Construction of a Pillared-layer Framework Based on Charge Balance: CO2 Adsorption and Luminescence①
FU Hong-Ru ZHULin WANG Kai-Lu WANG Hui-Fang HAN Min-Le
Construction of a Pillared-layer Framework Based on Charge Balance: CO2Adsorption and Luminescence①
FU Hong-Ru ZHULin WANG Kai-Lu WANG Hui-Fang HAN Min-Le②
(471934)
Via the strategy of charge balance,one new pillared layered metal-organic frame- work [Zn2(TNB)(2-nim)] (H3TNB = 4,4΄,4΄΄-nitrilotribenzoicacid, 2-nim = 2-nitroimidazole)(1) was successfully synthesized based on mixed ligands, where binuclear [Zn2(CO2)3]+cluster-based cationic layers [Zn2(TNB)]+are connected by deprotonated2-nitroimidazole. Meanwhile, CO2adsorption bahaviors and luminescent property of compound 1 were investigated.
pillared-layer, metal-organic frameworks, charge balance,CO2adsorption, luminescence;
1 INTRODUCTION
In recent years, porous metal-organic frameworks (MOFs) have been emerging as a class of crystalline materials, which are extensively attractive for poten- tial applications in gas storage/separation[1-3]. An increasingly inviting area in which MOFs have shown excellent potential is adsorption and selective capture of CO2[4-6]. For MOFs, structure predictabi- lity, an important requirement for the synthesis of functional materials with tailored properties, has been recently proved to be an fairly efficient strategy for the design of MOFs[7, 8]. Among the methods to construct this kind of porous MOFs, the pillared- layer architecture is an attractive approach to tune the properties of these materials. Because the functiona- lization of pillar-linker not only enhances the modifi- cation of physicochemical properties of the frame- work without a network rearrangement and maintains the topology, but also generates the important effect on the selective adsorption of guests[9-12].Commonly,according to the charge balance,thelinear dicar- boxylic ligands and the linear bis(N-containing heterocyclic ring) ligands, such as 4,4΄-bipyridine, divinyltriamine and so on, were selected to assemble the pillared-layer frameworks[13-15].
Here, one pillared-layer MOF [Zn2(TNB)(2- nim)]·DMF·2H2O (1, H3TNB = 4,4΄,4΄΄-nitrilotri- benzoicacid, 2-nim = 2-nitroimidazole) was desig- ned and synthesized via the method of charge balance and mixd ligands. The rigid tritopic aroma- tic polycarboxylate ligands coordinate binuclear zinc ions to form a cationic layer [Zn2(TNB)]+with special tritopic paddle-wheel zinc clusters, then the layers are further connected to a 3neutral pillared-layer framework by 2-nitroimidazole pillar. Meanwhile, CO2adsorption bahaviors and lumine- scent property of compound 1 were investigated.
2 EXPERIMENTAL
2. 1 Materials and physical measurements
All commercially available solvents and chemicals were of analytical grade. Elemental analyses for car- bon, hydrogen, and nitrogen atoms were performed on a Vario EL III elemental analyzer (Elementar, Germany). The crystal was determined on a Bruker SMART APEX II CCD diffractometer (Madison, WI, USA) equipped with a graphite-monochromatized Moradiation (= 0.71073 Å). Gas adsorption was measured using an ASAP 2020 instrument (Micro- meritics, USA). Powder X-ray diffraction(PXRD) pattern was recorded on a RigakuD/Max-2500 diffractometer at 40 kV and 30 mA with aCu-target tube and a graphite-monochromator. Thesolid-state luminescence emission/excitation spectrawere recorded on a Hitachi F-7000 Fluorescence spectro- photometer equipped with a continuous Xe-900xenon lamp. Thermalgravimetric analysis (TGA) was conducted on aNETZSCH STA 449F3 instru- ment in flowing N2ata heating rate of 5 ℃/min.
2. 2 Synthesis of the title complex
A mixture of 2-nitroimidazole (2-nim, 0.0135 g, 0.12 mmol), H3TNB (0.0200 g, 0.053 mmol) and Zn(NO3)2·6H2O (0.0560 g, 0.2 mmol), tetraethylam- monium bromide (0.0120 g, 0.06 mmol), which was used to facilitate the crystallization of product, and DMF/MeOH/H2O (v:v = 3:0.5:0.5, 4.0 mL) was heated in a 20 mL scintillation vials at 100 ℃ for 24 hours, followed by cooling to room temperature. The resulting mixture was washed with DMF and water, and colorless block crystals were collected and dried in air. Calcd. for [Zn2(TNB)][2-nim]·DMF·2H2O (%): C, 46.47; H, 3.18; N, 7.75. Found (%): C, 47.18; H, 3.38; N, 8.01. IR (KBr disk, cm-1): 3431(vs), 3156(w), 2927(w), 1653(m), 1593(vs), 1532(w), 1492(w), 1386(vs), 1315(s), 1274(s), 1176(s), 1125(w), 954(m), 832(m), 784(m).
2. 3 Crystal structure determination
Single-crystal X-ray diffraction analysis of the complex was carried out on a Bruker SMART APEX II CCD diffractometer (Madison, WI, USA) equip- ped with a graphite-monochromatized Mora- diation (= 0.71073 Å) by using thescan technique at room temperature. The structure was solved by direct methods with SHELXS-2014[16]. The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restrains. A full-matrix least-squares refinement on2was carried out using SHELXL-2014. The disorder guests were removed with SQUEEZE instruction by PLATON soft[17]. The selected bond lengths and bond angles are listed in Table 1.
Complex 1 crystallizes in orthorhombic system with space group212121,= 9.4101(3),= 13.8325(5),= 24.2383(10) Å,= 3155.0(2) Å3,= 4,D= 1.295 Mg/m3,(000) = 1236 and= 1.564 mm-1. A total of 24205 reflections were obtained and 7242 unique (int= 0.0340) were collected in the range of 2.62≤≤27.49º by using anscan mode, of which 6488 reflections with> 2() were used in the succeeding refinement. The final= 0.0385,= 0.1021 (= 1/[2(F2) + (0.0620)2+ 0.0000], where= (F2+ 2F2)/3), (Δ)max= 1.107, (Δ)min= –0.362 e/Å3, (Δ/)max= 0.001 and= 1.004.

Table 1. Selected Bond Distances (nm) and Bond Angles (o) for Compound 1
Symmetry transformations used to generate the equivalent atoms: #1:,–1+,; #2:3/2–,1–,–1/2+;#3:3/2–,2–,1/2+; #4: 3/2–, 1–, 1/2+; #5: 1/2–, 1–, 1/2+
3 RESULTS AND DISCUSSION
3. 1 Structural description of 1
Compound 1 crystallizes in space group21/and features a 3neutral framework with dinuclear Zn2(CO2)3units. As shown in Fig. 1a, the asym- metric unit of 1 contains one zinc atom, a half TNB and a half 2-nitroimidazole molecule. Two zinc cations are eight-coordinated by six carboxylate oxygen atoms from three TNB ligands and two nitrogen atoms from two 2-nitroimidazoles ligands, forming a similar paddle-wheel building unit Zn2(CO2)3. Compound 1 is a typical layered-pillar structure, the axial coordination sites are ligated by2-nim groups which act as a pillar inter-connecting the [Zn2(TNB)-] layers along an axis direction to form a three-dimension neutral framework [Zn2(TNB)(2-nim)] (Fig. 1c). Due to the large size of TNB3-ligand, the pores are large enough for a second net, and two such frameworks are mutually interpenetrated (Fig. 1d). From a topological point of view, the [Zn2(CO2)3]+paddle-wheel-units can be considered as a 5-connected node, and the TNB3-anions ligand can be reduced as a 3-connected node and the 2-nitroimidazole ligand as a line. Therefore, the framework of 1 can be simplified as a 2-fold interpenetrated hexagon-planar (3,5)-connected hms net with Point (Schläfli) symbol of (63) (69.8) (Fig. 1d).

Fig. 1. (a) Basic structure in compound 1. (b) Yellow polyhedra present [Zn2(CO2)3] units. (c) Pillared-layer framework along the-axis. (d) View of the interpenetrated topology along the-axis
3. 2 PXRD and thermal properties
In order to check the phase purity of 1, the X-ray powder diffraction (XRPD) pattern was checked at room temperature. As shown in Fig. 2, the peak positions of the simulated and experimental XRPD patterns are in agreement with each other, demon- strating the good phase purity of 1. Also, the XRPD peaks of the activated sample matches with the simulated shape, indicating the sample still maintains well the crystalline state.

Fig. 2. XRPD patterns for complex 1: the as-synthesized patterns, the activated isotherm and the simulated based on X-ray single-crystal data
TG curve for complex 1 is shown in Fig. 3. The TG curve of 1 shows the weight loss (15%) at 30~220℃, corresponding to the release of a free water molecule and the guest organic molecules (calcd.: 15.2%). The framework keeps stable in the range of 225~450 ℃, then collapses with rapid weight loss. In addition, the TG analyses of activated frameworks were performed. Theplateaus ranging from 40 to 400 ℃ show well that the initialguest molecules can be almost fully exchanged by methanol, andmethanol molecules can be completely removed by thethermal/vacuum activation at 60℃. The results of effective activation laid the foundation for gas adsorption.

Fig. 3. TGA plots of 1 and the activated sample
3. 3 Adsorption properties
The rigidity of these porous frameworks as well as the accessibility of guest molecules into the porous framework of 1 was assessed by solid-gas adsorption experiments with CO2as probe molecules. Prior to measurement, the as-synthesized crystalline samples were activated as follows: immersing in methanol for three days (solvent was exchanged nine times), followed by the thermal/vacuum activation at 303 K for 8 h to generate activated compound 1. The CO2adsorption was performed at 195 and 273 K, respec- tively. The CO2adsorption capacity is 145.56 cm3×g-1, and shows type-I behaviors, characterizing the typical of crystalline microporous materials. Accor- ding to the above CO2capacity, the calculated BET surface area is 646.38 m2×g-1. The CO2uptake for 1 is 56.48 cm3×g-1at 273 K. Compound 1 exhibits moderate capacity of CO2(Fig. 4).
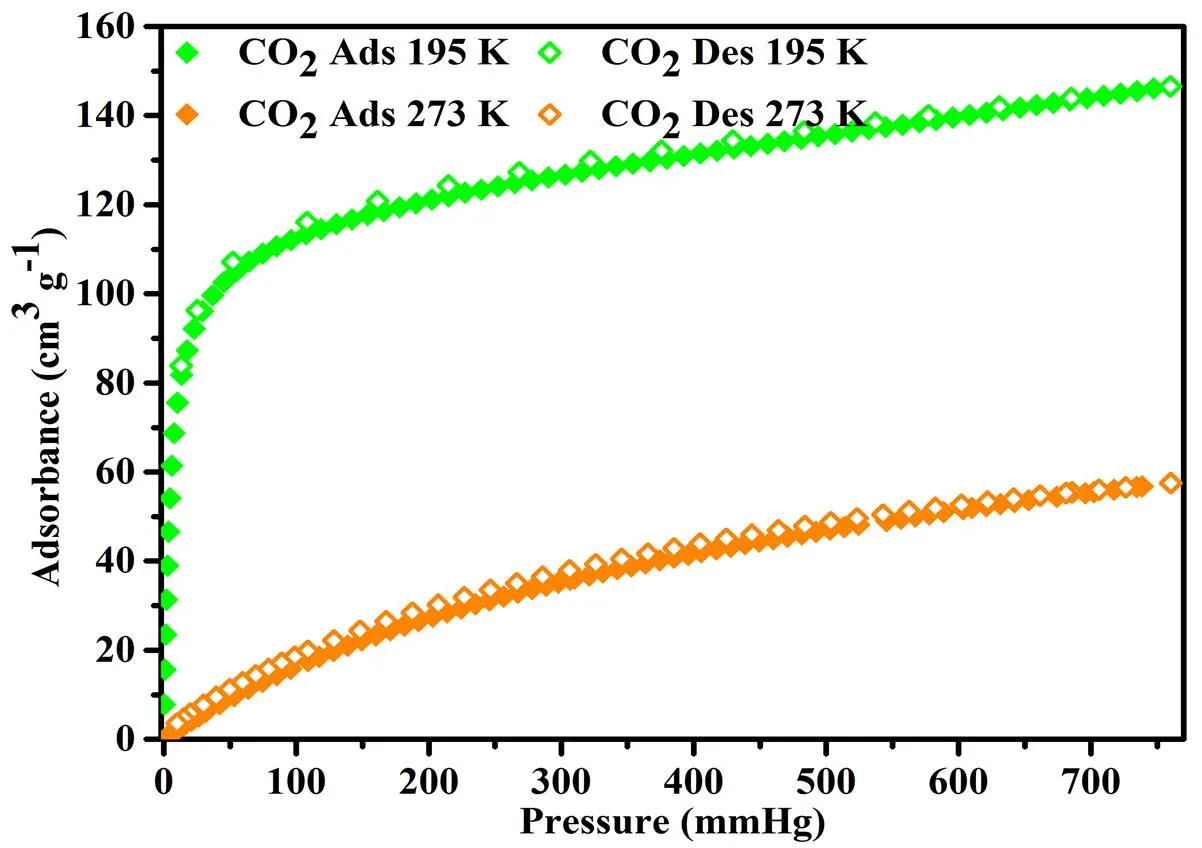
Fig.4. Gas adsorption isotherms of 1: CO2adsorptionisotherms at 195 and 77 K
3. 4 Fluorescent properties
Compounds constructed by10metal centers andconjugated organic linkers are promising candidatesfor photoactive materials with potential applicationssuch as chemical sensors and electro-luminescentdisplay[18]. The photoluminescent properties of free ligand H3TNB and compound 1were investigated in the solid stateat room temperature. The emission peaks of the compoundsare shown in Fig. 5. Compound 1exhibits an intenseemission between 400 and 575 nm (max= 467 nm uponexcitation at 325 nm). Themaxof free H3TNB is 458 nm, so the emission peaks for 1 can be attributed to the charge transfer (→* and→*) of internal ligands, and the slight change ofluminescence may be owing to the result of coordination and/or bridging effects of the relevantligands to a metal center, which effectively increasesthe rigidity and asymmetry of the ligands andreduces the loss of energy via radiation less pathway[19, 20]. The solid state UV-vis adsorption spectra of compound 1 were also measured (Fig.6). Two peaks at 325 and 394 nm were shown, indicating that compound 1 would show blue emission, and the former is closed to the excitation of compound 1. In addition, the quantum yields of TNB and compoundswere about 9.6 and 8.6%, respectively.
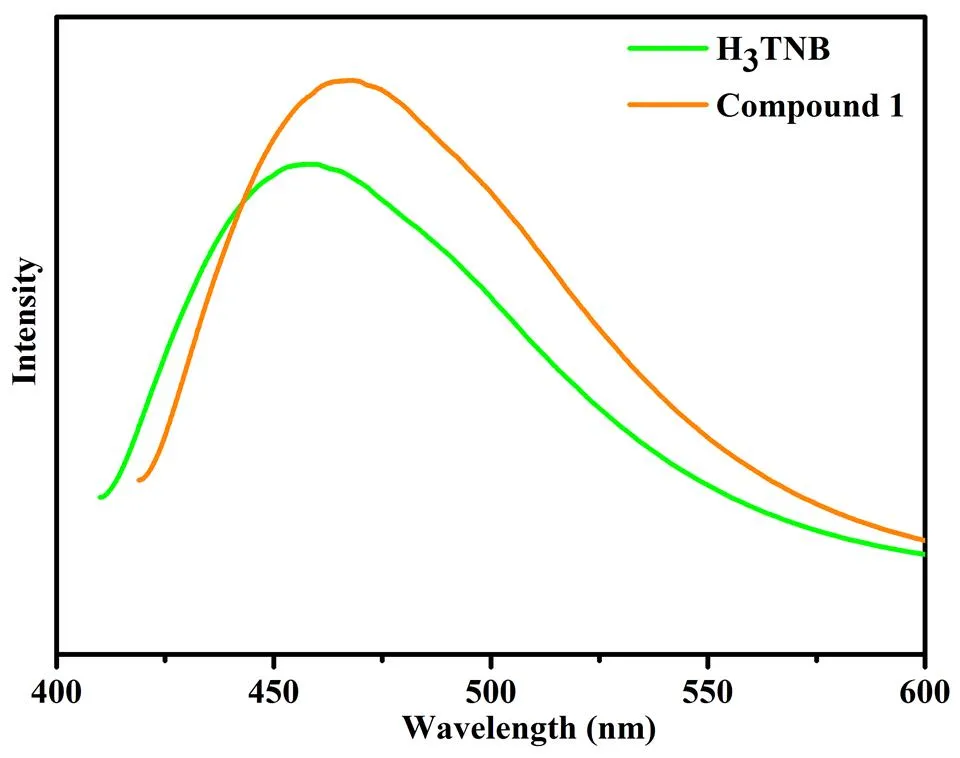
Fig. 5. Fluorescence emission spectra of compound 1
Fig. 6. Solid-state UV-vis adsorption spectra of compound 1
4 CONCLUSION
In summary, via the strategy of charge balance, one 2-fold interpenetrated pillared-layer compound was obtained by paddle-wheel building [Zn2(CO2)3] units and linear imidazole ligands. The adsorption of carbon dioxide was characterized. Meanwhile, com- pound 1displays strong fluorescence emission ability. This work prompts us to achieve more functional crystalline solids via such a reliable synthetic route by employing the mixed ligands, and further efforts on this perspective are still under way.
(1) Karim, A.; Youssef, B.; Renjith, S. P.; Amandine, C.; Prashant, M. B.; Ayalew, H. A.; Guillaume, M.; Mohamed, E. Gas/vapour separation using ultra-microporousmetal-organic frameworks: insights into the structure/separation relationship.2017, 46, 3402-3430.
(2) Sheng, D.; Dan, W. Y.; Luo, G.X.; Deng, M. A new coordination polymer built of 4-(5H-tetrazol)-benzoic acid and 3,5-dimethyl-1H,1,2,4-triazole showing a rarely observed (3,5)-connected lhh topology: synthesis, structure, CO2adsorption and luminescent property..2016, 2, 264-270.
(3) Fu, H. R.; Zhang, J. Selective sorption of light hydrocarbons on a family of metal-organic frameworks with different imidazolate pillars.2016, 55, 3928–3932.
(4) Lyndon, R.; Konstas, K.; Ladewig, B. P.; Southon, P. D.; Kepert, C. J.; Hill, M. R. Dynamic photo-switching in metal-organic frameworks as a route to low-energy carbon dioxide capture and release.2013, 52, 3695–3698.
(5) Zhao, H.; Jin, Z.; Su, H.; Zhang, J.; Yao, X.; Zhao, H.; Zhu, G. Target synthesis of a novel porous aromatic framework and its highly selective separation of CO2/CH4.2013, 49, 2780-2782.
(6) Henke, S.; Schneemann, A.; Wutscher, A.; Fischer, R. A. Directing the breathing behavior of pillared-layered metal-organic frameworks via a systematic library of functionalized linkers bearing flexible substituents.2012, 134, 9464−9474.
(7) Chang, X. H.; Ma, L. F.; Guo, H.; Wang, L. Y. Four low-dimensional cobalt(II) coordination polymers based on a new isophthalic acid derivative: syntheses, crystal structures, and properties.2012, 12, 3638-3646.
(8) Zhou, H. C.; Long, J. L.; Yaghi, O. M. Introduction to metal-organic frameworks.2012, 112, 673−674.
(9) Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: facts, analyses, rules and consequences.2009, 38, 1380–1399.
(10) Schneemann, A.; Bon, V.; Senkovska, I.; Senkovska, I.; Kaskel, S.; Fischer, R. A. Flexible metal-organic frameworks.2014, 43, 6062−6096.
(11) Farha, O. K.; Malliakas, C. D.; Kanatzidis, M. G.; Hupp, J. T. Control over catenation in metal-organic frameworks via rational design of the organic building block.2010, 132, 950−952.
(12) Biswas, S.; Vanpoucke, D. E. P.; Verstraelen, T.; Vandichel, M.; Couck, S.; Leus, K.; Liu, Y. Y.; Waroquier, M.; van Speybroeck, V.; Denayer, J. F. M.; van der Voort, P. New functionalized metal-organic frameworks MIL-47-X (X = -Cl, -Br, -CH3, -CF3, -OH, -OCH3): synthesis, characterization, and CO2adsorption properties.2013, 117, 22784−22796.
(13) Chen, B.; Ma, S.; Zapata, F.; Fronczek, F. R.; Lobkovsky, E. B.; Zhou, H.C. Rationally designed micropores within a metal-organic framework for selective sorption of gas molecules.2007, 46, 1233−1236.
(14) Ma, B. Q.; Mulfort, K. L.; Hupp, J. T. Microporous pillared paddle-wheel frameworks based on mixed-ligand coordination of zinc ions.2005, 44, 4912−4914.
(15) Henke, S.; Wieland, D. C. F.; Meilikhov, M.; Paulus, M.; Sternemann, C.; Yusenko, K.; Fischer, R. A. Multiple phase-transitions upon selective CO2adsorption in an alkyl ether functionalized metal-organic framework-an in situ X-ray diffraction study.2011, 13, 6399−6404.
(16) Spek, A. L. Crystal structure refinement with SHELXL.2015, 71, 3–8.
(17) Spek, A. L. PLATON squeeze: a tool for the calculation of the disordered solvent contribution to the calculated structure factors.2015, 71, 9–18.
(18) Gu, C. S.; Hao, X.M.; Zhang, Z. Y.; Ji, L. L.; Li, Y.; Song, W. D. Syntheses, structures and luminescent properties of the cadmium(II) complex with 3,3΄-thiodipropionic acid..2017, 3, 478-484.
(19) Fu, H. R.; Kang, Y.; Zhang, J. Highly selective sorption of small hydrocarbons and photocatalytic properties of three metal-organic frameworks based on tris(4-(1H-imidazol-1-yl)phenyl)amine ligand.2014, 53, 4209–4214.
(20) Li, B. H.; Wu, J.; Liu, J. Q.; Gu, C. Y.; Xu, J. W.; Luo, M. M.; Yadav, R.; Kumar, A.; Batten, S. R. A luminescent zinc(II) metal-organic framework for selective detection of nitroaromatics, Fe3+and CrO42−: a versatile three fold fluorescent sensor..2016, 81, 885–892.
23 June 2017;
11 December 2017 (CCDC1556976)
①This project was financially supported by the National Natural Science Foundation of China (No. 21601080), and the Key Scientific Research Projects of Higher Education of He'nan Province (16A150016)
. E-mail: minle_han@163.com
10.14102/j.cnki.0254-5861.2011-1762
- 结构化学的其它文章
- Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

