Effect of acupuncture on hippocampal mitochondrial proteome expression in SAMP8 mouse model with Alzheimer disease
Alzheimer disease (AD) is a neurodegenerative disease characterized by progressive cognitive decline.A large number of senile plaques (SPs) and neuronal fibrillary tangles (NFTs), as well as the extensive loss of neurons are the main pathological manifestations.Etiology of AD has not yet been identified. It currently cannot be cured[1]. A study has shown that neuronal cells have emerged a series of abnormal changes in mitochondrial function during the development of AD[2].Energy metabolism damage, reduced ATP production,and a large number of oxygen free radicals, leading to widespread and lasting oxidative stress damage, far before the formation of the characteristic pathological changes, such as Aβ aggregation, NFTs and synaptic dysfunction. Based on the central role of mitochondria in brain oxidative stress of AD patients, Swerdlow RH,et al[3]first proposed the ‘mitochondrial cascade hypothesis’ in 2004. It is believed that the abnormal changes of mitochondrial structure and function play important roles in the pathological process of AD, which may be the trigger event of AD occurrence. Senescenceaccelerated mouse prone 8 (SAMP8) is one of the ideal animal models of AD and begins to show age-related memory and learning decline at an early stage[4]. The characteristic pathological manifestations of AD such as Aβ deposition, abnormal tau phosphorylation and neuronal loss have also been demonstrated in SAMP8 mice[5]. In addition, elevated levels of mitochondrial dysfunction and oxidative stress markers are also evident in SAMP8 mice[6]. Therefore, SAMP8 mice can also be used as an ideal model for studying the important mechanisms of mitochondria in the pathogenesis of AD.
In recent years, acupuncture has shown significant effect in the clinical treatment of AD[7-8]. Studies have found that acupuncture has the characteristics of multi-target and multi-step treatment. Acupuncture has more advantages than single-target treatment for AD,which is an unclear etiology and multi-factor-related complicated disease. Our previous clinical trials found that acupuncture at Shenshu (BL 23), Baihui (GV 20),Xuehai (SP 10), Geshu (BL 17) and other acupoints effectively improved the memory, cognitive function and daily living ability of AD patients[9]; reduced the isoprostaglandin level in peripheral blood and urine of AD patients, so as to reduce the oxidative stress[10]. At the same time, previous animal studies have shown that acupuncture could shorten the escape latency in the water maze test and improve learning and memory in SAMP8 mice with AD[11-12]. In addition, we compared the effect of acupuncture on protein expression in the hippocampus of SAMP8 mice using comparative proteomics, and identified mitochondrial aconitine hydratase, cytochrome C oxidase[13], and neuroglobin[14],and other differentially expressed proteins related to oxidative stress. Mitochondria are the main source of oxygen free radicals in cells, indicating that mitochondria may be the main subcellular organelle target for acupuncture to play a therapeutic role.Based on this, we used the technique of subcellular organellar proteomics to observe the effect of acupuncture on the proteome expression of hippocampal mitochondria in SAMP8 mice, and to explore the possible mechanism of acupuncture for AD.
1 Materials and Methods
1.1 Laboratory animals and groups
Sixty male SAMP8 mice and 20 male senescenceaccelerated mouse resistant 1 (SAMR1) mice aged 6 months were purchased from the Animal Experimental Center of the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine. Laboratory animal quality certificate: No. 0004582. The mice are of clean grade, weighing (25±3.5) g. The mice were housed in cage padded with sawdust with free access to food and drinking water, at room temperature of 18-22 ℃,relative humidity of 40%-50%, and with adequate lighting and ventilation. After adaptive feeding for 1 week, the SAMP8 mice were divided into an acupuncture at acupoint group, an acupuncture at non-acupoint group and a model group according to random number table, 20 mice in each group. The 20 SAMR1 mice were used as a normal control group. This experiment was approved by the Experimental Animal Ethics Committee of Xiangya Third Hospital, Central South University [license number: LLSC (LA) 2014-030].The experiment was conducted in accordance with theGuiding Opinions on the Treatment of Experimental Animalsissued by the Ministry of Science and Technology in 2006.
1.2 Intervention methods
1.2.1 Acupuncture at acupoint group
Acupoints: Baihui (GV 20), Xuehai (SP 10), Shenshu(BL 23) and Geshu (BL 17), (Figure 1).

Figure 1. Positioning of acupoints
Methods: The location of acupoints in mice and the depth of acupuncture were referred to the acupoint map of animals in theExperimental Acupuncture Science[15]. Baihui (GV 20) was stimulated by horizontal insertion of needle backward into 3-5 mm. Xuehai(SP 10) and Shenshu (BL 23) were stimulated by perpendicular needling into 2-3 mm. Geshu (BL 17) was stimulated by insertion of needle upward with an 80°angle into 2-3 mm. The left Xuehai (SP 10), Shenshu(BL 23) and Geshu (BL 17) were stimulated on the first day, then the right ones on the second day, alternately on the two sides.
After needle insertion, the reinforcing and reducing method was performed according to the quantitative standard of acupuncture manipulation[16]: Xuehai (SP 10)and Geshu (BL 17) were applied with twirling reducing method, with a twisting amplitude of 180-360° and twisting frequency of 50-60 times/min; Shenshu (BL 23)and Baihui (GV 20) were applied with twirling reinforcing method, with a twisting amplitude of 60-90°,twisting frequency of 120-150 times/min, needle manipulation for 1min and needle retaining for 10 min.1.2.2 Acupuncture at non-acupoint group
Non-acupoint and non-meridian points: The fixed bilateral non-acupoint and non-meridian points below the costal region (3 mm above iliac crest and below bilateral costal regions, Figure 1).
Methods: The stimulation was performed by twisting the needle with even reinforcing-reducing method, the amplitude of 90°, the frequency of 90 times/min,needle manipulation for 1 min and needle retaining for 10 min.
1.2.3 Model group and normal control group
Mice in the model and normal control groups received grasp stimulations with the same time and degree as the two groups mentioned above.
Daily intervention was conducted for the mice in each group and had a 1-day rest after continuous 6 d intervention, for a total of 8 weeks intervention.
1.3 The main experimental reagents and equipments
Mitochondrial protein extraction kit (Nanjing Jiancheng Bioengineering Institute, China);bicinchoninic acid (BCA) protein concentration assay kit(Biyuntian Biotechnology Research Institute, China);neurofilament light polypeptide (NFL) antibody, ATP synthase beta subunit (ATP-β) antibody, tubulin beta-2A chain (TBB2A) antibody and NADH-ubiquinone oxidoreductase 75 kDa subunit (database ID: NDUS1)antibody (Abcam, UK); horseradish peroxidase-labeled goat anti-rabbit IgG (Jackson, USA); ECL chemiluminescent substrate (Thermo, USA);dithiothreitol (DTT) and complex two-dimensional gel electrophoresis reagent and protease inhibitor PMSF(Amresco, USA); acetone (J. T. Baker, USA); ammonium bicarbonate (NH4HCO3) for complex mass spectrometer assay, 5 µg/µL of fetal bovine serum (BSA) standard(Sigma, USA); IPG Buffer (pH 4-7, GE, Sweden); dried strip (24 cm, pH 4-7, BIORAD, USA); methanol,acetonitrile (ACN, Fisher, USA); pancreatin and trifluoroacetic acid TFA (Promega, USA); Imagescanner II scanner, ImageMaster 2D platinum 5.0 gel image analysis software (Bio-Rad, USA); Voyager-DE STR 4307 MALDI-TOF-MS mass spectrometer (Applied Biosystem,USA); Mascot MS/MS database search software (Matrix Science, UK); Hwato Brand acupuncture needles of 0.25 mm in diameter and 13 mm in length (Suzhou Medical Appliance Factory, China).
1.4 Detection of the differential protein expression in hippocampus mitochondria by proteomic method
After 8 weeks of acupuncture intervention, the mice were anesthetized with 10% chloral hydrate[300 mg/(kg·bw)]. The brains were harvested and the hippocampus was isolated after dislocation. The mitochondria were isolated and purified using the mitochondrial protein extraction kit. The purified mitochondria were centrifuged, sonicated, and centrifuged again to extract the mitochondrial protein,stored at –80 ℃ for later use. The total protein of hippocampal mitochondria in each group was separated by two-dimensional (2-DE) gel electrophoresis for three times. A total of 3 pieces of 2-DE maps with clear background, high resolution and good repeatability were obtained after silver staining and coomassie brilliant blue staining. ImageMaster 2D platinum 5.0 software was used for image analysis. The area and gray information of the identified protein spots were fitted into a new gel, and then the matching analysis was performed on the basis of the fitted gel map. The differentially expressed spots were selected according to the criteria: more than 2-time up-regulation or down-regulation of the differentially expressed proteins appeared at least in three different experiments. The protein spots were cut from the gel and put in a 1.5 mL Eppendorf tube. The prepared samples were analyzed by a MALDI-TOF-MS mass spectrometer. Mascot MS/MS database was searched on line using the term containing the peptide mass fingerprinting ‘*.DAT’. The software was automatically linked to the website of http://www.matrixscience.com and returned the query results.
1.5 Detection of the expressions of NFL, ATP-β, TBB2A and NDUS1 in hippocampus mitochondria by Western blot
The concentration of protein was determined by BCA protein concentration determination kit. Mitochondrial proteins of each group were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE), and then transferred onto the membrane.After 2 h blocking, the primary antibodies (NFL, ATP-β,TBB2A or NDUS1) diluted by 1:1 000 were added individually and incubated overnight at 4 ℃. After being rinsed for 3 times, 1:10 000 diluted horseradish peroxidase labeled goat anti-rabbit IgG secondary antibody was added and incubated at room temperature on the shaker for 1 h. After washing, the membrane was incubated for a few minutes with ECL color developing solution. Exposed and developed the X-ray film in dark room. The integrated optical density(IOD) of the bands was determined by ImagePro 5.0 image analysis software.
1.6 Statistical methods
Data were statistically analyzed using the SPSS 17.0 version software. Measurement data in normal distribution were presented as mean ± standard deviation, and analyzed by randomized analysis of variance. The least significant difference (LSD) or Student-Newman-Keuls (SNK) was used to compare data with homogeneity of variance between two groups.Data not fitting the normal distribution were compared using rank sum test.P<0.05 indicated a statistical significant difference.
2 Results
Analysis of the gel maps by ImageMaster 2D platinum 5.0 software identified that there were 32 differentially expressed spots between the normal control group and the model group, including 25 up-regulated and 7 down-regulated ones in the normal control group; there were 38 differentially expressed spots between the acupuncture at acupoint group and model group, of which, 29 were up-regulated and 9 were downregulated in the acupuncture at acupoint group; there were 13 differentially expressed spots between the acupuncture at non-acupoint group and the model group, of which, 7 were up-regulated and 6 were downregulated in the acupuncture at non-acupoint group.
The 2-DE map of the model group was used as a reference, the gel maps of the normal control group,the acupuncture at acupoint group and the acupuncture at non-acupoint group were respectively compared with it. Differentially expressed protein spots were randomly selected for identification. Eleven of the 16 differentially expressed spots selected from the normal control group were finally identified; thirteen of the 19 differentially expressed spots selected from the acupuncture at acupoint group were finally identified;four of the 10 differentially expressed spots selected from the acupuncture at non-acupoint group were finally identified (Figure 2).

Figure 2. Differentially expressed protein spots in mouse hippocampus mitochondria of each group
2.1 Identification of differentially expressed protein spots
The differentially expressed protein spots selected from the normal control group, acupuncture at acupoint group and acupuncture at non-acupoint group were preliminarily identified by Ultraflex III TOF/TOF mass spectrometer, and the peptide mass fingerprinting(PMF) was further determined (Figure 3-Figure 5). PMFs of the obtained protein spots were filtered from baseline peaks to identify the signal peaks using the software flex Analysis. The BioTools software was used to search the uniprot database for the Mascot scores.Figure 6-Figure 8 respectively showed the screenshots of the search results, by BioTools software, for the differentially expressed protein spots of No.3 in the normal control group, No.2 in the acupuncture at acupoint group and No.4 in acupuncture at non-acupoint group. Figure 9-Figure 11 were the PMFs of the three differentially expressed protein spots mentioned above from http://www.matrixscience.com.
Compared with the model group, which was used as a reference, 11 differential protein spots were eventually identified in the normal control group, 9 up-regulated and 2 down-regulated; 13 differential protein spots were eventually identified in the acupuncture at acupoint group, 9 up-regulated and 4 down-regulated; there were 4 differentially expressed protein spots in the acupuncture at non-acupoint group,2 up-regulated and 2 down-regulated. According to the protein function annotations provided in the protein database, and inquiring a large number of domestic and foreign literatures, the differentially expressed proteins can be roughly classified into the following categories:(1) proteins involved in mitochondrial energy metabolism and oxidative stress, such as NDUS1, ATP-β,pyruvate dehydrogenase E1 component subunit alpha(PDHE1-α), pyruvate dehydrogenase E1 component subunit beta (PDHE1-β), pyruvate dehydrogenase phosphatase 1 (PDP1) and cytochrome b5 type B(Cytb5); (2) cytoskeletal proteins, such as NFL, TBB2A,actin (cytoplasmic 1, database ID: ACTB); (3) signal(apoptosis) regulatory proteins, such as mitochondrialprocessing peptidase subunit alpha (MMP-α),adenosine kinase (ADK) and ataxin-10 (ATX10); (4)chaperones, such as heat shock cognate 71 kDa protein(HSC71). The results showed that the five proteins, NFL,TBB2A, ACTB, PDHE1-α and PDHE1-β, appeared in differentially expressed protein spots among the normal control group, acupuncture at acupoint group and acupuncture at non-acupoint group with the same expression directions. However, the number of differentially expressed protein spots between the acupuncture at non-acupoint and the model group was much smaller, and the identified proteins have little to do with the pathogenesis of AD, which suggests that acupuncture at non-acupoints has little influence on the mitochondrial protein expression in AD model mice.Details of differentially expressed protein spots in above groups are shown in Table 1, Table 2 and Table 3.
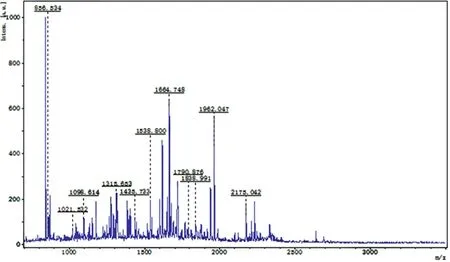
Figure 3. PMF of the No.3-differentially expressed protein spot in the 2-DE map of the normal control group
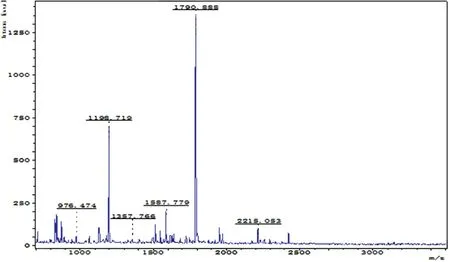
Figure 4. PMF of the No.2-differentially expressed protein spot in the 2-DE map of the acupuncture at acupoint group

Figure 5. PMF of the No.4-differentially expressed protein spot in the 2-DE map of the acupuncture at non-acupoint group

Figure 6. A screenshot of the search results for the No.3-differentially expressed protein spots in 2-DE map of the normal control group
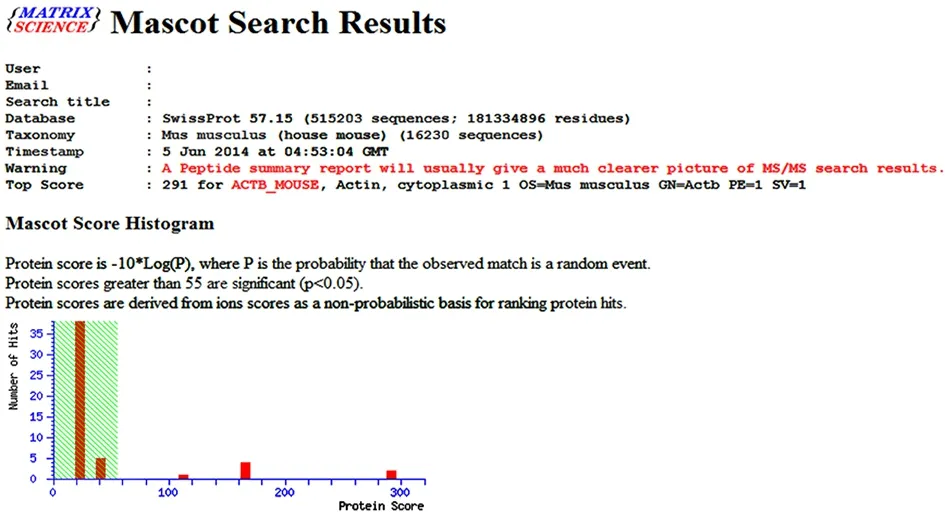
Figure 7. A screenshot of the search results for the No.2-differentially expressed protein spot in 2-DE map of the acupuncture at acupoint group
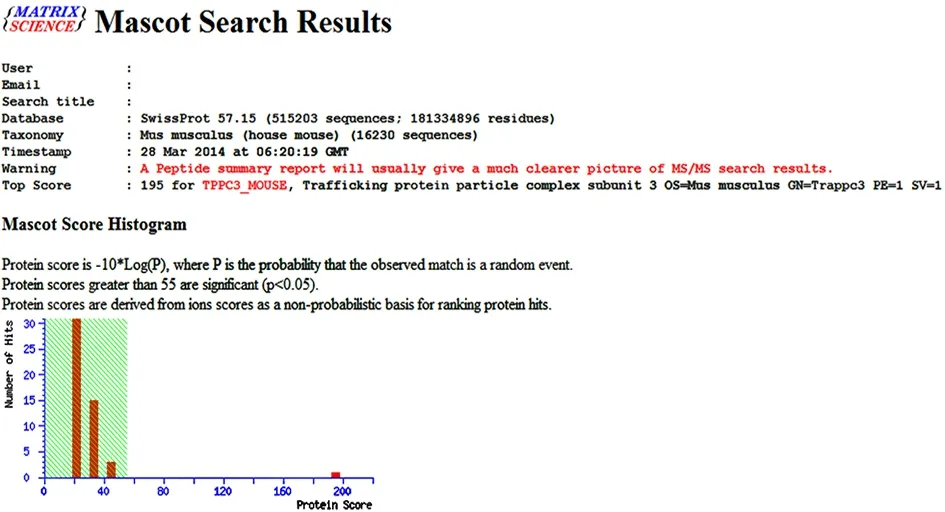
Figure 8. A screenshot of the search results for the No.4-differentially expressed protein spot in 2-DE map of the acupuncture at non-acupoint group
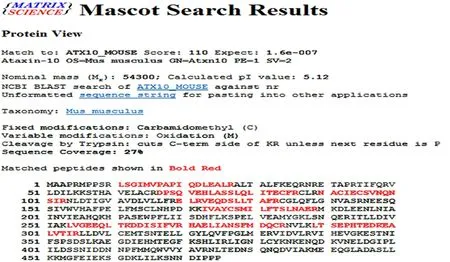
Figure 9. Database matching results of peptide mass fingerprinting for the No.3-differentially expressed protein spot in 2-DE Map of the normal control group (peptide mass fingerprinting results of the protein spot from http://www.matrixscience.com)

Figure 10. Database matching results of peptide mass fingerprinting for the No.2-differentially expressed protein spot in 2-DE map of the acupuncture at acupoint group (peptide mass fingerprinting results of the protein spot from http://www.matrixscience.com)
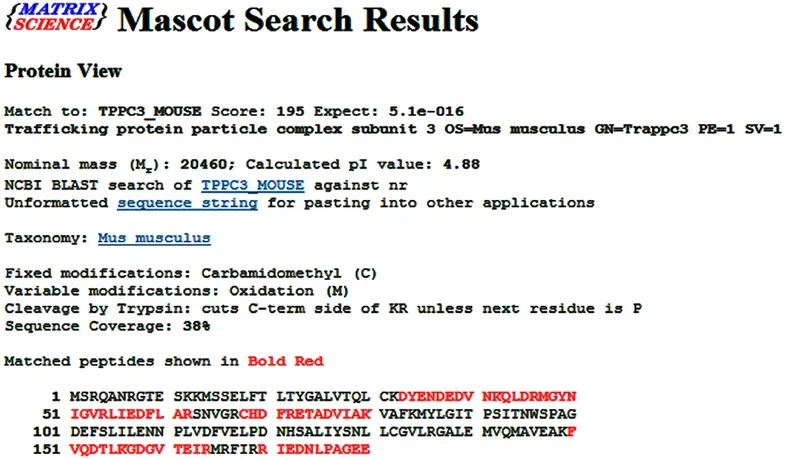
Figure 11. Database matching results of peptide mass fingerprinting for the No.2-differentially expressed protein spot in 2-DE map of the acupuncture at non-acupoint group (peptide mass fingerprinting results of the protein spot from http://www.matrixscience.com)

Table 1. Information of differentially expressed protein spots in hippocampal mitochondria identified by comparison of normal control group and model group
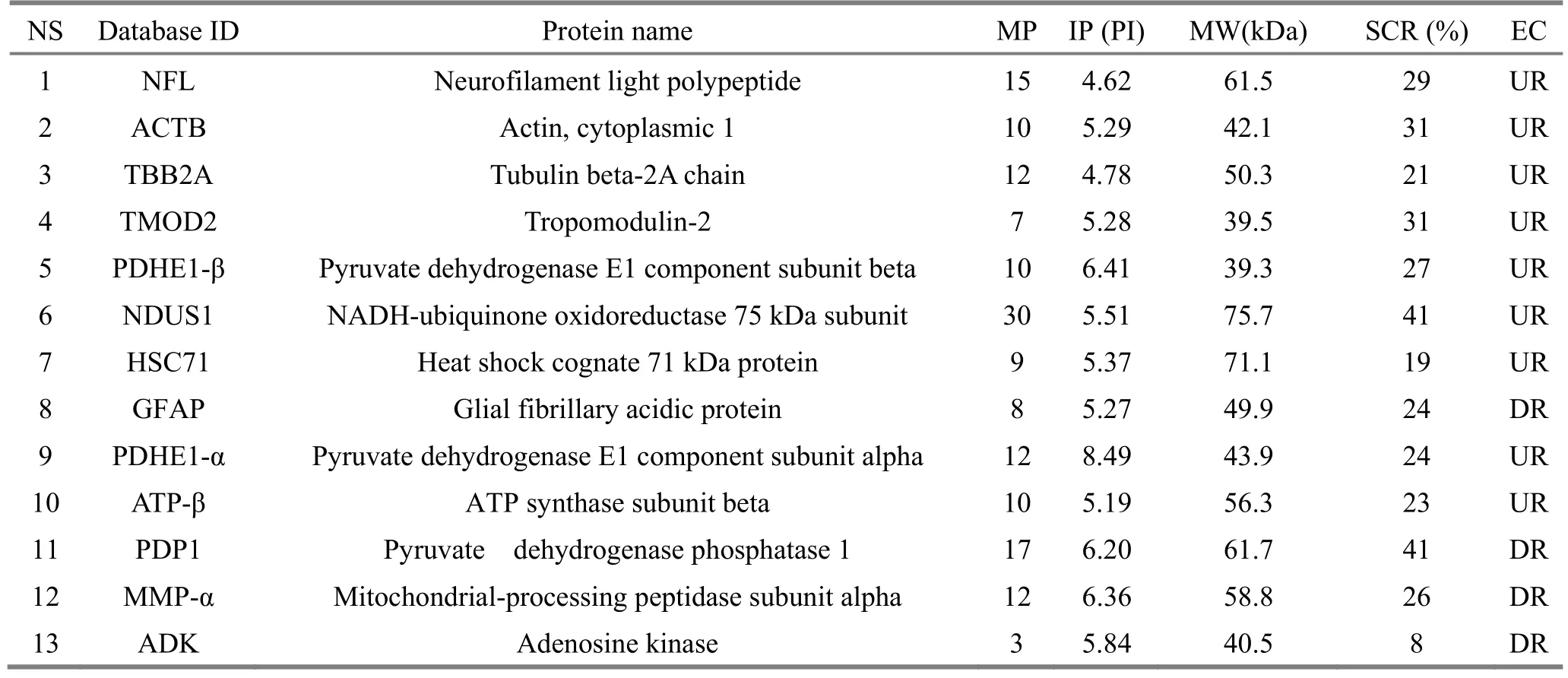
Table 2. Information of differentially expressed protein spots in hippocampal mitochondria identified by comparison of acupuncture at acupoint group and model group
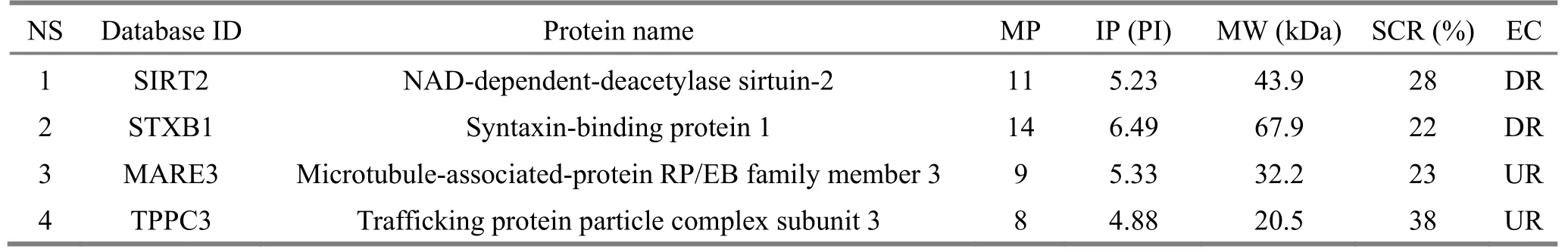
Table 3. Information of differentially expressed protein spots in hippocampal mitochondria identified by comparison of acupuncture at non-acupoint group and model group
2.2 NFL, ATP-β, TBB2A and NDUS1 expression levels detected by Western blot
Voltage-dependent anion-selective channel protein 1(VDAC1) was used as an internal control. Expression levels of the above indicators in mice of each group are shown in Figure 12.

Figure 12. NFL, ATP-β, TBB2A and NDUS1 expressions of mouse hippocampus mitochondria in each group
IOD test and data analysis showed that (1) NFL expression was significantly higher in the normal control group than in the model group, significantly higher in the acupuncture at acupoint group than in the acupuncture at non-acupoint group, and lower than in the normal control group (P<0.05), there was no significant difference between non-acupoint group and the model group (P>0.05); (2) ATP-β expression was significantly higher in the normal control group than in the model group, significantly higher in the acupuncture at acupoint group than in the model group, and lower than in the normal control group (P<0.05), had no significant difference between the acupuncture at non-acupoint group and model group (P>0.05); (3)TBB2A expression level was significantly higher in the normal control group than in the model group,significantly higher in the acupuncture at acupoint group than in the model group, and lower than in the normal control group (P<0.05), had no significant difference between the acupuncture at non-acupoint group and the model group (P>0.05); (4) NDUS1 expression level was significantly higher in the normal control group than in the model group, significantly higher in the acupuncture at acupoint group than in the model group (P<0.05), and no significant difference with the normal control group (P>0.05), no significant difference between the acupuncture at non-acupoint group and the model group (P>0.05). ATP-β and NDUS1 showed differences between the acupuncture at acupoint group and the model group. The results of TBB2A and NFL were consistent with the proteomics(section of 2.1), suggesting that acupuncture might regulate the structural protein of hippocampal mitochondria in SAMP8 model mice (Figure 13).
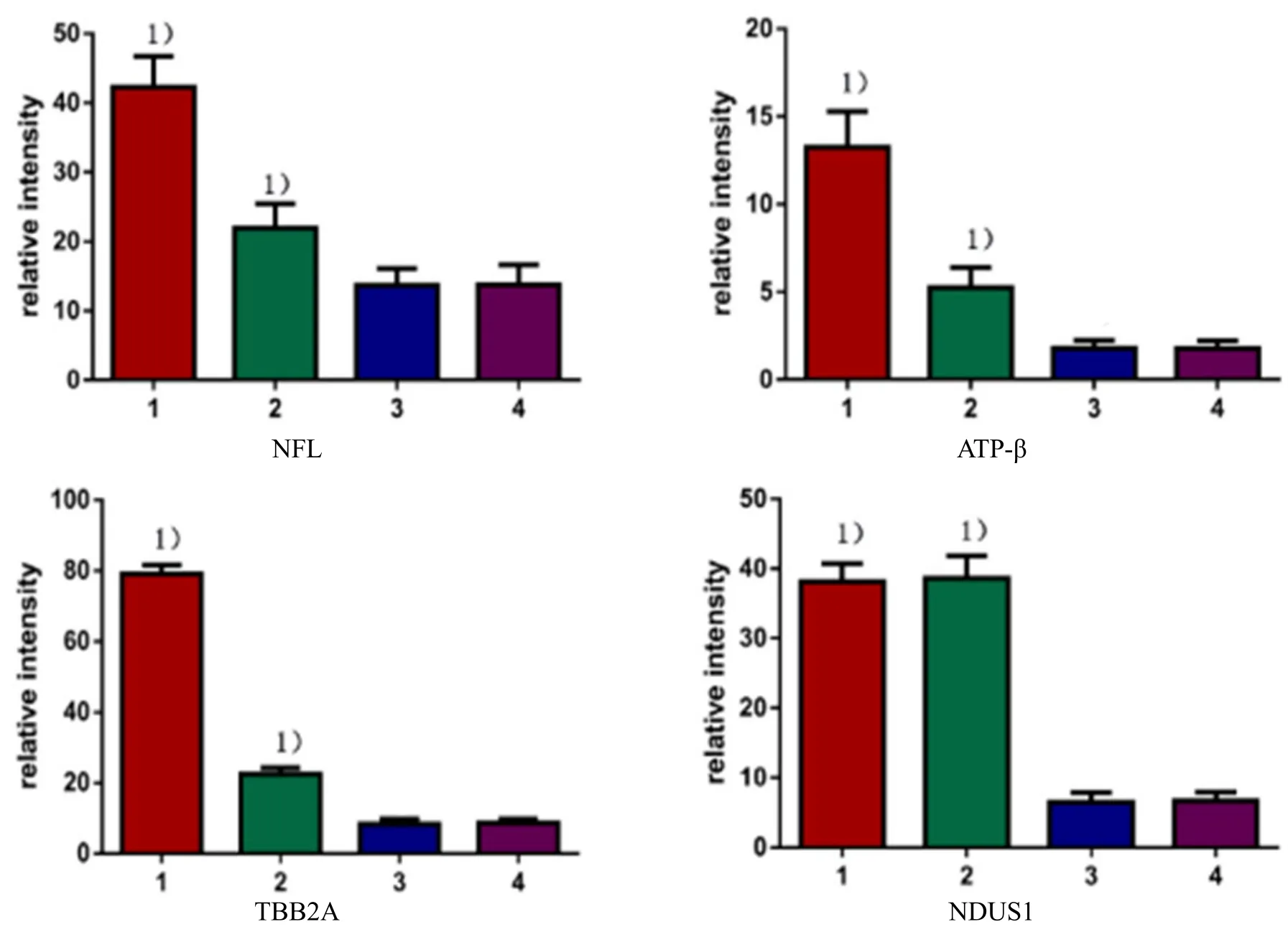
Figure 13. Statistical results of relative expression levels for NFL, ATP-β, TBB2A and NDUS1 in each group
3 Discussion
Among many hypotheses about AD, mitochondrial cascade hypothesis has been recognized by more and more scholars. Mitochondrial cascade hypothesis mainly emphasizes the important role of mitochondrial function changes in the pathogenesis of AD. Recent studies have shown that mitochondrial structural changes also play important roles in the development of AD[17-19]. Functionally, abnormalities in mitochondria can lead to widespread and long-lasting oxidative stress damage caused by impaired mitochondrial energy metabolism, reduced ATP production, production of large numbers of oxygen free radicals and storage of proteins regulating intracellular apoptotic pathways.Structurally, the main manifestations are mitochondrial fragmentation trend, unequal size, uneven, huge differences, reduced average volume, breakage,shortening, reduction or disappearance of the cristae,and decreased mitochondrial membrane fluidity and number of mitochondria. The experimental study found that most of the differentially expressed proteins were involved in the regulation of mitochondrial function and structure.
NDUS1 is the starting point of electron transfer and the main site of mitochondrial reactive oxygen species(ROS) production[20].
PDHE1-β, PDHE1-α and PDP1 are the components of the pyruvate dehydrogenase complex, which together regulate the tricarboxylic acid cycle. Under physiological conditions, mitochondria can promptly eliminate the production of ROS, therefore, ROS is under the dynamic balance of production and clearance. Their altered activity will result in barriers to electron transport,reduced oxygen utilization, reduced ATP synthesis, and impaired energy metabolism. This leads to a large number of ROS accumulating in the body, and mitochondrial functional domains are exposed to high concentration of ROS and destroyed. Thereby, it is easy to cause excessive oxidative stress in the body, leading to mitochondrial dysfunction and functional defects of oxidative phosphorylase released from mitochondria,causing cell damage and tissue destruction, further resulting in the destruction of mitochondrial structure and ultimately neuronal apoptosis[21]. In addition, when ROS is overproduced, lipid peroxidation can occur to impair the mitochondrial membrane, resulting in a substantial increase in calcium release from the mitochondria, triggering calcium overload, to accelerate the opening of the permeability transport pore,promote the release of cytochrome C, activate the apoptosis-related enzyme family, and trigger the cascade of apoptosis[22]. Acupuncture can promote the expressions of NDUS1, PDHE1-β and PDHE1-α and inhibit the expression of PDP1. The therapeutic effect of acupuncture may be related to the improvement of mitochondrial energy metabolism and the maintenance of intracellular calcium homeostasis. ATP-β is one of the constituent subunits of ATP synthase and also the catalytically active site of ATP synthase. The decrease of ATP-β activity leads to the disorder of mitochondrial energy metabolism, resulting in the barrier of electron transfer in the respiratory chain of mitochondria,increase of electron leakage and free radical generation.This triggers oxidative stress, destroys the structure of mitochondria and affects intracellular pH and mitochondrial membrane potential, which in turn causes structural damage and dysfunction of mitochondria[23]. Furthermore, abnormalities of ATP-β can lead to the increase of phosphorylated JNK and p-38 and the decrease of phosphorylated ERK and AKT,promote the apoptosis and eventually lead to the occurrence of neurodegeneration[24]. Acupuncture can up-regulate the expression of ATP-β, which may play a therapeutic role by stabilizing the mitochondrial membrane potential and increasing anti-apoptotic protein expression.
NFL and ACTB are important components of the cytoskeleton. In the absence or abnormal expression of NFL and ACTB, the synthesis of nerve fiber fails, and the fusion-division imbalance of mitochondria in subcellular structure leads to dysfunction of mitochondrial structure and function[25]. Gourlay CW, et al[25]found that slowing of the actin turnover led to the accumulation of a large amount of fibrous actin, and an increase in the ROS produced by mitochondria. In addition, studies have shown that abnormal expression of NFL may cause myelinated axonal regeneration disorders, leading to neuronal degeneration[26-27].Acupuncture can up-regulate NFL and ACTB, which may play a therapeutic role by improving the mitochondrial dynamics of hippocampal neurons.
TBB2A can regulate the morphology, structure and function stability of the neuronal microtubule system.The microtubule system is closely related to the structure, distribution and function of mitochondria.There are two specific binding sites for microtubuleassociated proteins in the mitochondrial outer membrane.
Microtubulin dysfunction brings about that microtubule-associated protein cannot combine with specific binding sites on the mitochondrial outer membrane. This is not good for kinesin nearby the cell body to combine with the microtubules, and directly move to the synapses for releasing, thus affecting the kinesin to transport the mitochondria to the synapses[28-29]. Dysfunction of nutrition transport channels in synaptic neurons leads to diminished neuronal function until atrophy and death, eventually leading to the development of AD. Acupuncture can increase TBB2A expression, indicating that acupuncture may have the ability to regulate mitochondrial transport disorders due to dysregulation of microtubulin.
HSC71 has the effect of maintaining the conformational stability of protein molecules, and protecting cell survival and function. The expression level of HSC71 is negatively correlated with the degree of neuronal damage, thus has a protective effect on hippocampal neurons. Studies have shown that HSC71 can inhibit hypoxia/reoxygenation-induced apoptosis through increasing Bcl-2 expression, therefore to inhibit mitochondria-related apoptosis pathways including changes in mitochondrial membrane potential and interruption of cytochrome c release[30].Acupuncture can promote the expression of HSC71 to achieve mitochondrial protection probably through blocking the mitochondria-related apoptotic pathways.There were no ATP-β and NDUS1 proteins in the information for the differentially expressed protein spots identified between the normal control group and the model group after the proteomics comparison,which was inconsistent with the results of Western blot assay. The possible reason is the limitation of 2-DE proteomics technology. When selecting the differentially expressed protein spots for the mass spectrometry analysis, we did not know their specific information. However, the number of the differentially expressed protein spots identified between the normal control group and the model group according to the selection standard was large. We could not analyze all the differentially expressed protein spots by mass spectrometry analysis due to the limited conditions.Only some randomly selected differential proteins were subjected to mass spectrometry. The number of differentially expressed proteins between the acupuncture at acupoint group and the model group was higher. Most of the identified proteins were related to the mitochondrial structure and function of hippocampus in AD model mice, suggesting that acupuncture at acupoints could regulate the function of mitochondrial protein in hippocampus of AD mice. The number of differentially expressed protein spots between the acupuncture at non-acupoint group and the model group was less, and the identified proteins had little to do with the pathogenesis of AD, suggesting that acupuncture at non-acupoints had little effect on mitochondrial protein expression in AD mice.
In summary, acupuncture can regulate the expression of multiple structural and functional proteins in hippocampal mitochondria of SAMP8 mice, thus may achieve the therapeutic effect in treating AD via double regulation of the hippocampus mitochondrial structure and functional proteins.
Conflict of Interest
The authors declared that there was no potential conflict of interest in this article.
This work was supported by National Natural Science Foundation of China (国家自然科学基金项目, No.81202730); the Fundamental Research Funds for the Central Universities (中央高校基本科研业务经费, No.2012QNZT162); the Natural Science Foundation of Hunan Province (湖南省自然科学基金, No. 2011JJ3097); Science and Technology Project of Traditional Chinese Medicine of Hunan Province (湖南省中医药科技项目, No. 201413).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
[1] Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer’s disease: past, present and future.Neuropharmacology, 2014, 76: 27-50.
[2] Moreira PⅠ, Carvalho C, Zhu X, Smith MA, Perry G.Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta, 2010,1802(1): 2-10.
[3] Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses, 2004, 63(1): 8-20.
[4] Morley JE, Farr SA, Kumar VB, Armbrecht HJ. The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des,2012, 18(8): 1123-1130.
[5] Pallas M, Camins A, Smith MA, Perry G, Lee HG,Casadesus G. From aging to Alzheimer’s disease: unveiling“the switch” with the senescence-accelerated mouse model(SAMP8). J Alzheimers Dis, 2008, 15(4): 615-624.
[6] Morley JE, Armbrecht HJ, Farr SA, Kumar VB. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim Biophys Acta, 2012, 1822(5): 650-665.
[7] Zhou J, Peng WN, Xu M, Li W, Liu ZS. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore), 2015,94(22): e933.
[8] Hua Q, He R. Alzheimer’s disease-mechanisms, drug targets and alternative treatments. Curr Top Med Chem,2016, 16(5): 470-471.
[9] Zhu H,Dong KL,Wu Y,Zhang T, Li RM, Hu SH, Wang HL. Cognitive function improvement of Bu Shen Huo Xue method on Alzheimer disease patients. Zhongguo Laonianxue Zazhi, 2010, 30(11): 1493-1495.
[10] Zhu H, Dong KL, Wu Y, Zhang T, Li RM, Dai SS, Wang HL. Ⅰnfluence of acupuncture on isoprostane in patients with Alzheimer’s disease. Zhongguo Zhen Jiu, 2010, 30(1):18-21.
[11] Dai SS, Dong KL, Zhu H. Effect of kidney-reinforcing and blood-activating acupuncture on learning and memory abilities and brain AChE activity in a SAMP8 mouse model of senile dementia. Shanghai Zhenjiu Zazhi, 2010, 29(1):57-59.
[12] Dai SS, Dong KL, Zhu H. Effects of Bu Shen Huo Xue acupuncture on the ability learning and memory and the expression of NEP in the hippocampal CA1 region of rapid aging SAMP8 mouse models. Hunan Zhongyiyao Daxue Xuebao, 2015, 35(1): 60-63, 72.
[13] Zhu H, Dong KL, Li GC, Xiao L. Effect of protein expressionin hippocampus tissue of SAMP8 by kidney-reinforcing and blood-activating acupuncture method. Zhongguo Laonianxue Zazhi, 2011, 31(23):4613-4616.
[14] Zhu H, Dong KL, Wu Y, Li GC, Xiao L, Zhang T, Wang HL. Effect of acupuncture for reinforcing kidney and activating blood on the neuroglobin expression in hippocampus tissue of Alzheimer’s disease model mice.Zhongyi Zazhi, 2011, 52(11): 955-957.
[15] Li ZR. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2007: 253-257.
[16] Bian JL, Zhang CH. Conception and core of academician Shi Xuemin’s acupuncture manipulation quantitative arts.Zhongguo Zhen Jiu, 2003, 23(5): 287-289.
[17] Riemer J, Kins S. Axonal transport and mitochondrial dysfunction in Alzheimer’s disease. Neurodegener Dis,2013, 12(3): 111-124.
[18] Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, Ⅰwaki T, Ohara T, Sasaki T, LaFerla FM, Kiyohara Y,Nakabeppu Y. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb Cortex, 2014, 24(9): 2476-2488.
[19] Leuner K, Müller WE, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol,2012, 46(1): 186-193.
[20] Distelmaier F, Koopman WJ, van den Heuvel LP,Rodenburg RJ, Mayatepek E, Willems PH, Smeitink JA.Mitochondrial complex Ⅰ deficiency: from organelle dysfunction to clinical disease. Brain, 2009, 132(Pt 4):833-842.
[21] Akhter F, Chen D, Yan SF, Yan SS. Mitochondrial perturbation in Alzheimer’s disease and diabetes. Prog Mol Biol Transl Sci, 2017, 146: 341-361.
[22] Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease.Nat Rev Mol Cell Biol, 2014, 15(10): 634-646.
[23] Vacirca D, Delunardo F, Matarrese P, Colasanti T, Margutti P, Siracusano A, Pontecorvo S, Capozzi A, Sorice M,Francia A, Malorni W, Ortona E. Autoantibodies to the adenosine triphosphate synthase play a pathogenetic role in Alzheimer’s disease. Neurobiol Aging, 2012, 33(4): 753-766.
[24] Wang WJ, Ma Z, Liu YW, He YQ, Wang YZ, Yang CX, Du Y, Zhou MQ, Gao F. A monoclonal antibody (Mc178-Ab)targeted to the ecto-ATP synthase beta-subunit-induced cell apoptosis via a mechanism involving the MAPKase and Akt pathways. Clin Exp Med, 2012, 12(1): 3-12.
[25] Gourlay CW, Carpp LN, Timpson P, Winder SJ, Ayscough KR. A role for the actin cytoskeleton in cell death and aging in yeast. J Cell Biol, 2004, 164(6): 803-809.
[26] Koutsis G, Pandraud A, Karadima G, Panas M, Reilly MM,Floroskufi P, Wood NW, Houlden H. Mutational analysis of PMP22, EGR2, LⅠTAF and NEFL in Greek Charcot-Marie-Tooth type 1 patients. Clin Genet, 2013, 83(4):388-391.
[27] Filali M, Dequen F, Lalonde R, Julien JP. Sensorimotor and cognitive function of a NEFL (P22S) mutant model of Charcot-Marie-Tooth disease type 2E. Behav Brain Res,2011, 219(2): 175-180.
[28] Sheng ZH, Cai Q. Mitochondrial transport in neurons:impact on synaptic homeostasis and neurodegeneration.Nat Rev Neurosci, 2012, 13(2): 77-93.
[29] Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau.Science, 2008, 319(5866): 1086-1089.
[30] Yuan ZQ, Zhang Y, Li XL, Peng YZ, Huang YS, Yang ZC.HSP70 protects intestinal epithelial cells from hypoxia/reoxygenation injury via a mechanism that involves the mitochondrial pathways. Eur J Pharmacol, 2010, 643(2-3):282-288.
 Journal of Acupuncture and Tuina Science2018年2期
Journal of Acupuncture and Tuina Science2018年2期
- Journal of Acupuncture and Tuina Science的其它文章
- Effect of acupuncture in intervening heroin-induced brain damage via regulating ubiquitin-proteasome pathway
- Effect of An-pressing manipulation on the serum levels of T-AOC and CK-MM in volunteers with delayed onset muscle soreness in biceps brachii
- Effect of acupuncture plus Tai Ji Quan on the recovery of neurological function and depression state in post-stroke depression patients
- Yi Jin Jing (Sinew-transforming Qigong Exercises) for primary osteoporosis in the elderly: a clinical trial
- Observation on clinical effect of tuina plus Western medication for functional dyspepsia due to liver qi stagnation and spleen deficiency
- Clinical observation on cervical chiropractic for cervical spondylosis of vertebral artery type
