Synthesis, Crystal Structureand Theoretical Calculations of a Cadmium Complex Containing 3-Hydroxybenzoic Acid and 1,4-Bis(imidazol-1-yl)-butane①
WANG Zhi-To WANG Sho-Jie LIXiu-Mei PAN Y-Ru
Synthesis, Crystal Structureand Theoretical Calculations of a Cadmium Complex Containing 3-Hydroxybenzoic Acid and 1,4-Bis(imidazol-1-yl)-butane①
WANG Zhi-TaoaWANG Shao-Jieb②LIXiu-MeiaPAN Ya-Rua
a(134002)b(134002)
A new complex [Cd(hba)2(bib)]n·nH2O (1,Hhba = 3-hydroxybenzoic acid, bib = 1,4-bis(imidazol-1-yl)-butane) has been hydrothermally synthesized and characterized by elemental analysis, IR spectrum, thermogravimetric analysis, fluorescence spectrum, and single- crystal X-ray diffraction. Yellow crystal crystallizes in the orthorhombic system, space groupwith= 16.220(5),= 14.980(5),= 20.521(5) Å,= 4986(3) Å3, C24H26CdN4O7,M= 594.89,D= 1.585g/cm3,(Mo) = 0.927 mm-1,(000) = 2416,= 8,the final= 0.0242 and= 0.0589 for 4076 observed reflections (> 2()). In 1, the Cd(II) ion takes a six-coordination mode, and bib ligand bridges adjacent Cd(II) ions to generate 1Dchains; these neighboring chains are connected by O–H···O hydrogen bonding interactions, producing a 2D folded layered structure. Furthermore, by O–H···O hydrogen bonding between layers and layers, a 3D supramolecular architecture is formed. In addition, we analyzed Natural Bond Orbital (NBO) in using the PBE0/LANL2DZ method built in Gaussian 03 Program. The calculation results indicated obvious covalent interaction between the coordinated atoms and Cd(II) ion.
crystal structure, Cd(II) complex, 3-hydroxybenzoic acid, 1,4-bis(imidazol-1-yl)-butane, natural bond orbital;
1 INTRODUCTION
In the past few decades, the design and construc- tion of coordination polymers have attracted great attention for their potential request, framework, and molecular topologies[1-3]. A lot of factors such as the coordination geometry of metal ion, solvent system, pH value, counter anion, and number of coordination sites supplied by organic ligands can play the key role in constructing coordination networks[4-6]. The selection of special ligands is very important in the construction of these coordination polymers.
Many aromatic carboxylic acids have been exten- sively applied as multifunctional building blocks in the construction of metal-organic networks due to their abundant coordination modes to metal ions, allowing different types of structural topologies due to their ability to act as good hydrogen-bond acceptors as well as donor, relying on the degree of deprotonation of carboxylate groups to assemble supramolecular structure[7-9]. Among them, polycar- boxylic acids, such as 1,2,4,5-benzenetetracarboxylic acid, 4,4΄-oxydibenzoic acid, 5-nitroisophthalic acid, and 3,3΄,4,4΄-benzophenone tetracarboxylic acid, are paid much attention to because of their abundant coordination modes[10-13]. Besides the carboxylate linkers, bis(imidazole) bridging ligands with dif- ferent length and flexibly, for example, 1,3-bis(imi- dazol-1-ylmethyl)-benzene,1,4-bis(imidazol-1-ylme- thyl)-benzene and 1,4-bis(imidazol-1-yl)-butane, are frequently used in the assembly process of CPs as bridging linkers[14-17].
In view of these factors, we herein report the synthesis and characteristics of a new coordination polymer containing 3-hydroxybenzoic acid and1,4-bis(imidazol-1-yl)-butane, namely, [Cd(hba)2(bib)]n·nH2O (1),which shows supramole- cular architecture by hydrogen bondinginteractions.
2 EXPERIMENTAL
2. 1 General procedures
All chemicals were of AR grade and used without further purification. The content of carbon, hydrogen and nitrogen were determined using an Elementar Vario EL III elemental analyzer. IR spectrum was recorded in the range of 4000~400 cm-1on a Nicolet 6700 FTIR spectrophotometer using a KBr pellet. Thermogravimetric analysis data were collected on a TG/DTA7300 analyzer at a heating rate of 10oC·min-1. Powder X-ray diffraction (PXRD) patterns were collected in the 2range of 5~50º with a scan speed of 0.1 º·s-1on a Bruker D8 Advance instrument using a Curadiation (= 1.54056 Å) at room temperature. The fluorescent spectrum was obtained on a computer-controlled JY Fluoro-Max-3 spectro- meter at room temperature.
2. 2 Synthesis
A mixture of Cd(OAc)2·2H2O (0.026 g, 0.1 mmol), Hhba (0.028 g, 0.2 mmol), bib (0.038 g, 0.2 mmol)and 10 mL H2O was stirred for 15 min at room temperature, then sealed in a 20 mL Teflon-lined stainless-steel vessel, and heated to 130 °C for 3 days, followed by slow cooling (a descent rate of 5oC/h) to room temperature. Pale yellow parallelogram-shaped crystals were obtained. Yield of 23% (based on Cd). Anal. Calcd. for C24H26CdN4O7(%): C, 48.45; H, 4.41; N, 9.42. Found (%): C, 47.93; H, 4.04; N, 8.92. IR (cm–1): 3500(w), 3136(w), 1550(s), 1396(s), 1236(m), 1098(m), 938(w), 821(w), 770(w), 650(w), 542(w), 498(w), 460(w).
2. 3 Structure determination
The data of the single crystal with dimensions of 0.36mm × 0.27mm × 0.13mm were collected at 293(2) K on a Bruker D8 QUEST CMOS dif- fractometer with Moradiation (= 0.71073 Å). The structure was solved by direct methods and refined by full-matrix least-squares on2using theSHELX-97 program[18, 19]. The non-hydrogen atoms of the complex were refined with anisotropic temperature parameters. The hydrogen atoms were generated geometrically and refined isotropically using the riding model. For 1, a total of 26051 reflections were collected in the range of 1.98≤≤26.07°, of which 4930 were independent (int= 0.0219). The final= 0.0242 and= 0.0589 for observed reflections with> 2(), and= 0.0326 and= 0.0647 for all data with (Δ)max= 0.490 and (Δ)min= –0.494 e·Å-3. Selected bond lengths and bond angles of complex 1 are shown in Table 1. Hydrogen bond parameters of 1 are listed in Table 2.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1

Table 2. Hydrogen Bonds for Complex 1
2. 4 Theoretical calculation determination
The calculations in this manuscript were per- formed with the Gaussian03 program[20].The para- meters of the molecular structure for calculation were all rooted in the experimental data of the complex. Natural bond orbital (NBO) analysis was acted by density functional theory (DFT)[21]with the PBE0[22-25]hybrid functional and the LANL2DZ basis set[26]. We select one Cd(II) ion, one hba ligand and one bib ligand as unit structures of calculation.
3 RESULTS AND DISCUSSION
3. 1 Description of the structure
X-ray crystallography analyses show that complex 1 exhibits a one-dimensionalchain structure. Its asymmetric unit consists of one crystallogra- phically unique Cd(II) ion, two hba ligands, one bib molecule, and one crystal water molecule. As depicted in Fig. 1, the six-coordinated Cd(1) ion owns a distorted octahedral {CdO4N2} geometry filled by four carboxylate oxygen atoms from two different hba ligands and two nitrogen donors from two flexible bib molecules. The lengths of the Cd–O bonds range from 2.3425(17) to 2.4021(17) Å, yet the Cd–N distances vary from 2.262(2) to 2.2670(19) Å. The three carboxylate O atoms (O(2), O(3), O(4)) and one imidazole N atom (N(3)) are located in the basal plane, however another carboxylate O atom (O(1)) and imidazole N atom (N(1)) occupy the axial positions from the opposite direction. In 1, the hba ligand shows a2-linker, in which deprotonated carboxylate group acts as a1:1bidentate mode. In addition, the bib ligands take a-conformation bridging mode with a dihedral angle between the two imidazole rings of 9.80º. In these patterns, bib ligands link neighboring Cd(II) ions to produce a 1chain structure with the Cd···Cd distance of 13.857 Å (Fig. 2); these adjacent chains are connected together by O–H···O hydrogen bonding interactions, producing a 2D folded layered structure. Moreover, by O–H···O hydrogen bonding between layers and layers, a 3D supramolecular architecture is formed (Fig. 3).
3. 2 IR spectrum
IR spectrum of 1 exhibits a broad absorption band at 3500 cm-1corresponding to the H···O stretching of crystal water molecule in 1. The C–N absorption peaks of imidazole can be observed at 1236 cm-1. Asymmetric and symmetric COO–stretching modes of the lattice hba anion were evidenced by very strong, slightly broadened bands at 1550 and 1396 cm-1[27], which is consistent with the results of X-ray analysis.
3. 3 TGA analysis and PXRD results
To determine the thermal stability of complex 1, its thermal behaviors were investigated under nitrogen atmosphere by thermogrametric analysis (TGA). As depicted in Fig. 4, the TGA curve of 1 reveals that one coordinated water molecule is released between 99 and 260 ℃ (obsd. 3.2%; calcd. 3.0%), and the dehydrated solid begins to decompose at 261 ℃. The patterns for the as-synthesized bulk material closely match the simulated ones from the single-crystal structure analysis, which points out the pure solid-state phase (Fig. 5).

Fig. 1. View of the asymmetric unit of complex 1. All hydrogen atoms are omitted for clarity
Fig. 2. View of the one-dimensionalchain of complex 1

Fig. 3. View of the 3D supramolecular architecture of 1 formed by O–H···O hydrogen bonds
Fig. 4. TGA curve of complex 1

Fig. 5. PXRD patterns of complex 1 at room temperature: bottom-simulated, top-experimental
3. 4 Photoluminescent properties
Metal-organic coordination polymers, especially10metal centers, such as AgI, AuI, ZnIIand CdII, and conjugated organic linkers have been researched because of their fluorescent properties and potential applications as fluorescent-emitting materials, che- mical sensors and electroluminescent displays[28]. Solid-state photoluminescent emission spectrum of complex 1 was discussed in the solid state at room temperature (Fig. 6). When excited at 360 nm, complex 1 exhibits intense blue luminescence and shows one emission peak at 452 nm. The free ligands Hhba and bib show photoluminescence with the emission maximum at 410 nm and 438 nm respectively (ex= 315 nm), which can be assigned to intraligand (*→) transition[29]. Thus, the emission band of complex 1 could be assigned to the emission of ligand-to-ligand charge transfer[30]. Owing to strong fluorescent intensity, it appears to be good candidates for novel hybrid inorganic-organic photoactive materials.
4 THEORETICAL CALCULATIONS
The major natural atomic charges, natural electron configuration, Wiberg bond indices and NBO bond orders (a.u) for complex 1 are shown in Table 3. It is shown that the electronic configurations of Cd(1) ion,nitrogenandoxygen atoms are 50.3349.9950.30, 21.3824.19~4.20and 21.69~1.7025.03~5.07, respectively. In view of the above effects, one can infer that the Cd(1) ion coordination with nitrogen and oxygen atoms is mainly on 4, 5, and 5orbitals. Nitrogen atoms form coordination bonds with Cd(1) ion using 2and 2orbitals. The oxygen atoms provide electrons of 2and 2to Cd(1) ion and form the coordination bonds. So, the Cd(1) ion gained some electrons from two nitrogen atoms of bib ligand, and four oxygen atoms of hba ligands[31, 32]. So, on the basis of valence-bond theory, the atomic net charge distribution and NBO bond orders of complex 1 (Table 3) exhibit obvious covalent interaction between the coordinated atoms and Cd(1) ion. The differences of NBO bond orders for Cd–O and Cd–N bonds make their bond lengths different[32, 33], which is in good agreement with the X-ray crystal structural data of compound 1.

Fig. 6. Solid-state emission spectrum of 1 at room temperature
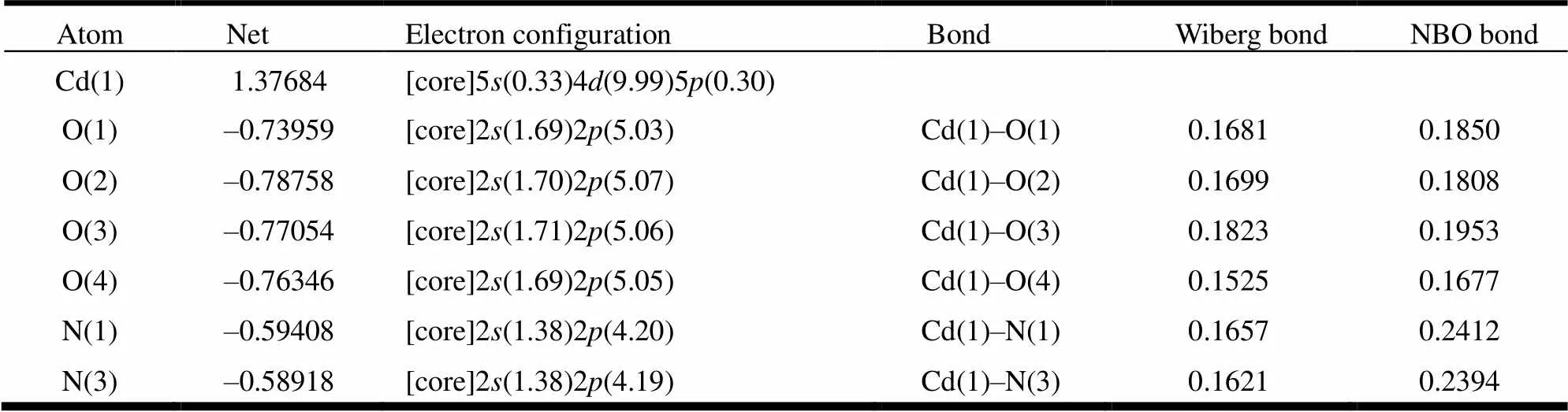
Table 3. Natural Atomic Charges, Natural Valence Electron Configurations, Wiberg Bond Indexes and NBO Bond Orders (a.u) for 1
As can be seen from Fig. 7, the lowest unoccupied molecular orbital (LUMO) is mainly made up oforbitals of imidazole rings in the bib molecule, whereas the highest occupied molecular orbital (HOMO) mainly composed oforbitals of benzene rings in hba ligand. Thus, the charge transfer from ligand to ligand may be deduced from some contours of molecular orbital of compound 1.
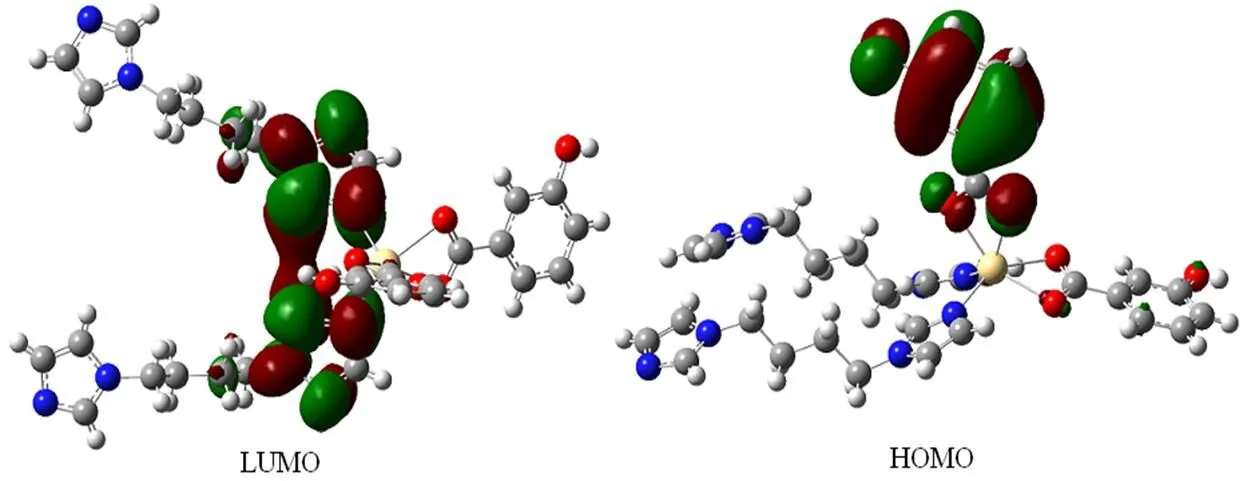
Fig. 7. Frontier molecular orbitals of complex 1
(1) Hagrman, P. J.; Hagrman, D.; Zubieta, J. Organic-inorganic hybrid materials: from “simple” coordination polymers to organodiamine-templated molybdenum oxides.1999, 38, 2638-2684.
(2) Rao, C. N. R.; Natarajan, S.; Vaidhyanathan, R.Metal carboxylates with open architectures.2004, 43, 1466–1496.
(3) Kitagawa, S.; Kitaura, R.; Noro, S. I.Functional porous coordination polymers.. 2004,43, 2334–2375.
(4) Gu, J. Z.; Gao, Z. Q.; Tang, Y. pH and auxiliary ligand influence on the structural variations of 5(2΄-carboxylphenyl) nicotate coordination polymers.. 2012, 12,3312–3323.
(5) Gao, Q.; Xie, Y. B.; Li, J. R.; Yuan, D. Q.; Yakovenko, A. A.; Sun, J. H.; Zhou, H. K. Tuning the formations of metal-organic frameworks by modification of ratio of reactant, acidity of reaction system, and Use of a secondary ligand.. 2012, 12, 281–288.
(6) Lee, J.; Kang, Y. J.; Cho, N. S.; Park, K. M.Role and effect of anions in the construction of silver complexes based on a pyridylimidazole ligand with L-type coordination vectors and their photoluminescence properties.. 2016, 16, 996–1004.
(7) Li, L.; Cao, X. Y.; Huang, R. D. Research progress of metal-organic frameworks based on aromatic.. 2016, 34, 143–156.
(8) Lu, W. G.; Su, C. Y.; Lu, T. B.; Jiang, L.; Chen, J. M.Two stable 3D meta-organic frameworks constructed by nanoscale cages via sharing the single-layer walls.2006, 128, 34–35.
(9) Wang, H. H.; Yang, H. Y.; Shu, C. H.; Chen, Z, Y.; Hou, L.; Wang, Y. Y.Five new Cd(II) complexes induced by reaction conditions and coordination modes of 5-(1-tetrazol-5-yl)isophthalic acid ligand: structures and luminescence.. 2016, 16, 5394–5402.
(10) Wang, J. J.; Zhang, D. J.; Zhang, R. C.; Jin, H. H.; Gao, X. F.Co-crystals based on 1,2,4,5-benzenetetracarboxylic acid: synthesis, supramolecular frameworks and optical properties.2014,640, 497–503.
(11) Wang, H.; Wang, Y. Y.; Yang, G. P.; Wang, C. J.; Wen, G. L.; Shi, Q. Z.; Batten, S. R.A series of intriguing metal-organic frameworks with 3,3΄,4,4΄-benzophenonetetracarboxylic acid: structural adjustment and pH-dependence.. 2008, 10, 1583–1594.
(12) Chen, S. P.; Ren, Y. X.; Wang, W. T.; Gao, S. L.Nanoporouslanthanide-carboxylateframeworks based on5-nitroisophthalic acid.. 2010, 39, 1552–1557.
(13) Li, X. M.; Ji, J. Y.; Niu, Y. L.; Wang, Z. T. Hydrothermal synthesis and crystal structure of nickel(Ⅱ) coordination polymer assembled by 5-nitroisophthalic acid and 1,4-bis(imidazol-1-ylmethyl)-benzene ligands.. 2013, 29, 165–169.
(14) Li, X. M.; Ji, J. Y.; Niu, Y. L.; Wang, Q. W.; Wang, Z. T.Hydrothermal synthesis and crystal structure of a 2D network Mn(Ⅱ) coordination polymer based on oxalic acid and bis(imidazol) ligands..2013, 29, 1302–1306.
(15) Wang, Z. T.; Ji, J. Y.; Li, X. M.; Niu, Y. L.; Wang, Q. W.; Liu, B. Hydrothermal synthesis and crystal structure of a cobalt(II) coordination polymer: [Co(C2O4)(bix)]n(bix = 1,4-bis(imidazol-1-ylmethyl)-benzene).. 2013, 32, 296–300.
(16) Li, X. M.; Ji, J. Y.; Niu, Y. L.; Wang, Q. W.; Wang, Z. T. Synthesis, crystal structure and fluorescent property of a new two-dimensional Zn(II) coordination polymer based on oxalic acid and 1,4-bis(imidazol-1-ylmethyl)-butane.2014, 44, 891–894.
(17) Pan, Y. R.; Li, X. M.; Ji, J. Y.; Wang, Q. W.Synthesis, crystal structure and theoretical calculations of a two cobalt, nickel coordination polymers with 5-nitroisophthalicacid and bis(imidazol)ligands.. 2016, 69, 1296–1304.
(18) Sheldrick,G. M.. University of Göttingen, Germany1997.
(19) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(20) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A., Revision B.03. Gaussian, Inc., Pittsburgh, PA 2003
(21) Parr, R. G.; Yang, W.. Oxford University Press: Oxford 1989.
(22) Ernzerhof, M.; Scuseria, G. E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional.. 1999, 110, 5029-5036.
(23) Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model.. 1999, 110, 6158-6170.
(24) Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple.1996, 77, 3865-3868.
(25) Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple.1997, 78, 1396-1397.
(26) Dunning, T. H.; Hay Jr, P. J. in, Ed. by H.F. Schaefer III (Plenum, New York 1976), p. 1.
(27) Devereux, M.; Shea, D. O.; Kellett, A.; McCann, M.; Walsh, M.; Egan, D.; Deegan, C.; Kędziora, K.; Rosair, G.; Müller-Bunz, H. Synthesis, X-ray crystal structures and biomimetic and anticancer activities of novel copper(II) benzoate complexes incorporating 2-(4΄-thiazolyl)benzimidazole (thiabendazole), 2-(2-pyridyl)benzimidazole and 1,10-phenanthroline as chelating nitrogen donor ligands.. 2007, 101, 881–892.
(28) Kreno, L. E.; Leong, K.; Farha, O. K.; Allendorf, M.; Duyne, R. P. V.; Hupp, J. T. Metal-organic framework materials as chemical sensors.. 2012, 112, 1105–1125.
(29) Lin, J. D.; Long, X. F.; Lin, P.; Du, S. W.A series of cation-templated, polycarboxylate-based Cd(II) or Cd(II)/Li(I) frameworks with second-order nonlinear optical and ferroelectric properties.. 2010, 10, 146–157.
(30) Li, G. L.; Liu, G. Z.; Huang, L. L.; Li, L.; Zhang, X.Ancillary ligand-mediated syntheses, structures and fluorescence of three Zn/Cd(II) coordination polymers based on nitrobenzene dicarboxylate.. 2014, 24, 617–623.
(31) Huang, Y. J.; Pan, Y. R.; Du, G.; Cao, Y. X. Extended structures of two coordination polymers based on 1,10-phenanthroline derivatives: preparation, structural characterization and properties.2016, 128, 459–465.
(32) Li, Z. P.; Xing, Y. H.; Zhang, Y. H. Synthesis, structure and quantum chemistry calculation of scorpionate oxovanadium complexes with benzoate.2009, 25, 741–746.
(33) Huang, Y. J.; Ni, L.; Zhu, Y. Y.; Yao, J. Hydrothermal processing, characterization and theoretical calculation of a copper(II) compound with 1,10-phenanthroline derivative ligand: 2-(4-methoxyphenyl)-1H-imidazo[4,5-][1,10] phenanthroline.. 2012, 31, 353–360.
18 August 2017;
24 October 2017 (CCDC 1535329)
① The project was supported by the Science and Technology Development Project of Jilin Provincial Science & Technology
Department (201205080) and the Science and Technology Research Projects of the Education Office of Jilin Province (No. 2013. 384)
. Wang Shao-Jie, born in 1968. Tel: 0435-3209655, E-mail: lixm20032006@163.com
10.14102/j.cnki.0254-5861.2011-1786
- 结构化学的其它文章
- Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

