苯并三唑类紫外稳定剂在环境中的检测、分布及其毒性效应
李雯镜,李志彤,梁雪芳
内蒙古大学生态与环境学院,呼和浩特 010021
苯并三唑及其衍生物(benzotriazole and its derivatives, BTRs)是一类具有优良的金属防腐蚀功能和光稳定功能的化学品,广泛应用于工业和生活产品中。BTRs生产量大,分布广泛,近年来在环境中不断被检出,成为一类新型污染物[1]。苯并三唑类紫外稳定剂(benzotriazole ultraviolet stabilizers, BUVSs)是苯并三唑的一类具有良好紫外吸收能力的衍生物(可吸收280~400 nm光谱范围内的紫外线)[2],常作为添加剂用于各种工业产品中,如塑料、建筑材料、汽车配件、粘合剂、涂料等,以防止紫外辐射造成的黄化和降解[2]。此外,BUVSs也用于个人护理品中,如防晒霜、沐浴乳、洗发露、香水等[3]。BUVSs由于其应用范围广、产量高、分布广泛,以及其潜在的环境持久性和生物累积性,近年来得到了广泛的关注。BUVSs已在多种环境介质中检出,例如地表水[4-5]、污水[6-7]、沉积物[8-9]、污泥[10-11]、填埋浸出液[8]、土壤[12],以及贝类[13]、鱼类[3,14]和海鸟[2]等生物体。不仅如此,还有研究表明,在服装纺织品[15]、室内灰尘[16]、人类母乳[17]、尿液[18-19]和脂肪组织[20]中也有BUVSs检出。同时,研究表明BUVSs具有潜在的内分泌干扰效应,可影响鱼类和人的芳烃受体通路[21-23]。尽管BUVSs的急性毒性较低,但长期暴露仍然会给人类健康和生态环境带来潜在风险。因此,本文在总结BUVSs的环境分布和污染水平的基础上,重点介绍了BUVSs的毒理学研究进展并对典型BUVSs的生物活性进行了预测,以期为BUVSs的毒性研究和风险管理提供理论依据和科学基础。
1 环境中BUVSs的污染水平(Pollution level of BUVSs in environment)
1.1 BUVSs的分析方法
在环境样品中,由于基质复杂等原因污染物难以直接检测,因此通常需要在测定前进行适当的预处理,以尽可能消除基质影响,提高分析精度。当前,建立和优化BUVSs的仪器分析技术,有助于研究获得更准确的数据信息,更好地对BUVSs在环境中的污染水平、分布规律、生态风险等方面进行更好地评估(表1)。
1.1.1预处理方法
1.1.1.1固相萃取
固相萃取(solid-phase extraction, SPE)是最常用于处理水样中目标化合物的预处理方法,包括液相和固相的物理萃取过程,样品通过吸附剂时被选择保留分离物和一些干扰物,用适当的溶剂淋洗吸附剂,保留的干扰物被洗掉,可以得到纯化、浓缩的分离物[33]。SPE的性能受到大量变量的影响,因此经常根据不同的目标化合物和样品基质从萃取柱、洗脱溶剂、洗脱体积、pH、萃取周期等方面进行优化,以获得高回收率,提高分析的灵敏度。Liu等[24]在测定4种苯并三唑(benzotriazole, BT)和6种紫外吸收剂时,将SPE作为地下水和污水的预处理方法,调节水样pH至2,使用HLB柱提取水样,并用甲醇/二氯甲烷(V∶V=50∶50)洗脱获得目标化合物,分别获得了70%~150%和82%~127%的回收率。王金成等[34]测定地表水中苯并三唑类和苯并噻唑类衍生物时,选用OASIS HLB为固相萃取柱,10%的甲醇水溶液为淋洗溶剂,20%的丙酮的甲醇溶液为洗脱溶剂,溶液上样体积为200 mL,调节pH至3.0,获得了59.8%~98.7%的回收率。
SPE技术在广泛用于环境分析领域的同时,还存在着一些缺点,例如在样品处理期间可能加大损失量以及需要大体积样本,而在线SPE技术将常规的程序自动化,缩小了预处理过程中的样本损失,同时将污染最小化,提高了分析的可重复性[27]。
1.1.1.2液液萃取
液液萃取(liquid-liquid extraction, LLE)是利用液体混合物中各组分在某种溶剂中的溶解度不同而实现样品分离提纯目的的技术。简单来讲,就是将萃取剂加入到样品溶液中,充分混合,不同组分进入不同的相中,进而达到分离目标物的目的。Nakata等[28]基于LLE技术提取污水处理厂进出水中的BUVSs,使用己烷进行萃取,获得了98%~115%的回收率。
1.1.1.3微萃取技术
近些年,微萃取技术在不断发展,因其操作简单、用量小等优点越来越多地应用于提取分析化合物当中。固相微萃取(solid-phase microextraction, SPME)是以SPE技术为基础发展的微萃取技术,也常被用于在水样中测定BUVSs。SPME通常在纤维涂层、pH、样品温度以及采样模式上进行优化,以提高监测效率。SPE和SPME在提取有效样本的同时,仅存在中等甚至没有有机溶剂的消耗[29]。与SPE相比,SPME技术程序更为简单,且消耗的溶液少,灵敏度更高。
1.1.1.4搅拌棒吸附萃取
搅拌棒吸附萃取(stir bar sorption extraction, SBSE)是Baltussen等[35]开发的相对新型的预处理技术,SBSE基于与SPME相同的原理,但相比SPME具有更高的聚二甲基硅氧烷(polydimethylsiloxane, PDMS)相体积,因此有更好的样本容量和萃取效率[36]。SBSE是成本相对较低的提取技术,适用于现场取样,可以在不具有复杂设备的实验室实施,Montesdeoca-Esponda等[26]从水样品中提取BUVSs,对于极性强的BUVSs回收率高(68.4%~92.2%),而对极性弱的回收率较低(18.3%~47.0%)。
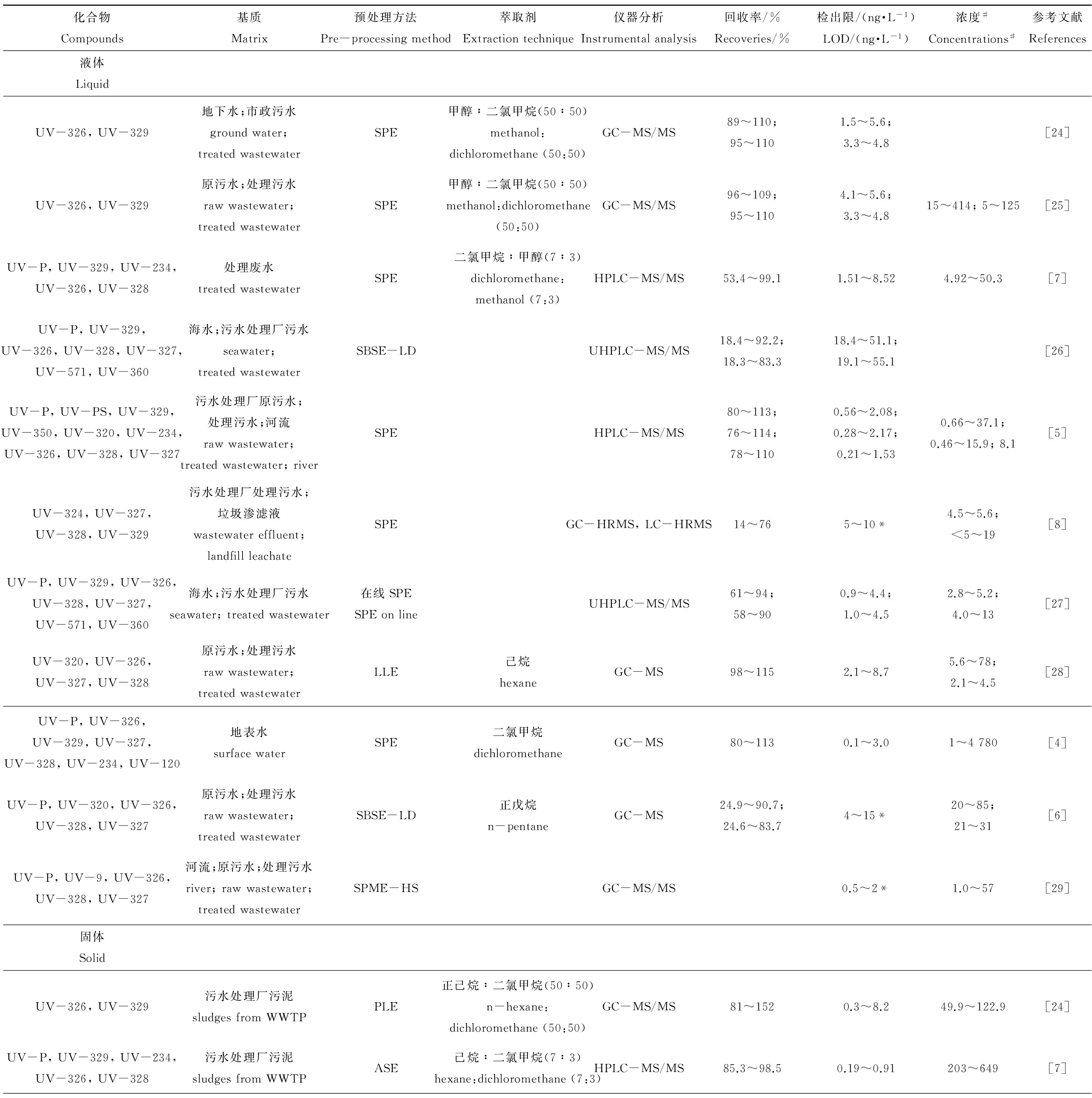
表1 不同基质中苯并三唑类紫外稳定剂(BUVSs)的分析方法Table 1 Methods of the determination of benzotriazole ultraviolet stabilizers (BUVSs) in different matrix
注:#液体浓度单位为ng·L-1,固体和生物体浓度单位为ng·g-1,* LOQ为定量限。SPE为固相萃取,SBSE-LD为搅拌棒吸附萃取-液体解吸,SPME-HS为固相微萃取-顶空,LLE为液液萃取,PLE为加压溶剂萃取,ASE为加速溶剂萃取,HSSE为快速溶剂萃取;GC-MS/MS为气相色谱-串联质谱法,LC-MS/MS为液相色谱-串联质谱法,HPLC-MS/MS为高效液相色谱-串联质谱法,UHPLC-MS/MS为超高效液相色谱-串联质谱法,GC-HRMS为气相色谱-高分辨质谱法,LC-HRMS为液相色谱-高分辨质谱法,GC-QTOF-MS为气相-四极杆飞行时间串联质谱,UFLC-MS/MS为超高速液相色谱-串联质谱法;MSPD为基质固相分散;UV-P,2-(2'-羟基-5'-甲基苯基)苯并三唑;UV-234,2-(2'-羟基-3',5'双(a,a-二甲基苄基)苯基)苯并三唑;UV-320,2-(2'-羟基-3',5'-二叔丁基苯基)-苯并三唑;UV-326,2'-(2'-羟基-3'-叔丁基-5'-甲基苯基)-5-氯苯并三唑;UV-327,2-(2'-羟基-3',5'-二特丁基苯基)-5-氯苯并三唑;UV-328,2-[2-羟基-3,5-二(1,1-二甲基丙基苯基)]-2H-苯并三唑;UV-329,2-(2'-羟基-5'-特辛基苯基)苯并三唑;UV-350,2-(2'-羟基-3'-异丁基-5'-叔丁基苯基)苯并三唑;UV-360,2,2'-亚甲基双(4-叔辛基-6-苯并三唑苯酚);UV-571,2-(2H-苯并三唑-2-基)-6-十二烷基-4-甲酚;UV-PS,2-(2H-苯并三唑-2-基)-4-(1,1-二甲基乙基)-苯酚;UV-120,3,5-二叔丁基-4-羟基苯甲酸-2,4-二叔丁基苯酯;UV-9,2-羟基-4-甲氧基二苯甲酮;UV-928,2-(2H-苯并三唑-2-基)-6-(1-甲基-1-苯乙基)-4-(1,1,3,3-四甲基丁基)苯酚;TBHPBT,2-(2H-苯并三唑-2-基)-4-(1,1-二甲基乙基)-苯酚。
Note: # concentration unit for liquid is ng·L-1, while for solid and biotas is ng·g-1; * LOQ, limits of quantification. SPE, solid-phase extraction; SBSE-LD, stir bar sorption extraction-liquid desorption; SPME-HS, solid-phase microextraction-headspace; LLE, liquid-liquid extraction; PLE, pressurized liquid extraction; ASE, accelerated solvent extraction; HSSE, high-speed solvent extraction; GC-MS/MS, gas chromatography-tandem mass spectrometry; LC-MS/MS, liquid chromatography-tandem mass spectrometry; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; UHPLC-MS/MS, ultra-high performance liquid chromatography-tandem mass spectrometry; GC-HRMS, gas chromatography-high resolution mass spectrometry; LC-HRMS, liquid chromatography-high resolution mass spectrometry; GC-QTOF-MS, gas chromatography-quadrupole time-of-flight mass spectrometry; UFLC-MS/MS, ultra-fast liquid chromatography-tandem mass spectrometry; MSPD, matrix solid-phase dispersion; UV-P, 2-(benzotriazol-2-yl)-4-methylphenol; UV-234, 2-(benzotriazol-2-yl)-4,6-bis (2-phenylpropan-2-yl)phenol; UV-320, 2-(benzotriazol-2-yl)-4,6-ditert-butylphenol; UV-326, 2-tert-butyl-6-(5-chlorobenzotriazol-2-yl)-4-methylphenol; UV-327, 2,4-ditert-butyl-6-(5-chlorobenzotriazol-2-yl)phenol; UV-328, 2-(benzotriazol-2-yl)-4,6-bis(2-methylbutan-2-yl)phenol; UV-329, 2-(benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol; UV-350, 2-(benzotriazol-2-yl)-6-butan-2-yl-4-tert-butylphenol; UV-360, 2-(benzotriazol-2-yl)-6-[[3-(benzotriazol-2-yl)-2-hydroxy-5-(2,4,4-trimethylpentan-2-yl) phenyl]methyl]-4-(2,4,4-trimethylpentan-2-yl) phenol; UV-571, 2-(benzothiazol-2-yl)-6-dodecyl-4-methyl phenol; UV-PS, 2-(5-tert-butyl-2-hydroxyphenyl) benzotriazole; UV-120, 2,4-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate; UV- 9, 2-hydroxy-4-methoxybenzophenone; UV-928, 2-(2H-benzotriazol-2-yl)-6-(1-methyl-1-phenylethyl)-4-(1,1,3,3-tetramethylbutyl)phenol; TBHPBT, 2-(5-t-butyl-2-hydroxyphenyl)benzotriazole.
1.1.2分析方法
现阶段,对于不同的环境介质,测定BUVSs所采用的方法主要有气相色谱-质谱法(gas chromatography mass spectrometry, GC-MS),气相色谱-串联质谱法(gas chromatography-tandem mass spectrometry, GC-MS/MS),液相色谱-串联质谱法(liquid chromatography-tandem mass spectrometry, LC-MS/MS)以及高效液相色谱法(high-performance liquid chromatography, HPLC)等。色谱和质谱的结合可以使其分别发挥高效的分离能力和特异的鉴别能力,在分析监测领域起着越来越重要的作用。
1.1.2.1气相色谱-质谱
GC-MS将气相色谱与质谱结合,可以同时进行有机物定量和定性的分析,已被广泛用于在不同环境基质(水、空气、土壤等)中污染物的监测。Zhang等[9]在研究沉积物和污泥中的BUVSs时采用GC-MS技术进行监测,检出限为0.1~0.5 ng·g-1,回收率范围在82%~106%。薛建平[37]用GC-MS测定纺织品中UV-320的含量,检出限可达0.05 ng·g-1,平均回收率为90.3%~103.0%。Carpinteiro等[6]进行SBSE和液体解吸后,将样品大量注入GC-MS进行分析,在离子监测(SIM)模式下测定污水基质中的6种BUVSs,定量限在4~15 ng·L-1,在原污水和处理后污水分别得到29.3%~90.7%、24.6%~83.7%的回收率。
1.1.2.2高效液相色谱
HPLC是20世纪60年代末以经典液相色谱法为基础,引入气相色谱的理论与试验方法,以液体作为流动相的色谱技术。与气相色谱法相比,HPLC具有使用范围广,分离效率高,流动相选择范围广,分析速度快等优点,现阶段已广泛应用于环境样品中有机污染物的分析。
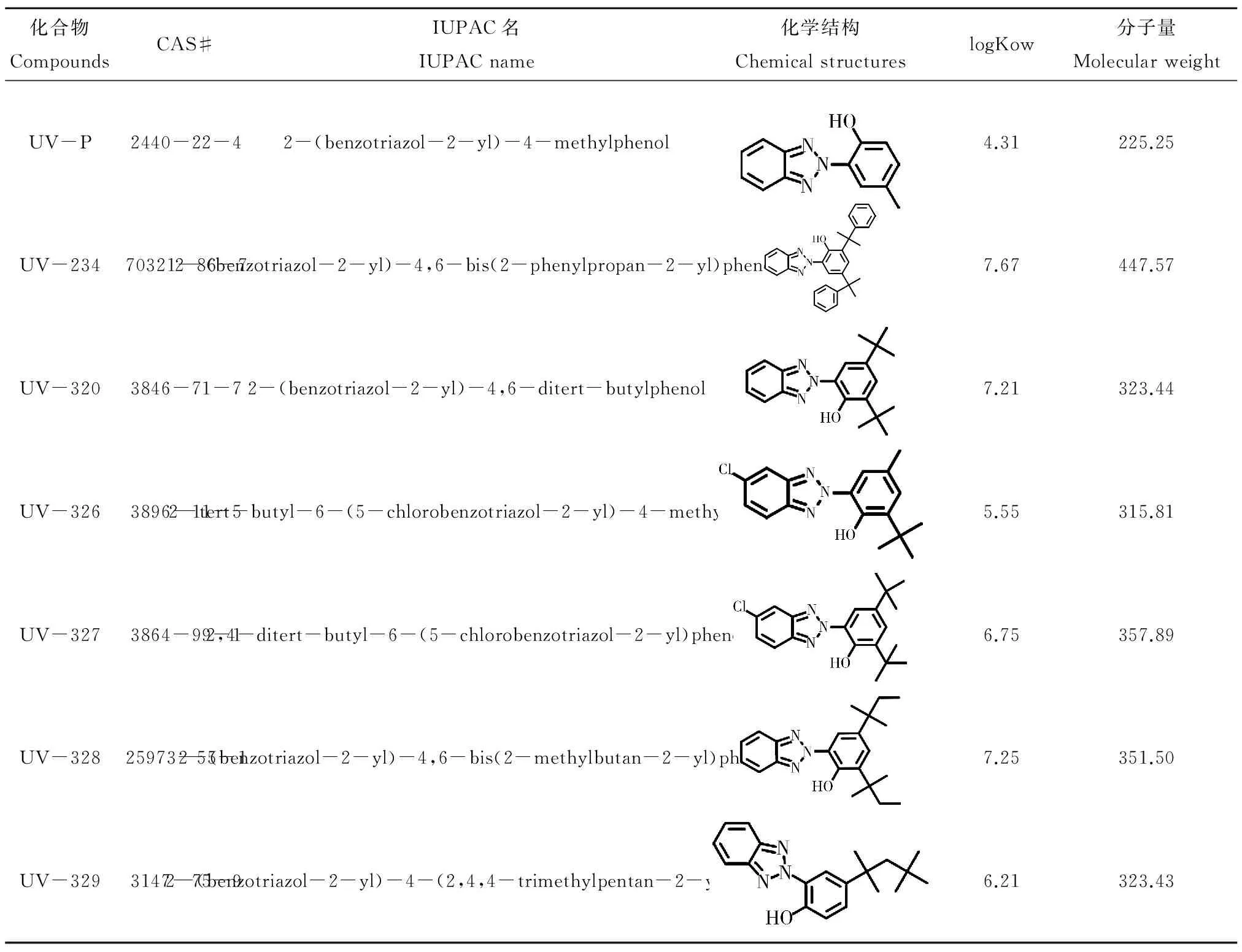
表2 典型苯并三唑类紫外稳定剂的物理化学特性Table 2 Physicochemical characteristics of the most frequently detected benzotriazole UV stabilizers
Liu等[5]使用自动在线固相萃取和高效液相色谱-串联质谱(HPLC-MS/MS)技术连用,对不同污水处理厂和河流采集的水样进行分析,监测到了UV-P, UV-329, UV-350, UV-234和UV-328这5种BUVSs,浓度高达37.1 ng·L-1,提高了UV-328和UV-327的回收率。还有Ruan等[11]用HPLC-MS/MS技术对城市污水处理厂污泥样品中的BUVSs进行检测,达到了平均93%的回收率,检出限为0.15~0.77 ng·g-1。该方法简便,实用,适用于水样中BUVSs的同时分析与监测。
超高效液相色谱(ultra-high performance liquid chromatography, UHPLC)是借助HPLC的原理,在色谱柱装填固定相、超高压输液泵等方面进行改进,实现了更快速、高分离度和高灵敏度的色谱技术。有研究使用UHPLC与串联质谱分析结合,监测鱼类[14]、污水处理厂污水以及沿海水域海水、海洋沉积物[26, 38-39]中的BUVSs。
1.2 环境介质中的BUVSs
随着分析方法的优化和检测限的降低,近年来,BUVSs在环境中不断被检出,在污水[6-7]、地表水[4-5]、污泥[10-11]、沉积物[8-9]和水生生物[2-3,13]中均发现了BUVSs的存在。环境中常检出的BUVSs主要包括UV-P, UV-234, UV-320, UV-326, UV-327, UV-328和UV-329(表2),检出浓度为ng·L-1(或ng·g-1dw)水平。根据文献报道,环境中较高浓度的BUVSs主要来源于污水、污泥和沉积物中。BUVSs在污泥和沉积物中的检出率明显高于地表水,有些BUVSs在污泥和沉积物中的浓度高达数十μg·g-1dw(表3)。相比之下,地表水中的BUVSs检出率较低,浓度在2.3~307.7 ng·L-1。尽管BUVSs的水溶性极低,但是通过污水排放、沉积物释放等,仍不断有BUVSs在地表水中检出(表3)。
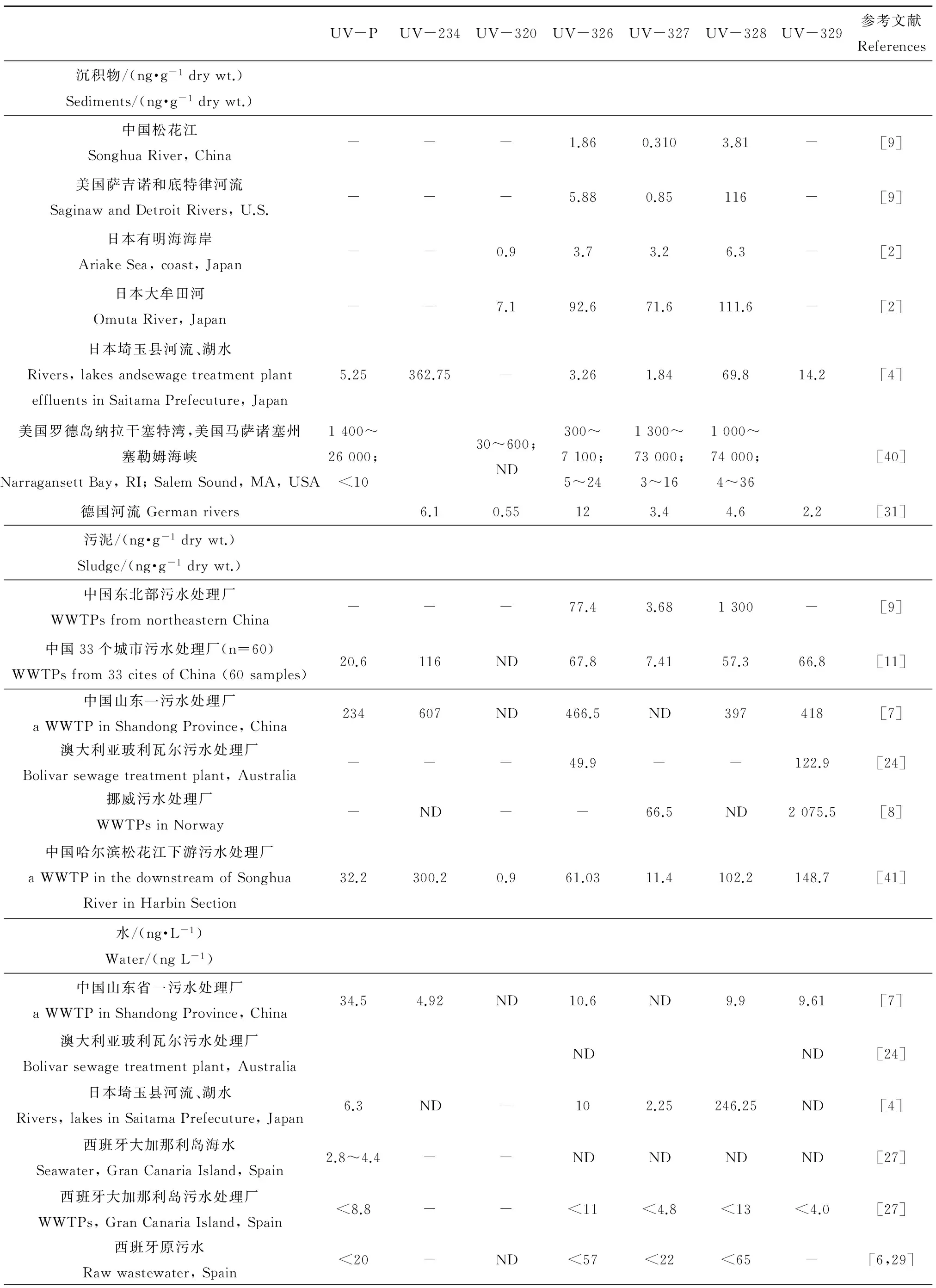
表3 沉积物、污泥、水和生物体样本中常测苯并三唑类紫外稳定剂(BUVSs)的浓度Table 3 Concentrations of most frequently detected benzotriazole ultraviolet stabilizers (BUVs) in sediment, sludge, water and biota samples
注:ND,未量化(低于定量检测限);lw,脂重;ww,湿重;dw,干重。
Note: ND, not quantified (below the limits of quantification, LOQ); lw, lipid weight; ww, wet weight; dw, dry weight.
由于BUVSs的疏水性较高(logKow>4.31,表2),在水环境中更容易吸附到污泥和沉积物中(表3)。Kameda等[4]在调查日本埼玉县水体中BUVSs的浓度时发现沉积物中UV-234的平均检出浓度为362.75 ng·g-1dw,而在水中的浓度则低于检测限。另外,许多水域的沉积物中都发现有高浓度的UV-328,这可能与UV-328的高使用量及其高的logKow(7.22)有关。在美国罗德岛纳拉干塞特湾的沉积物中,UV-328的浓度高达74 000 ng·g-1dw[40]。同时,在污泥和沉积物中,UV-P, UV-326, UV-327和UV-328是检出率最高且浓度较高的几种BUVSs。在污染严重的水体中,其平均浓度均能达到数千ng·g-1dw以上(表3)。研究显示,在沉积物中UV-326, UV-327和UV-328的浓度之间有着显著的相关性[2,4,11],说明其来源可能相似或者具有相似的环境归趋。
在污水中,UV-P, UV-326, UV-327和UV-328也是检出率最高的几种化合物,浓度为2~85 ng·L-1(表3)。而UV-234和UV-329是近几年水环境,尤其是我国污水样品中检出率较高的化合物。在污水处理厂,污水中的BUVSs浓度往往较低,而在污泥中BUVSs的浓度较高。Ruan等[11]收集了我国33个城市的污水处理厂的60个污泥样本,几乎所有样本中均检测到了UV-234和UV-329(59/60),中值浓度分别为116 ng·g-1dw和66.8 ng·g-1dw。Zhao等[41]报道,在哈尔滨某污水处理厂的污水样品中UV-234和UV-329的检测率分别为98%和100%,进水平均浓度为37.8和38.9 ng·L-1;而在污泥样品中UV-234和UV-329的检测率均为100%,且经不同工艺处理后污泥中的浓度分别为297~303.4和130.6~166.8 ng·g-1dw。
1.3 生物体中的BUVSs
由于具有高度亲脂性,BUVSs容易在生物体内富集,近年来BUVSs在海洋无脊椎动物、鱼类以及鸟类等多种生物体内检出(表3)。
UV-320和UV-327的生物富集系数(bioconcentration factor, BCF)相对较高,鲤鱼在UV-320暴露下,BCF值为1 380~10 000,与UV-320相似,UV-327的BCF值为3 400~9 000[2]。对于日本有明海的江豚(finless porpoises, Neophocaena phocaenoides),UV-327的BCF值高达为33 300,比相同区域内的小鱼(3 250)高约一个数量级[32]。基于不同的暴露,UV-326和UV-328的BCF值相对较低,为54~2 700[2]。Nakata等[2]报道了在日本有明海的无脊椎动物如平蛤、牡蛎和腹足类体内检测到0.30~80 ng·g-1ww的BUVSs。在菲律宾马尼拉湾周围市场,鱼体中UV-328, UV-P, UV-320, UV-234的检出率分别为88%、86%、79%和55%,其中UV-328的平均浓度为34.2 ng·g-1lw[3]。在亚太地区和美国沿海海域贻贝样品中的UV-326, UV-327和UV-328,根据采样点的不同,化合物浓度呈现出较大的差异,反映了BUVSs的使用率、来源及释放途径的差异[13]。UV-326, UV-327和UV-328高度亲脂,在高营养级物种生物中累积模式类似,这表明它们在海洋食物链中具有较强的持久性和生物累积性[2, 8]。
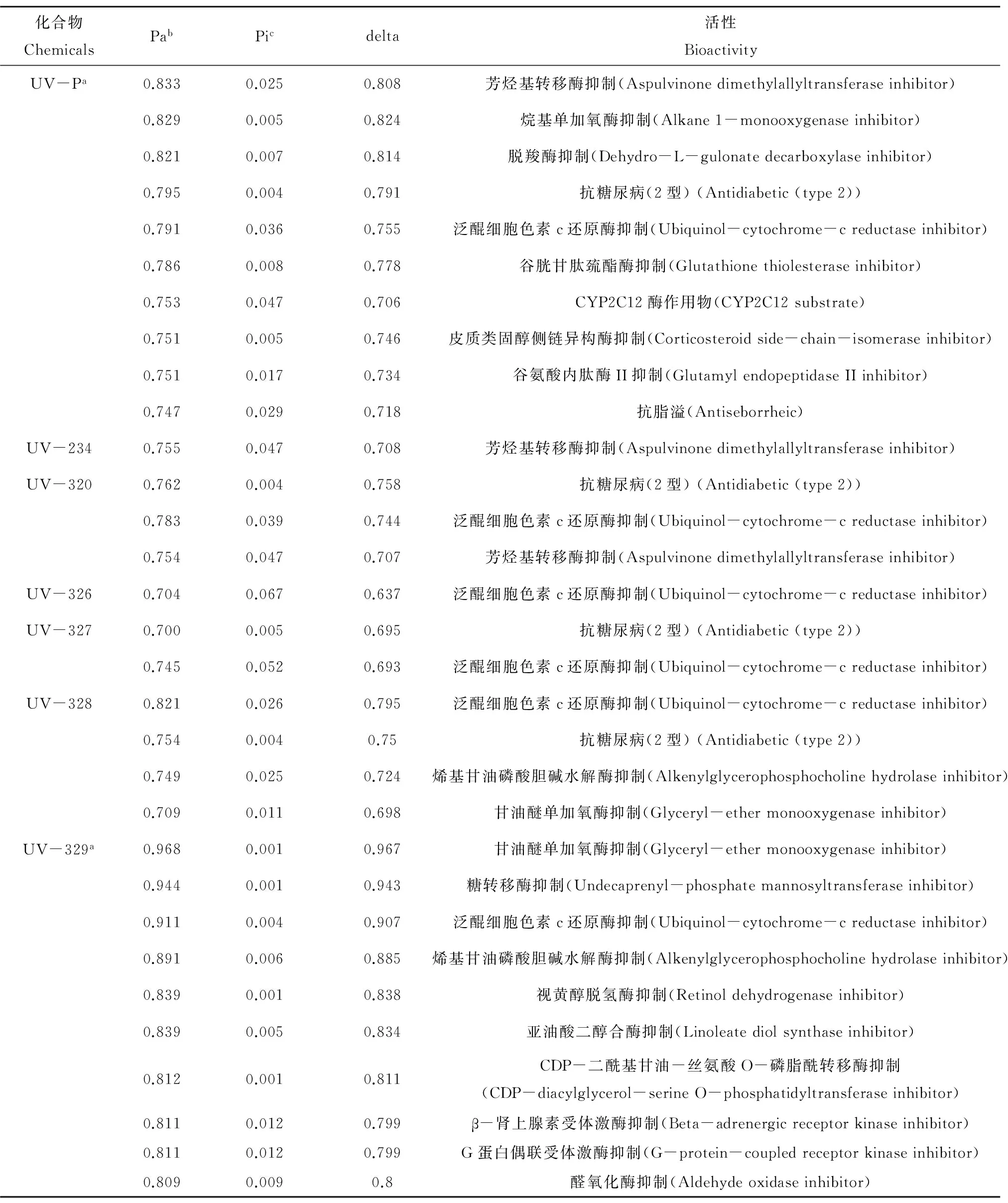
最近,有报道表明在人类母乳中也有BUVSs(UV-P, UV-9, UV-320, UV-326, UV-327, UV-328, UV-329)的存在,总浓度范围在 随着BUVSs在环境和生物体中不断被检出,其潜在的健康效应和生态毒理效应引起了广泛的关注(表4)。目前已有多个研究报道了关于苯并三唑及其衍生物对水生生物的急性毒性,研究表明其急性毒性较低,为mg·L-1水平。Pillard等[43]报道了BT对于网纹溞(Ceriodaphnia dubia)的48 h半数致死浓度LC50为102 mg·L-1,而黑头呆鱼(Pimephales promelas)对BT更为敏感,其96 h-LC50为65 mg·L-1。另外,还有研究显示5-甲基-1H-苯并三唑(5-methyl-1H-benzotriazole, 5-MeBt)对黑头呆鱼的96 h-LC50为22.0 mg·L-1,对网纹溞的48 h-LC50为81.3 mg·L-1[44]。Seeland等[45]的研究显示,在48 h BT暴露下大型溞(Daphnia magna)和盔形溞(Daphnia galeata)的半数有效浓度EC50分别为107 mg·L-1和15.8 mg·L-1,而暴露在5-MeBt下EC50分别为51.6和8.58 mg·L-1,表明盔形溞对这2种化合物更为敏感,尤其是5-MeBt。 相对于BT和其他衍生物,BUVSs在毒理学方面的信息还很少,已有报道表明其急性毒性较低(mg·L-1水平)。在淡水甲壳类动物的急性毒性实验中,UV-571对蚤状溞(Daphnia pulex)暴露24 h和48 h的LC50分别为6.35和2.59 mg·L-1,而其他BUVSs(UV-9, UV-234, UV-320, UV-326, UV-327, UV-328, UV-329, UV-360)的24 h和48 h-LC50均>10 mg·L-1[46]。同时,有报道证明UV-329对大型溞的24 h-EC50为15 mg·L-1(U.S. Environmental Protection Agency)[47]。另外,研究表明直接接触UV-P可能会引起皮炎等皮肤刺激问题[48]。 尽管BUVSs的急性毒性较低,但研究表明BUVSs具有潜在的慢性毒性,长期暴露仍可能对人类健康以及生态环境造成不利影响。BT具有植物毒性并且对沙门氏菌和大肠杆菌具有诱变性(Health Council of the Netherlands, 2000)[49]。在长期暴露下,BT和5-MeBt可对水生植物和水生无脊椎动物产生繁殖毒性和生长抑制[45]。此外,BT可干扰稀有鮈鲫(rare minnow, Gobiocypris rarus)脑组织的细胞呼吸、信号传导和细胞凋亡通路,具有潜在的神经毒性效应[50]。同时,经长期暴露,BT可影响稀有鮈鲫肝脏蛋白组的表达,干扰氧化应激、凋亡和翻译等生物学过程,并造成组织损伤,产生肝脏毒性[51]。 相比BT (logKow=1.44),BUVSs的亲脂性更强(logKow>4.31),更易在生物体内积累。例如,尽管UV-320对蚤状溞的急性毒性不高(LC50>10 mg·L-1)[46],但大鼠经UV-320 28天和52周的长期暴露,肝脏、肾脏、甲状腺和脾脏的血液指标和组织病理学均发生显著变化,且其毒性效应与性别相关[52-53]。Hirata-Koizumi等[54-55]证明这种性别差异是由于UV-320具有肝脏过氧物酶体增殖物活性,通过影响过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptor, PPARα)的表达,对雌雄个体产生不同的效应。UV-320因其生物累积性和毒性,在日本已被列为I类指定化学物质,2007年被日本政府禁止[32]。另外,雄性大鼠在25 mg·kg-1UV-327重复剂量暴露下,血清白蛋白以及白血球比值会显著升高,同时肝脏比重也会显著增加[56],还有报道显示UV-327的毒性差异与性别相关,可导致大鼠的肝细胞肥大[57],这些可能与BUVSs影响体内酶的活性增殖相关。 最近,研究表明,BT和BUVSs具有潜在的内分泌干扰效应,且不同结构的BUVSs其毒性作用机制存在差异。在体外实验(in vitro)中,Harris等[58]报道BT有明显的抗雌激素作用,而在活体实验(in vivo)中,BT对海洋青鳉(marine medaka, Oryzias melastigma)[59]和稀有鮈鲫(rare minnow, Gobiocypris rarus)[60]都显示出雌激素干扰效应;BT引起海洋青鳉VTG和CYP19A基因的表达显著上调,同时抑制CYP1A1的表达[59]。而在对稀有鮈鲫的暴露中,BT通过干扰稀有鮈鲫的HPG轴受体通路,影响血液中雌激素水平,对其产生内分泌干扰效应,造成肝脏和性腺的损伤[60]。另外,BUVSs可激活人和斑马鱼的芳香烃受体(aryl hydrocarbon receptor, AHR)通路,产生显著的抗雄激素活性[21-23]。然而,不同结构的BUVSs,其生物学活性也有差异。例如,Liang等[61]的研究显示BUVSs对斑马鱼胚胎甲状腺通路相关基因的表达产生显著影响,且不同结构的BUVSs的作用模式不同。Zhuang等[23]报道了8种BUVSs (UV-P, BT, UV-234, UV-326, UV-327, UV-328, UV-329, UV-350)在CYP3A4酶的存在下具有抗雄激素活性,UV-328经CYP3A4酶代谢后抗雄激素活性显著提高,而UV-P经代谢后抗雄激素活性降低,其他BUVSs则没有显示抗雄激素活性。这些研究表明,BUVSs的毒性效应、毒性作用方式和作用机制可能与其结构相关,因此研究不同结构的BUVSs的生物活性对于正确评估其对生物体的毒性作用具有重要的意义。 为考察不同结构的BUVSs的毒性效应,利用PASS (Prediction of Activity Spectra for Substances, http://www.way2drug.com/PASSOnline/index.php)对环境中典型BUVSs (UV-P, UV-234, UV-320, UV-326, UV-327, UV-328和UV-329)的生物活性进行了预测。将各化合物的Canonical SMILES输入PASS预测程序,即可得到相应的生物活性的预测结果[62-63]。在输出的结果中,Pa (probability "to be active")表示预测化合物具有该活性的可能性,Pi (probability "to be inactive")表示预测化合物不具有该活性的可能性,通过Pa值和Pi值进行筛选得到相应化合物具有的可能性较高的生物活性,以预测结果显示,UV-P和UV-329的生物活性种类较多,Pa >0.7的预测生物学活性分别为13和23个,而其他BUVSs Pa >0.7的预测生物学活性只有1~4个(表5)。比较各化合物预测生物学活性,UV-P具有较强的芳烃基转移酶抑制(aspulvinone dimethylallyltransferase inhibitor),烷基单加氧酶抑制(alkane 1-monooxygenase inhibitor)和脱羧酶抑制(dehydro-L-gulonate decarboxylase inhibitor)的活性(delta >0.8,表5);而UV-329具有强烈的甘油醚单加氧酶抑制(glyceryl-ether monooxygenase inhibitor),糖转移酶(undecaprenyl-phosphate mannosyltransferase inhibitor)和泛醌细胞色素c还原酶抑制(ubiquinol-cytochrome-c reductase inhibitor)活性(delta >0.9,表5)。相比于UV-P和UV-329,其他BUVSs的预测生物活性稍弱(delta <0.8,表5)。 表4 BUVSs对于不同生物体的毒性效应Table 4 Toxicity effects of BUVSs on different organisms 注: BT,苯并三唑;UV-090,2-[3-(2H-苯并三唑-2-基)-4-羟基苯基]乙基-2-甲基丙烯酸酯。 Note: BT, benzotriazole; UV-090, 2-[3-(2H-benzotriazol-2-yl)-4- hydroxyphenyl]ethyl methacrylate. 表5 PASS(Prediction of Activity Spectra of Substances)预测的BUVSs的生物学活性Table 5 The theoretical bioactivities of BUVSs estimated using Prediction of Activity Spectra of Substances (PASS) 注:a. 如果生物学活性种类>5,只显示Pa值最高的10项(UV-P和UV-329,Pa >0.7的预测生物学活性分别为13、23个);b. Pa (probability "to be active")表示预测化合物具有该活性的可能性;c. Pi (probability "to be inactive")表示预测化合物不具有该活性的可能性;delta = Pa - Pi。 Note: a. If the biological activity type is>5, only show the top 10 of the Pa value (UV-P and UV-329, the predicted biological activity of Pa >0.7 was 13, 23 respectively); b. Pa (probability "to be active") indicates that the predicted compound has the possibility of this activity; c. Pi (probability "to be inactive") indicates that the predicted compound doesn’t have the possibility of this activity; delta = Pa - Pi. 该化合物的毒性作用方式。各BUVSs的Canonical SMILES如下:UV-P,CC1=CC(=C(C=C1)O)N2N=C3C=CC=CC3=N2;UV-234,CC(C)(C1=CC=CC=C1)C2=CC(=C(C(=C2)N3N=C4C=CC=CC4=N3)O)C(C)(C)C5=CC=CC=C5;UV-320,CC(C)(C)C1=CC(=C(C(=C1)N2N=C3C=CC=CC3=N2)O)C(C)(C)C;UV-326,CC1=CC(=C(C(=C1)N2N=C3C=CC(=CC3=N2)Cl)O)C(C)(C)C;UV-327,CC(C)(C)C1=CC(=C(C(=C1)N2N=C3C=CC(=CC3=N2)Cl)O)C(C)(C)C;UV-328,CCC(C)(C)C1=CC(=C(C(=C1)N2N=C3C=CC=CC3=N2)O)C(C)(C)CC;UV-329,CC(C)(C)CC(C)(C)C1=CC(=C(C=C1)O)N2N=C3C=CC=CC3=N2。 值得注意的是,UV-P, UV-234, UV-320和UV-329 (Pa =0.742,表5未显示)都具有芳烃基转移酶抑制(aspulvinone dimethylallyltransferase inhibitor)活性,该酶催化二甲基烯丙基焦磷酸酯(dimethylallyl pyrophosphate)芳香基团的转移[64],而该化合物参与固醇类的生物合成[65-66]。同时,已有研究证明UV-P和UV-326可以激活人和斑马鱼的AHR活性[21],这些结果表明这4种BUVSs可能通过干扰固醇合成,产生内分泌干扰作用;另外,PASS预测结果显示除UV-234外,所有BUVSs都具有泛醌细胞色素c还原酶抑制(ubiquinol-cytochrome-c reductase inhibitor)活性(表5),该酶是线粒体呼吸电子传递链中的重要组分[67],这表明BUVSs很可能作用于线粒体,抑制线粒体呼吸,产生毒性作用。 (1) BUVSs的毒性数据仍然十分有限,毒性作用机制尚不清晰,尤其是对于不同结构的BUVSs,其毒性作用方式可能存在差异,因此进一步开展对BUVSs毒性效应和毒性通路的研究,有利于阐明其对人和其他生物的毒性作用机制。 (2) 根据PASS预测的结果,BUVSs可能作用于线粒体并产生毒性作用,而目前还未见相关报道,因此进一步开展BUVSs对线粒体作用的研究可为探明此类化合物的毒性作用模式提供新的视角。 (3) 尽管现有毒性数据与PASS预测均表明BUVSs可能对人和其他生物产生毒性效应,但是当前关于BUVSs的潜在生态风险和健康风险的研究仍相对较少。最近Molins-Delgado等[68]应用风险商(hazard quotient, HQs)对西班牙巴塞罗那附近水域的BT和MeBT (methylbenzotriazole)进行风险评估,结果表明污水出水中BT和MeBT的HQs >1,对水生态环境具有一定的风险,而BUVSs的生态风险研究还未见报道,亟待开展。 参考文献(References): [1]Montesdeoca-Esponda S, Vega-Morales T, Sosa-Ferrera Z, et al. Extraction and determination methodologies for benzotriazole UV stabilizers in personal-care products in environmental and biological samples [J]. Trac-Trends in Analytical Chemistry, 2013, 51: 23-32 [2]Nakata H, Murata S, Filatreau J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan [J]. Environmental Science & Technology, 2009, 43(18): 6920-6926 [3]Kim J W, Isobe T, Ramaswamy B R, et al. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography-tandem mass spectrometry [J]. Chemosphere, 2011, 85(5): 751-758 [4]Kameda Y, Kimura K, Miyazaki M. Occurrence and profiles of organic sun-blocking agents in surface waters and sediments in Japanese rivers and lakes [J]. Environmental Pollution, 2011, 159(6): 1570-1576 [5]Liu R Z, Ruan T, Wang T, et al. Determination of nine benzotriazole UV stabilizers in environmental water samples by automated on-line solid phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry [J]. Talanta, 2014, 120: 158-166 [6]Carpinteiro I, Ramil M, Rodriguez I, et al. Combining stir-bar sorptive extraction and large volume injection-gas chromatography-mass spectrometry for the determination of benzotriazole UV stabilizers in wastewater matrices [J]. Journal of Separation Science, 2012, 35(3): 459-467 [7]Song S J, Ruan T, Wang T, et al. Occurrence and removal of benzotriazole ultraviolet stabilizers in a wastewater treatment plant in China [J]. Environmental Science-Processes & Impacts, 2014, 16(5): 1076-1082 [8]Langford K H, Reid M J, Fjeld E, et al. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway [J]. Environment International, 2015, 80: 1-7 [9]Zhang Z F, Ren N Q, Li Y F, et al. Determination of benzotriazole and benzophenone UV filters in sediment and sewage sludge [J]. Environmental Science & Technology, 2011, 45(9): 3909-3916 [10]Casado J, Rodriguez I, Carpinteiro I, et al. Gas chromatography quadrupole time-of-flight mass spectrometry determination of benzotriazole ultraviolet stabilizers in sludge samples [J]. Journal of Chromatography A, 2013, 1293: 126-132 [11]Ruan T, Liu R Z, Fu Q, et al. Concentrations and composition profiles of benzotriazole UV stabilizers in municipal sewage sludge in China [J]. Environmental Science & Technology, 2012, 46(4): 2071-2079 [12]Lai H J, Ying G G, Ma Y B, et al. Occurrence and dissipation of benzotriazoles and benzotriazole ultraviolet stabilizers in biosolid-amended soils [J]. Environmental Toxicology and Chemistry, 2014, 33(4): 761-767 [13]Nakata H, Shinohara R I, Nakazawa Y, et al. Asia-Pacific mussel watch for emerging pollutants: Distribution of synthetic musks and benzotriazole UV stabilizers in Asian and US coastal waters [J]. Marine Pollution Bulletin, 2012, 64(10): 2211-2218 [14]Kim J W, Ramaswamy B R, Chang K H, et al. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry [J]. Journal of Chromatography A, 2011, 1218(22): 3511-3520 [15]Avagyan R, Luongo G, Thorsen G, et al. Benzothiazole, benzotriazole, and their derivates in clothing textiles—A potential source of environmental pollutants and human exposure [J]. Environmental Science and Pollution Research, 2015, 22(8): 5842-5849 [16]Kim J W, Isobe T, Malarvannan G, et al. Contamination of benzotriazole ultraviolet stabilizers in house dust from the Philippines: Implications on human exposure [J]. Science of the Total Environment, 2012, 424: 174-181 [17]Lee S, Kim S, Park J, et al. Synthetic musk compounds and benzotriazole ultraviolet stabilizers in breast milk: Occurrence, time-course variation and infant health risk [J]. Environmental Research, 2015, 140: 466-473 [18]Asimakopoulos A G, Bletsou A A, Wu Q, et al. Determination of benzotriazoles and benzothiazoles in human urine by liquid chromatography-tandem mass spectrometry [J]. Analytical Chemistry, 2013, 85(1): 441-448 [19]Asimakopoulos A G, Wang L, Thomaidis N S, et al. Benzotriazoles and benzothiazoles in human urine from several countries: A perspective on occurrence, biotransformation, and human exposure [J]. Environment International, 2013, 59: 274-281 [20]Wang L, Asimakopoulos A G , Kannan K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue [J]. Environment International, 2015, 78: 45-50 [21]Fent K, Chew G, Li J, et al. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos [J]. Science of the Total Environment, 2014, 482: 125-136 [22]Nagayoshi H, Kakimoto K, Takagi S, et al. Benzotriazole ultraviolet stabilizers show potent activities as human aryl hydrocarbon receptor ligands [J]. Environmental Science & Technology, 2015, 49(1): 578-587 [23]Zhuang S L, Lv X, Pan L M, et al. Benzotriazole UV 328 and UV-P showed distinct antiandrogenic activity upon human CYP3A4-mediated biotransformation [J]. Environmental Pollution, 2017, 220(0269-7491): 616-624 [24]Liu Y S, Ying G G, Shareef A, et al. Simultaneous determination of benzotriazoles and ultraviolet filters in ground water, effluent and biosolid samples using gas chromatography-tandem mass spectrometry [J]. Journal of Chromatography A, 2011, 1218(31): 5328-5335 [25]Liu Y S, Ying G G, Shareef A, et al. Occurrence and removal of benzotriazoles and ultraviolet filters in a municipal wastewater treatment plant [J]. Environmental Pollution, 2012, 165: 225-232 [26]Montesdeoca-Esponda S, del Toro-Moreno A, Sosa-Ferrera Z, et al. Development of a sensitive determination method for benzotriazole UV stabilizers in enviromental water samples with stir bar sorption extraction and liquid desorption prior to ultra-high performance liquid chromatography with tandem mass spectrometry [J]. Journal of Separation Science, 2013, 36(13): 2168-2175 [27]Montesdeoca-Esponda S, Sosa-Ferrera Z, Santana-Rodriguez J J. On-line solid-phase extraction coupled to ultra-performance liquid chromatography with tandem mass spectrometry detection for the determination of benzotriazole UV stabilizers in coastal marine and wastewater samples [J]. Analytical and Bioanalytical Chemistry, 2012, 403(3): 867-876 [28]Nakata H, Shinohara R. Concentrations of benzotriazole UV stabilizers and polycyclic musks in wastewater treatment plant samples in Japan [J]. Interdisciplinary Studies on Environmental Chemistry, 2010, 4: 51-59 [29]Carpinteiro I, Abuin B, Rodriguez I, et al. Headspace solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the sensitive determination of benzotriazole UV stabilizers in water samples [J]. Analytical and Bioanalytical Chemistry, 2010, 397(2): 829-839 [30]Reddy C M, Quinn J G, King J W. Free and bound benzotriazoles in marine and freshwater sediments [J]. Environmental Science & Technology, 2000, 34(6): 973-979 [31]Wick A, Jacobs B, Kunkel U, et al. Benzotriazole UV stabilizers in sediments, suspended particulate matter and fish of German rivers: New insights into occurrence, time trends and persistency [J]. Environmental Pollution, 2016, 212: 401-412 [32]Nakata H, Shinohara R, Murata S, et al. Detection of benzotriazole UV stabilizers in the blubber of marine mammals by gas chromatography-high resolution mass spectrometry (GC-HRMS) [J]. Journal of Environmental Monitoring, 2010, 12(11): 2088-2092 [33]刘长武, 翟广书, 买光熙, 等. 固相萃取技术的原理及进展[J]. 农业环境与发展, 2003(1): 42-44 [34]王金成, 张海军, 陈吉平. 固相萃取高效液相色谱-串联质谱法测定地表水中苯并三唑类及苯并噻唑类衍生物[J]. 分析测试学报, 2013, 32(9): 1056-1061 Wang J C, Zhang H J, Chen J P. Simultaneous determination of benzotriazoles and benzothiazoles in surface water by solid phase extraction and high performance liquid chromatography-tandem mass spectrometry [J]. Journal of Instrumental Analysis, 2013, 32(9): 1056-1061 (in Chinese) [35]Baltussen E, Sandra P, David F, et al. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles [J]. Journal of Microcolumn Separations, 1999, 11(10): 737-747 [36]Fries E. Determination of benzothiazole in untreated wastewater using polar-phase stir bar sorptive extraction and gas chromatography-mass spectrometry [J]. Analytica Chimica Acta, 2011, 689(1): 65-68 [37]薛建平. GC-MS测定苯并三唑类紫外线吸收剂[J]. 印染, 2013, 39(6): 42-44 Xue J P. Determination of benzotriazole ultraviolet absorber in textiles by GC-MS [J]. Dyeing & Finishing, 2013, 39(6): 42-44 (in Chinese) [38]Montesdeoca-Esponda S, Sosa-Ferrera Z, Kabir A, et al. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples [J]. Analytical and Bioanalytical Chemistry, 2015, 407(26): 8137-8150 [39]Montesdeoca-Esponda S, Sosa-Ferrera Z, Santana-Rodriguez J J. Microwave-assisted extraction combined with on-line solid phase extraction followed by ultra-high-performance liquid chromatography with tandem mass spectrometric determination of benzotriazole UV stabilizers in marine sediments and sewage sludges [J]. Journal of Separation Science, 2013, 36(4): 781-788 [40]Cantwell M G, Sullivan J C, Katz D R, et al. Source determination of benzotriazoles in sediment cores from two urban estuaries on the Atlantic Coast of the United States [J]. Marine Pollution Bulletin, 2015, 101(1): 208-218 [41]Zhao X, Zhang Z F, Xu L, et al. Occurrence and fate of benzotriazoles UV filters in a typical residential wastewater treatment plant in Harbin, China [J]. Environmental Pollution, 2017, 227: 215-222 [42]Lu Z, Peart T E, Cook C J, et al. Simultaneous determination of substituted diphenylamine antioxidants and benzotriazole ultra violet stabilizers in blood plasma and fish homogenates by ultra high performance liquid chromatography-electrospray tandem mass spectrometry [J]. Journal of Chromatography A, 2016, 1461: 51-58 [43]Pillard D A, Cornell J S, Dufresne D L, et al. Toxicity of benzotriazole and benzotriazole derivatives to three aquatic species [J]. Water Research, 2001, 35(2): 557-560 [44]Cancilla D A, Baird J C, Geis S W, et al. Studies of the environmental fate and effect of aircraft deicing fluids: Detection of 5-methyl-1H-benzotriazole in the fathead minnow (Pimephales promelas) [J]. Environmental Toxicology and Chemistry, 2003, 22(1): 134-140 [45]Seeland A, Oetken M, Kiss A, et al. Acute and chronic toxicity of benzotriazoles to aquatic organisms [J]. Environmental Science and Pollution Research, 2012, 19(5): 1781-1790 [46]Kim J W, Chang K H, Isobe T, et al. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnia pulex) [J]. Journal of Toxicological Sciences, 2011, 36(2): 247-251 [47]U.S Environmental Protection Agency. Detailed chemical results. (2011-07-26) [2017-08-17]. https://iaspub.epa.gov/oppthpv/quicksearch.display?pChem=100707 [48]Niklasson B, Bjorkner B. Contact allergy to the UV-absorber Tinuvin P in plastics [J]. Contact Dermatitis, 1989, 21(5): 330-334 [49]Health Council of The Netherlands: Dutch Expert Committee on Occupational Standards (DECOS). 1,2,3-Benzotriazole. Publication no. 2000/14OSH [R]. The Hague: Health Council of The Netherlands, 2000 [50]Liang X F, Martyniuk C J, Zha J M, et al. Brain quantitative proteomic responses reveal new insight of benzotriazole neurotoxicity in female Chinese rare minnow (Gobiocypris rarus) [J]. Aquatic Toxicology, 2016, 181: 67-75 [51]Liang X, Zha J, Martyniuk C J, et al. Histopathological and proteomic responses in male Chinese rare minnow (Gobiocypris rarus) indicate hepatotoxicity following benzotriazole exposure [J]. Environmental Pollution, 2017, 229: 459-469 [52]Hirata-Koizumi M, Watari N, Mukai D, et al. A 28-day repeated dose toxicity study of ultraviolet absorber 2-(2'-hydroxy-3',5'-di-tert-butylphenyl) benzotriazole in rats [J]. Drug and Chemical Toxicology, 2007, 30(4): 327-341 [53]Hirata-Koizumi M, Ogata H, Imai T, et al. A 52-week repeated dose toxicity study of ultraviolet absorber 2-(2'-hydroxy-3',5'-di-tert-butylphenyl)benzotriazole in rats [J]. Drug and Chemical Toxicology, 2008, 31(1): 81-96 [54]Hirata-Koizumi M, Matsuno K, Kawabata M, et al. Gender-related difference in the toxicity of 2-(2'-hydroxy-3',5'-di-tert-butylphenyl)benzotriazole in rats: Relationship to the plasma concentration, in vitro hepatic metabolism, and effects on hepatic metabolizing enzyme activity [J]. Drug and Chemical Toxicology, 2009, 32(3): 204-214 [55]Hirata-Koizumi M, Ise R, Kato H, et al. Transcriptome analyses demonstrate that peroxisome proliferator-activated receptor α (PPARα) activity of an ultraviolet absorber, 2-(2'-hydroxy-3',5'-di-tert-butylphenyl) benzotriazole, as possible mechanism of their toxicity and the gender differences [J]. Journal of Toxicological Sciences, 2016, 41(5): 693-700 [56]Ema M, Fukunishi K, Hirose A, et al. Repeated-dose and reproductive toxicity of the ultraviolet absorber 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)-5-chlorobenzotriazole in rats [J]. Drug and Chemical Toxicology, 2008, 31(3): 399-412 [57]Hirata-Koizumi M, Matsuyama T, Imai T, et al. Gender-related difference in the toxicity of ultraviolet absorber 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)-5-chlorobenzotriazole in rats [J]. Drug and Chemical Toxicology, 2008, 31(3): 383-398 [58]Harris C A, Routledge E J, Schaffner C, et al. Benzotriazole is antiestrogenic in vitro but not in vivo [J]. Environmental Toxicology and Chemistry, 2007, 26(11): 2367-2372 [59]He T T, Liang B, Liu W H, et al. Estrogenic potential of benzotriazole on marine medaka (Oryzias melastigma) [J]. Ecotoxicology and Environmental Safety, 2012, 80: 327-332 [60]Liang X F, Wang M, Chen X, et al. Endocrine disrupting effects of benzotriazole in rare minnow (Gobiocypris rarus) in a sex-dependent manner [J]. Chemosphere, 2014, 112: 154-162 [61]Liang X, Li J, Martyniuk C J, et al. Benzotriazole ultraviolet stabilizers alter the expression of the thyroid hormone pathway in zebrafish (Danio rerio) embryos [J]. Chemosphere, 2017, 182: 22-30 [62]Filimonov D A, Lagunin A A, Gloriozova T A, et al. Prediction of the biological activity spectra of organic compounds using the PASS online web resource [J]. Chemistry of Heterocyclic Compounds, 2014, 50(3): 444-457 [63]Lagunin A, Stepanchikova A, Filimonov D, et al. PASS: Prediction of activity spectra for biologically active substances [J]. Bioinformatics, 2000, 16(8): 747-748 [64]Takahashi I, Ojima N, Ogura K, et al. Purification and characterization of dimethylallyl pyrophosphate-aspulvinone dimethylallyltransferase from aspergillus-terreus [J]. Biochemistry, 1978, 17(13): 2696-2702 [65]Clayton R B. Biosynthesis of sterols, steroids, and terpenoids. Part I. Biogenesis of cholesterol and the fundamental steps in terpenoid biosynthesis [J]. Quarterly Reviews Chemical Society, 1965, 19(2): 168-200 [66]Jeong J W, Kwak I, Lee K Y, et al. The genomic analysis of the impact of steroid receptor coactivators ablation on hepatic metabolism [J]. Molecular Endocrinology, 2006, 20(5): 1138-1152 [67]Palsdottir H, Lojero C G, Trumpower B L, et al. Structure of the yeast cytochrome bc(1) complex with a hydroxyquinone anion Q(o) site inhibitor bound [J]. Journal of Biological Chemistry, 2003, 278(33): 31303-31311 [68]Molins-Delgado D, Tavora J, Diaz-Cruz M, et al. UV filters and benzotriazoles in urban aquatic ecosystems: The footprint of daily use products [J]. Science of the Total Environment, 2017, 601: 975-9862 BUVSs的毒性效应(Toxicological effects of BUVSs)
2.1 急性毒性
2.2 慢性毒性
3 BUVSs的生物活性预测(Bioactivity prediction of BUVSs)

4 展望(Future prospects)

