004, 43, 1466–1496.
(5) Kitagawa, S.; Kitaura, R.; Noro, S. I.Functional porous coordination polymers.2004, 43, 2334–2375.
(6) Kreno, L. E.; Leong, K.; Farha, O. K.; Allendorf, M.; Van Duyne, R. P.; Hupp, J. T. Metal-organic framework materials as chemical sensors.2012, 112, 1105–1125.
(7) Fujita, M.; Oka,H.; Yamaguchi,K.; Ogura, K.Self-assembly of ten molecules into nanometre-sized organic host frameworks.1995,378, 469–471.
(8) Power, K.N.; Hennigar,T.L.; Zaworotko,M.J.X-ray crystal structure of {Cu[1,2-bis(4-pyridyl)ethane]2(NO3)2}n: the first example of a coordination polymer that exhibits the NbO 3D network architecture..1998, 595–596.
(9) Kuduva, S.S.; Craig, D.C.; Nangia,A.; Desiraju,G.R. Cubanecarboxylic acids. Crystal engineering considerations and the role of C−H···O hydrogen bonds in determining O−H···O networks.. 1999, 121, 1936–1944.
(10) Grayson, M. N.;Yang, Z. Y.; Houk, K. N.Chronology of CH···O hydrogen bonding from molecular dynamics studies of the phosphoric acid-catalyzed allylboration of benzaldehyde.2017, 139, 7717–7720.
(11) Jiang, D. Y.; Sui, W.; Li, X. M.; Liu, B.; Wang, Q. W.; Pan, Y. R. Synthesis, crystal structure and theoretical calculations of a zinc(II) coordination polymer assembled by pyrazine-2,3-dicarboxylicacid and bis(imidazol) ligands.2016, 35, 505–513.
(12) Perkins, C. G.; Warren, J. E.; Fateeva, A.; Stylianou, K. C.; Mclennan, A.; Jelfs, K.; Bradshaw, D.; Rosseinsky, M. J. A porous layered metal-organic framework from-stacking of layers based on a Co6building unit.2012, 157, 24–32.
(13) Tse, M.C.; Cheung, K.K.; Chan, M.C.W.; Che, C. M.Phosphinocarboxylic acids as building blocks in organometallic crystal engineering. Self-organisation of one-dimensional photoluminescent cyclometallated platinum(II) polymeric structures.. 1998,2295–2296.
(14) Reddy, D.S.; Panneerselvam,K.; Pilati, T.; Desiraju,G.R.Molecular tapes based on C≡N···Cl interactions.1993, 661–662.
(15) Pan, Y. R.; Li, X. M.; Ji, J. Y.; Wang, Q. W. Synthesis, crystal structure, and theoretical calculationsof two cobalt, nickel coordination polymers with5-nitroisophthalic acid and bis(imidazol) ligands.2016, 69, 1296–1304.
(16) Lehn, J.M.. VCH, Weinheim1995.
(17) Whitesides,G.M.; Mathias, J.P.; Seto, C.T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures.1991, 254, 1312–1319.
(18) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(19) Devereux, M.; Shea, D. O.; Kellett, A.; McCann, M.; Walsh, M.; Egan, D.; Deegan, C.; Kędziora, K.; Rosair, G.; Müller-Bunz, H. Synthesis, X-ray crystal structures and biomimetic and anticancer activities of novel copper(II) benzoate complexes incorporating 2-(4΄-thiazolyl)benzimidazole (thiabendazole), 2-(2-pyridyl)benzimidazole and 1,10-phenanthroline as chelating nitrogen donor ligands.. 2007, 101, 881–892.
(20) Zheng,S. L.; Chen, X. M. Recent advances in luminescent monomeric, multinuclear, and polymeric Zn(II) and Cd(II) coordination complexes..2004, 57, 703–712.
19 June 2017,
20 September 2017 (CCDC 1556725 (1) and 1556726 (2))
① The project was supported by the Science and Technology Development Project of Jilin Provincial Science & Technology
Department (201205080) and the Science and Technology Research Projects of the Education Office of Jilin Province (No. 2013. 384)
. Wang Qing-Wei, born in 1961. Tel: 0434-3291973, E-mail: wqw611223@163.com
10.14102/j.cnki.0254-5861.2011-1758
- 结构化学的其它文章
- Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

