Risk stratification of patients with primary arrhythmia syndrome
Among primary arrhythmia syndromes, the follows are frequently seen: long QT syndrome(LQTS), ST elevation in the right precordium, right bundle branch block(RBBB), sudden cardiac death(SCD) and catecholaminergic polymorphic ventricular tachycardia(CPVT)/ventricular fibrillation(VF).
LQTS is autosomal dominant, rarely autosomal recessive and is usually associated with deafness. It has been found by testing gene-specific features of LQTS that 17 genes of over 60% genotypes are involved in genetic heterogeneity.
Established risk factors of LQTS are aborted sudden death and syncope event. Family history of SCD is not a risk factor in LQTS which has been proved by many studies. Other risk factors include the presence of Torsades de pointes(TdP) and T-wave alternans(TWA) on ECG, and the prolongation of QT interval. The longer the QT, the higher the risk. And deafness inherited in the autosomal recessive mode, that is congenital deafness, is also a risk factor of LQTS. Among the above risk factors, TdP, TWA and the prolongation of QT interval can be identified on ECG.
The QT prolongation over 500 ms on ECG indicates the patient being at risk. T-wave morphology is an important indicator, particularly TWA. In the following example, we’re not sure about the existence of QT-dispersion, however, reduced heart rate suggests the possibility of conduction block. In addition, TdP is also a risk factor of LQTS.
As shown in Fig.1, the patient is a 26-year-old female with congenital deafness and life-long “seizure disorder” (probably wrongly diagnosed). She was presented with recurrent syncope one week after the birth of her 3rd child. ECG abnormalities were recognized by the computer and physicians, particularly extremely abnormal T-waves. A patient like this should be considered as being at high risk for syncope events and that is echo arrhythmias.
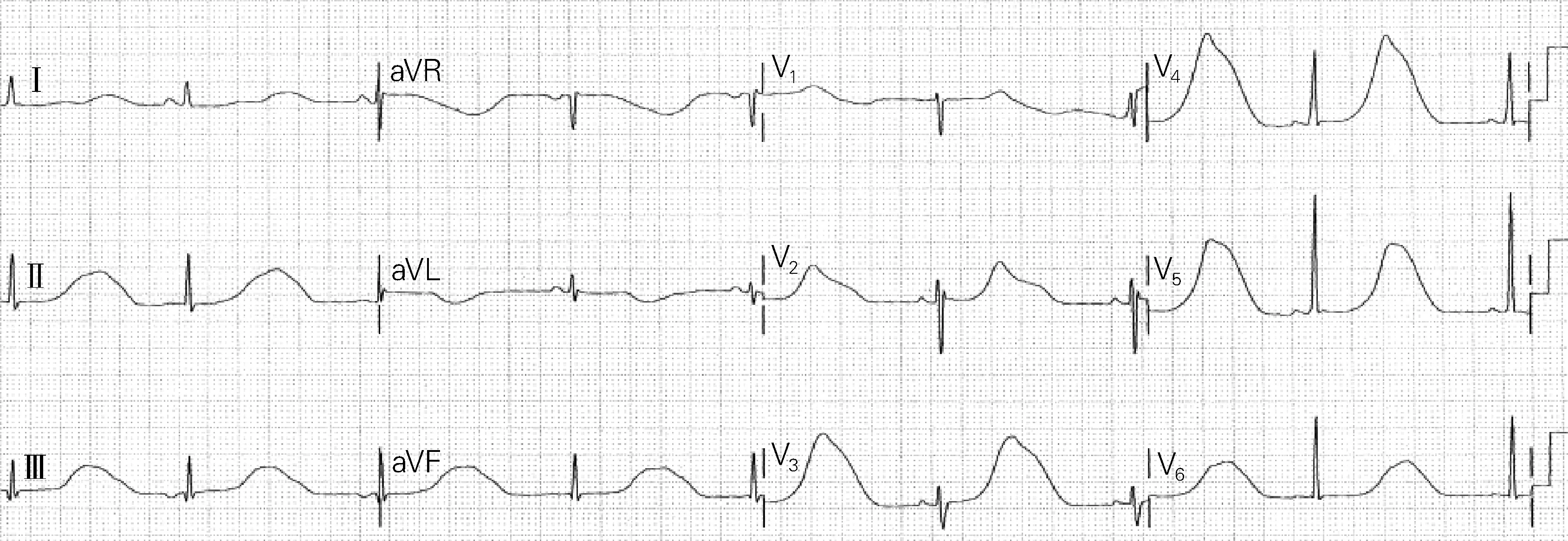
Fig.1 ECG of a patient at high risk for syncope events
1 Risk stratification of long QT syndrome
QT interval has already been proved to be an important risk factor of LQTS[1]. The quartile of QTc is ≤446 ms, 447-468 ms, 469-498 ms and ≥499 ms, respectively. As shown in Fig.2, the cumulative cardiac-event-free survival is plotted along the vertical axis and age on the horizontal axis. The longer the QTc, the more likely the patient is becoming symptomatic. But importantly, the patient in the first quartile with normal QTc is not cardiac-event-free.

Fig.2 The cumulative cardiac-event-free survival with age
In a 500-case group at high risk of cardiac events, symptomatic individuals account for about 70%, with an annual incidence of cardiac events of 2%. For the cases falling in the normal QTc interval range, the proportion and rate are separately about 20% and 0.5% per year.
Based on QT interval and genetic theories, Silvia G Priori came up with the “pyramid” of risk stratification of LQTS. The high-risk patient is identified by QTc≥500 ms with genotype of LQT1 or LQT2, but LQT3 is found in males only. For the females with LQT3 and long QT interval, the risk is apparently lower, that is,intermediate risk. Males with LQT2, or patients with LQT1 and QTc<500 ms have low risk of LQTS.
Fig.3 shows another patient at high risk, and it is the first ECG of a neonate shortly after birth. Prenatal bradycardia had already been recognized. The patient has syndactyly which is a sign that he/she may suffer from LQTS with genotype of LQT8. Marked alternans can be clearly observed on ECG(as pointed by black arrow in Fig.3), which are determinants of high risk for adverse event in near future. Fig.4 is from the same patient and the risk of LQTS appears to be much lower if compared with Fig.3. There are two P waves, one functional atrioventricular block and one QRS complex. These P waves can’t conduct downward because the repolarization of the ventricle has not been completed yet. And the functional atrioventricular block is also a risk indicator of abnormally prolonged QT interval.

Fig.3 The first ECG of a neonate shortly after birth
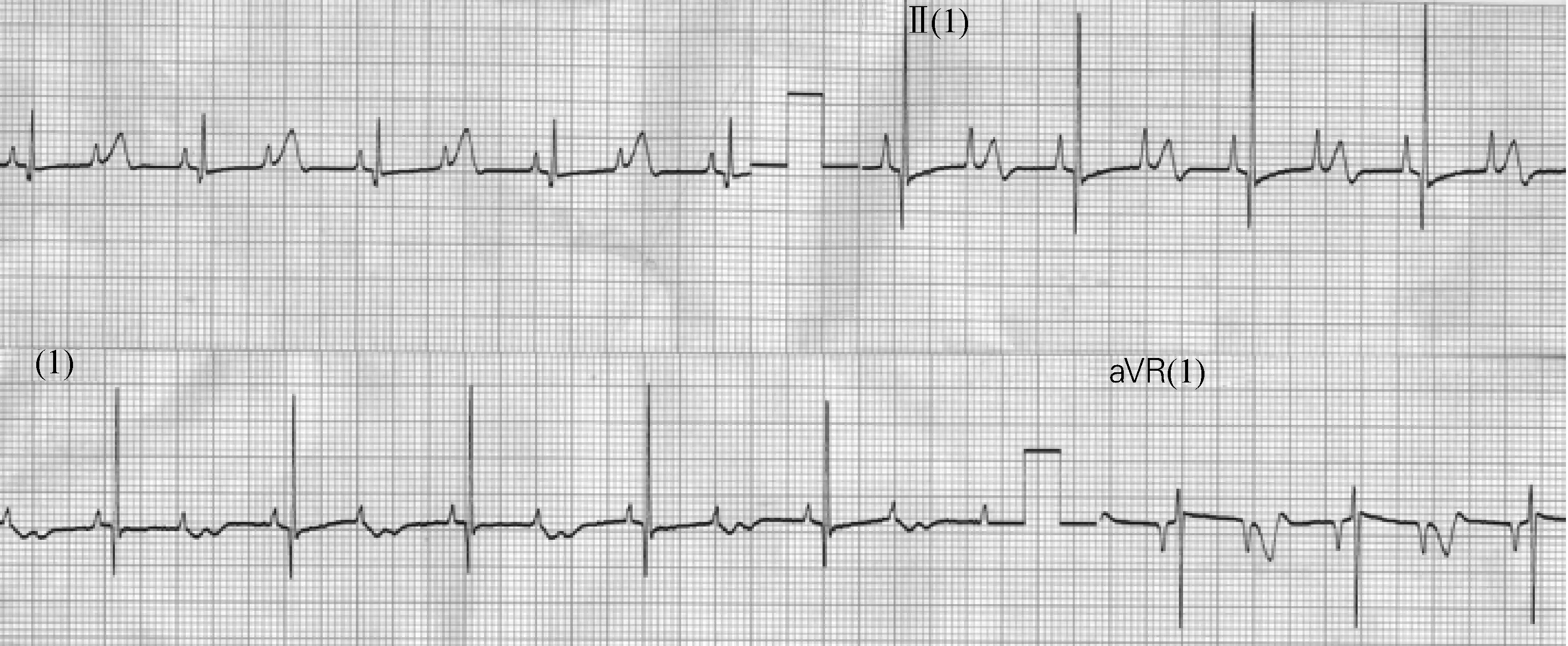
Fig.4 ECG of the same patient as Fig.3
Fig.5 shows an elderly individual treated with Sotalol also under indicator of risk. Once again, there are marked prolonged QT intervals and bradycardia. And then the alternans on top of the U wave or T wave can be observed twice. In the occurrence of alternans, it is almost be sure that the next ECG in the next minute will be like Fig.6. The patient should be monitored accurately.

Fig.5 ECG of an elderly individual

Fig.6 ECG of a patient with ventricular tachycardia
Hence, the risk of LQTS depends on genic phenotype, gender(female gender), QTc (≥500 ms, female gender) and age(male gender at very young age). Specific ECG features such as alternans and serum inhibition factor suggest functional atrioventricular block. However, as mentioned earlier, genotype is also important. Specific genic mutations usually indicate high malignancy. For example,A34 1Vis one of the best-known malignant genic mutations.The chances of high malignancy are higher in the appearance of pore mutations, autosomal dominant variant mutations and C-loop mutations. It has been revealed that LQT2 belongs to pore mutation while we are not very clear about LQT3.Specific genic mutations can be used to screen out the high risk population for LQTS.
2 Risk stratification of Brugada syndrome
Fig.7 is the ECG of typical type 1 Brugada syndrome acquired from a 40-year-old male with a family history of nocturnal sudden death. It is unknown whether he has undergone drug therapy or not, and the ECG might be acquired from the 4th intercostal space, or higher in the 2nd, 3rd or intercostal space. His ECG may vary from day to day and sudden death events may occur at rest or at exercise. Fever can increase the rate of sudden death but rarely are children reported.
The ECG of type 1 Brugada syndrome can be easily discriminated from type 2 and 3, however, the ECG of type 2 and 3 can be transformed into type 1 by taking class Ⅰ anti-arrhythmia drugs.
The risk stratification of Brugada syndrome is somewhat disputative. It’s widely agreed that the patients with cardiac arrest or VF are high-risky. Patients with symptoms like syncope are at high risk. EPS inducibility could probably be utilized in predicting Brugada syndrome although it’s still controversial. However, in my personal opinion, its predictive value is limited. Spontaneous alterations have been enrolled in the list of risk factors by different studies, like fragmented QRS complex(fQRS). Recently, Japanese study found thatSCN5Amutations may add some extra risk of Brugada syndrome.
The fQRS and spontaneous alteration are obvious on ECG[2-3]. If a patient comes in with usually normal ECGs and the above abnormal ECGs at onset, it seems that the patient is high risky of Brugada syndrome. Fig.8 shows spontaneous variation of ST elevation in the same patient at different ages—55, 56, 57 and 58 years old[2]. Sometimes the ECG is normal although this might be influenced by different locations of electrodes. However, most of the time the ECG is abnormal, and on the ECG with marked variation there are a lot of fractionations which indicate high risk for ventricular arrhythmias.
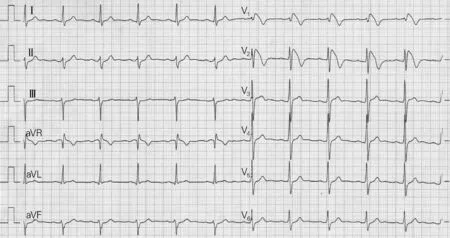
Fig.7 ECG of a 39-year-old male

Fig.8 Spontaneous variations of fragmented QRS complex
Fig.9 is another example of fQRS in Brugada syndrome[2]. There is marked fQRS here on this QRS complexes and it’s omnipresent. Morita et al[2]illustrated that the presence of fQRS predicted sudden death in the future.

Fig.9 Example of fQRS in Brugada syndrome
3 Risk stratification of catecholaminergic polymorphic ventricular tachycardia
As shown in Fig.10, this is a patient with exercise-induced arrhythmias and the ECG has very typical features of CPVT. However, it manifests as alternating morphology of bidirectional VT and QRS complex, which is referred to as “bidirectional”. When clinically confronting with this ECG, we can strongly doubt the diagnosis of CPVT.
CPVT is autosomal dominant. Its initial clinical manifestations are syncope or aborted sudden cardiac death during exercise or under emotional stress, and usually appear during the first or second decade of life. At present, 6 subtypes of CPVT have been reported. It usually starts at young age and importantly, the baseline of ECG is normal.
The patients at high risk of CPVT are in accordance with at least one of the following conditions: (ⅰ) a medical history of cardiac arrest; (ⅱ) initial presentation at young age; (ⅲ) occurrence of complex arrhythmias during exercise test, as shown in Fig.10; (ⅳ) patients not using beta blocker are definitely at higher risk. In addition, it has been prove by our research that CPVT is related to some mental retardation. The result has not been published yet but has been submitted recently.
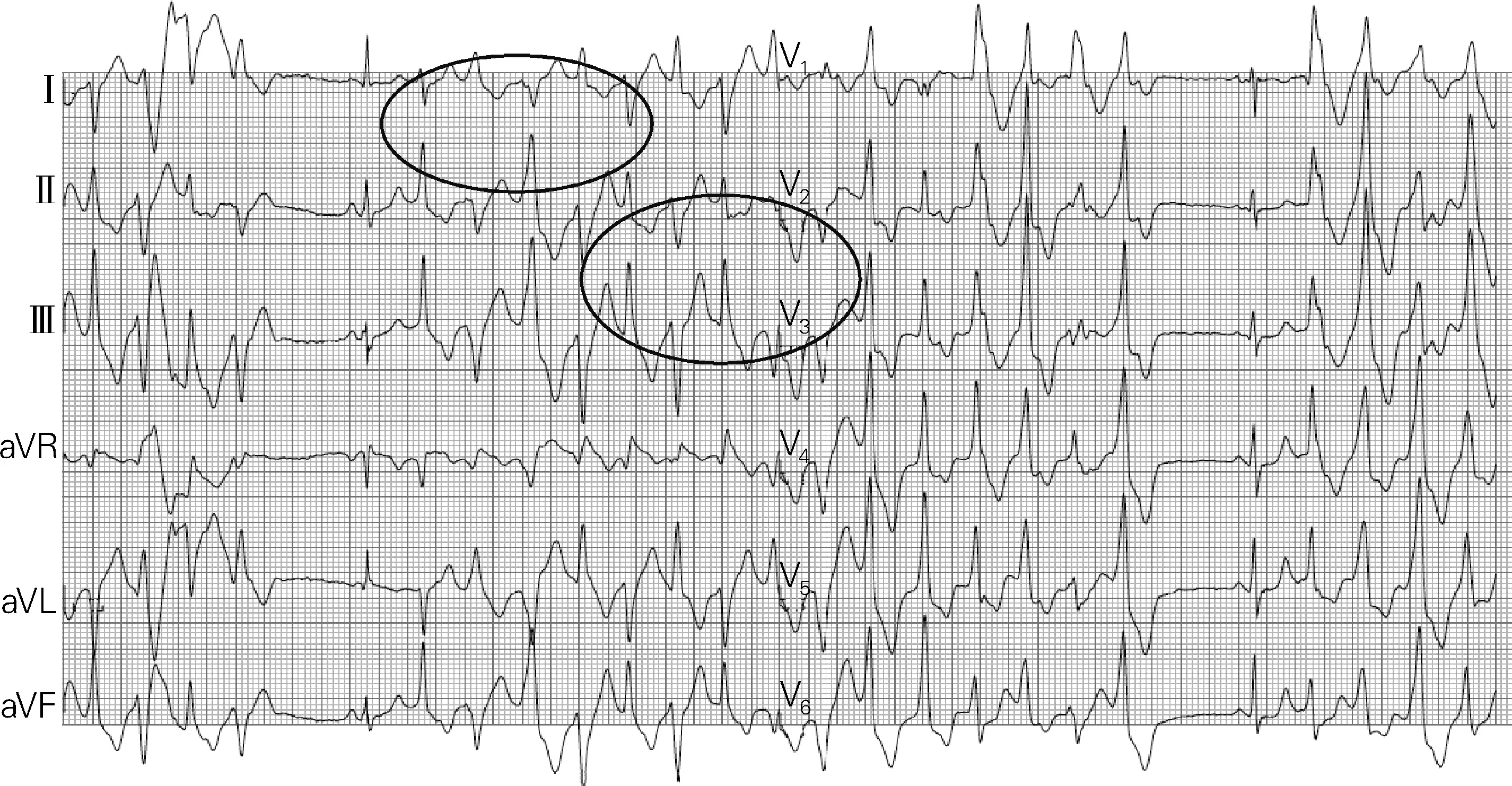
Fig.10 The ECG of catecholaminergic polymorphic ventricular tachycardia
4 Relationship between lamin A/C cardiomyo-pathy and primary arrhythmia syndrome
Cardiomyopathy induced by lamin A/C genic mutation is not primary arrhythmia syndrome, but its first manifestations are atrioventricular block and atrial fibrillation. Obvious cardiomyopathy fails to appear at an early phase while left ventricular dysfunction emerges in later period. So initially these patients may present with symptoms similar to primary arrhythmia syndrome. In the neurologic phenotype, a known clinical manifestation is limb girdle muscular dystrophy, for instance, progressive muscular dystrophy and elevation of creatine kinase. The cardiac phenotype has the initial manifestation of first degree atrioventricular block and atrial fibrillation, followed by left ventricular dysfunction. ECG is typical recognized by P-waves with low amplitude, first degree atrioventricular block, and narrow or normal QRS.
Fig.11 is the ECG of a 32-year-old male with strong family history of sudden death. His father died of heart failure at age 42 and uncle died at age 38 implanted with pacemaker from age 35. The ECG shows P-wave with very low amplitude and marked first degree atrioventricular block, but the QRS complex and repolarization is completely normal. This is very typical of lamin A/C cardiomyopathy and the ECG indicates high risk of sudden death.
van Rijsingen et al[4]carried out a multicenter study involving 275 patients, and proposed that risk factor is left ventricular ejection fraction(LVEF) <50%, not the usual 35% in other academic hypothesis. Non-sustained VTs on the Holter, male gender and LVEF<50% are three risk factors, which enormously increased the risk for ventricular arrhythmias. During a follow-up 20 months , over 30% of the individuals have developed VT/VF. So these patients should be considered as high-risk cases.
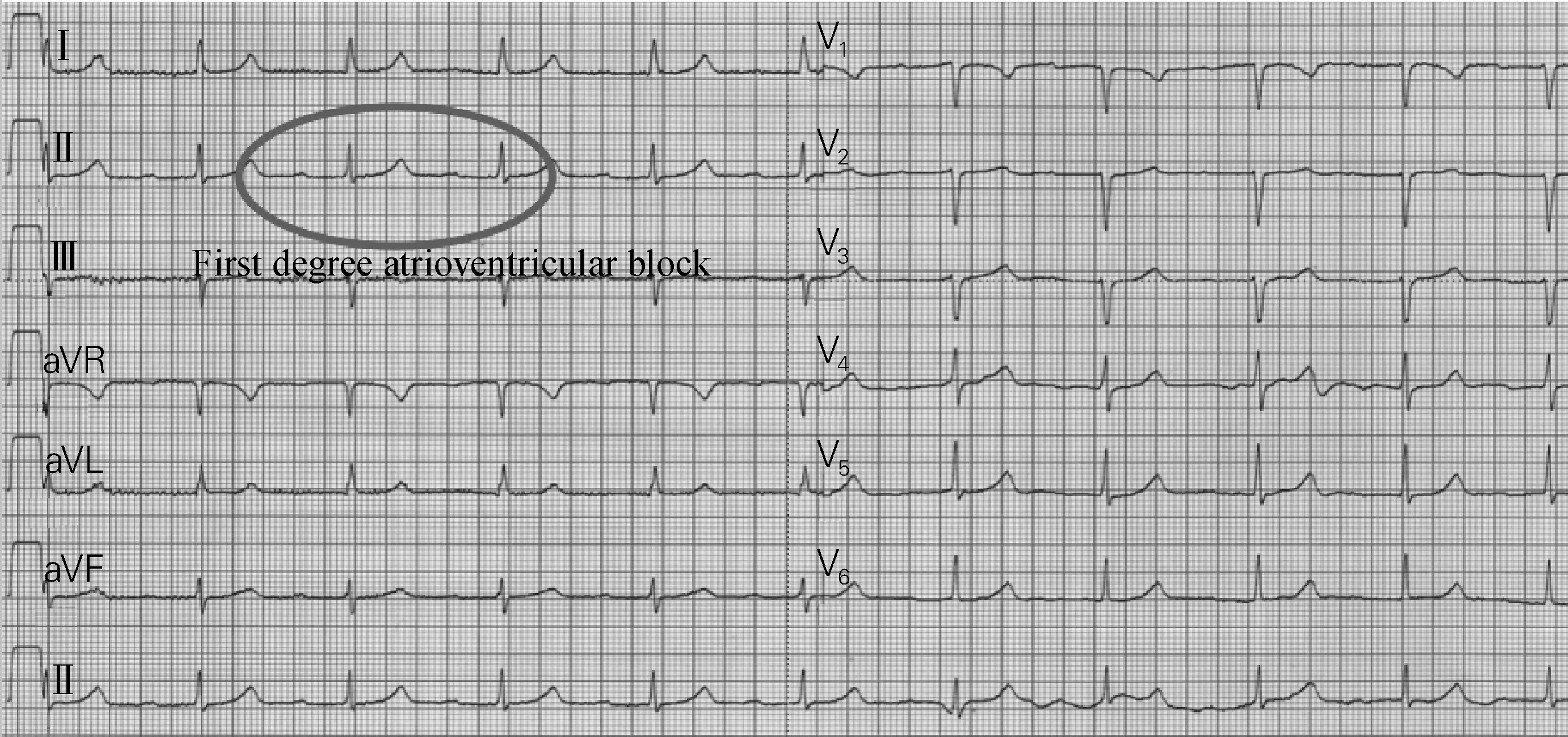
Fig.11 The ECG with prolonged PR, normal QRS and low amplitude P-wave
5 Conclusion
The risk assessment of primary arrhythmia syndrome is as follows, (ⅰ) long QT syndrome: QTc≥500 ms, T-wave alternans and genetic abnormality; (ⅱ) Brugada syndrome: spontaneous ST-segment elevation, variable ECG morphology and fQRS, and the symptom is like long QT syndrome; (ⅲ) CPVT: all patients need to be treated. In addition, other patients especially those with complex arrhythmias during exercise are also at high risk. The specificity of ECG in dilated cardiomyopathy conduction disease is not that high, but in the presence of LMNA as a virulence gene, high risk for dilated cardiomyopathy conduction disease is strongly indicated.
[1] Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome[J]. N Engl J Med, 2003,348(19):1866-1874.
[2] Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome[J]. Circulation, 2008,118(17):1697-1704.
[3] Take Y, Morita H, Wu J, et al. Spontaneous electrocardiogram alterations predict ventricular fibrillation in Brugada syndrome[J]. Heart Rhythm, 2011,8(7):1014-1021.
[4] van Rijsingen IA, Arbustini E, Elliott PM, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study[J]. J Am Coll Cardiol, 2012, 59(5):493-500.
[摘要] 遗传性原发性心律失常综合征主要包括长QT综合征、Brugada综合征及儿茶酚胺敏感性心动过速(catecholaminergic polymorphic ventricular tachycardia,CPVT)等。由于该类疾病猝死风险极高,因此及早诊断并进行危险分层极其重要。确定极高危或低危个体相对容易,但对多数中危患者进行分层较困难,基因分析和临床评估对危险分层有一定的临床价值。本文结合病例分析,主要探讨对长QT综合征、Brugada综合征及CPVT危险分层的相关因素。其中,长QT综合征危险分层与QTc、明显的T波改变及基因变异有关;Brugada综合征危险分层因素包括基因变异及碎裂QRS波;CPVT危险分层因素包含心脏骤停病史、发病年龄及是否应用β受体阻滞剂。此外,文章还阐述了核纤层蛋白A/C基因突变致心肌病与遗传性原发性心律失常综合征的关系。
[关键词] 遗传性原发性心律失常综合征;长QT综合征;Brugada综合征;儿茶酚胺敏感性心动过速;危险分层
[中图分类号] R540.4 [文献标志码] A [文章编号] 2095-9354(2018)03-0169-09
DOI:10.13308/j.issn.2095-9354.2018.03.004
作者单位: 1100DE 荷兰 阿姆斯特丹,阿姆斯特丹大学心内科,学术医学中心
作者简介: Arthur AM Wilde,医学教授,主要从事心血管疾病遗传学的相关研究,E-mail:a.a.wilde@amc.uva.nl
就目前的认识,常见的遗传性原发性心律失常综合征包括:长QT综合征(LQTS)、右心前区ST段抬高、右束支阻滞(RBBB)、心源性猝死(SCD)和儿茶酚胺敏感性多形性室性心动过速(CPVT)/心室颤动(VF)。
LQTS通过常染色体显性遗传,很少发生隐性遗传,且通常与耳聋有关。通过测试LQTS的基因特异性特征后发现,其遗传异质性主要涉及17个基因,超过60%的基因型。
目前已知的LQTS的危险因素包括猝死和晕厥事件。许多研究已证实,SCD家族史并不是LQTS的危险因素。其他危险因素包括心电图证实的尖端扭转型室性心动过速(TdP)和T波电交替(TWA)以及QT间期延长,并且,QT间期越长,风险越高。通过常染色体隐性遗传导致的耳聋,即先天性耳聋亦为LQTS的危险因素。在这些危险因素中,TdP、TWA以及QT间期延长均可通过心电图识别出来。
当心电图提示QT间期延长超过500 ms时,预示着患者可能处于危险之中。T波形态是提示LQTS的重要危险因素,特别是TWA。对于下面这个病例,我们并不确定是否有QT间期离散,但减慢的心率提示可能存在传导阻滞。此外,TdP也是LQTS的一个危险因素。
如图1所示,患者女,26岁,患有先天性耳聋和终生“癫痫症”(可能被误诊)。在她的第三个孩子出生后一周,患者开始出现反复晕厥。计算机和人工均识别到心电图异常,尤其是T波极度异常。像这样的患者应该被归为晕厥事件的高危人群,也就是回声障碍。
1 长QT综合征的危险分层
既往已证明QT间期是LQTS的重要危险因素[1]。QTc数值被划分为四层:≤446 ms、447~468 ms、469~498 ms和≥499 ms。如图2所示,以无心脏事件的累积生存率为纵轴、以年龄为横轴作图。QTc越长,患者越有可能出现症状。然而需要注意的是,QTc正常患者(QTc≤446 ms)也可能发生心脏事件。
在QTc>500 ms的高危组中,心脏事件的年发生率为2%,其中,70%的个体有临床症状。而在QTc正常者中,20%的个体有临床症状,心脏事件的年发生率为0.5%。
根据QT间期和遗传学理论,Silvia G Priori提出了LQTS风险分层的“金字塔”。高风险患者的QTc>500 ms,基因型为LQT1或LQT2,但只有男性有LQT3。对于长QT间期且基因型为LQT3的女性,其风险明显较低,属于中风险。基因型为LQT2的男性,或基因型为LQT1且QTc<500 ms的患者,其LQTS风险较低。
再举一例 LQTS风险较高的病例。图3为一名新生儿出生后不久的第一份心电图,其已诊断出产前心动过缓。该病例患有并指,提示其可能患有LQT8型LQTS。从心电图中可清楚地观察到明显的交替现象(箭头所指处),这是近期不良事件高风险的决定性因素。图4来自同一患者,从心电图来看,其LQTS风险较图3明显降低。心电图显示有两个P波、一个功能性的房室阻滞和一个QRS波群,其中,这些P波不能下传,因为心室复极尚未完成;该房室阻滞同时也是QT间期异常延长的风险指标,预示着高风险。
图5为一例接受索他洛尔治疗的老年患者心电图,亦提示存在LQTS风险因素。与之前的病例类似,该患者心电图上可明显观察到QT间期延长和心动过缓。此外,两次出现了U波或T波的顶部交替现象。当出现这一现象时,我们几乎可以肯定下一分钟的下一个心电图就类似于图6。如果患者有这样的心电图,就意味着需要对其进行心电监护。
因此,LQTS风险高低取决于基因表型、性别(女性)、QTc(≥500 ms,女性)和年龄(年轻男性)。特定的心电图特征,如交替现象和血清抑制因子则提示功能性房室阻滞。但正如前文所述,基因型也很重要。当出现特定基因突变时,通常提示高度恶性:A34 1V就是人们熟知的恶性突变之一;当出现细胞孔蛋白突变、常染色体显性变异突变或C-环突变时,高度恶性的风险似乎更高。研究表明,LQT2属于孔蛋白突变,LQT3则尚不清楚。特定的基因突可用于鉴别诊断LQTS的高风险人群。
2 Brugada综合征的危险分层
图7为一例典型的1型Brugada综合征患者心电图。患者男,40岁,有夜间猝死家族史。我们不清楚该患者是否采取药物治疗,心电图可能采集自第四肋间间隙,或者在第二、第三或肋间间隙较高的位置。他的心电图可能每天都不同,猝死事件可能在休息时发生,也可能在运动时发生。发烧可能会使猝死发生率升高,但儿童少见这方面的报道。
1型Brugada综合征心电图较2型和3型更易识别,但通过应用Ⅰ类抗心律失常药物,2型和3型心电图也可以转化为1型心电图特征。
目前,Brugada综合征的危险分层仍存在争议,普遍接受的观点是心脏骤停或VF的患者发生事件的风险非常高。表现为晕厥等症状的患者有较高的危险性。电生理检查(EPS)诱导性或许可用于预测Brugada综合征,尽管该指标尚存在争议,但笔者认为价值有限。自发交替现象在不同的研究中均被列为危险因素,正如碎裂QRS波。最近,日本的研究表明SCN5A突变可能会使Brugada综合征的患病风险升高。
碎裂QRS波和自发性交替现象在心电图上表现得较为明显[2-3]。如果一例平时心电图正常的患者在发病时出现上述异常心电图,则提示该患者属于Brugada综合征的高危人群。图8显示同一患者在不同年龄(55、56、57和58岁)发生ST段抬高的情况[2]。由图8可见,有时心电图是正常的(尽管这可能受到不同电极位置的影响),但大多数时间都是异常的,且这种明显变异的心电图中存在很多的碎裂波,即室性心律失常的高危因素。
图9是Brugada综合征中碎裂QRS波的另一个例子[2]。图中可见明显的碎裂QRS,并且它无处不在。Morita等[2]的研究表明,碎裂QRS波提示未来可能发生猝死事件。
3 儿茶酚胺敏感性多形性室速的危险分层
某病例患有运动性心律失常,如图10所示,其心电图具有非常典型的CPVT的特征,但表现为双向VT和QRS波群交替的形态。这就是所谓的“双向性”。临床上面对这份心电图时,我们可以强烈质疑CPVT的诊断。
CPVT为常染色体显性遗传。CPVT最初的临床表现是在运动或情绪激动时,发生晕厥或心脏性猝死事件,通常在10岁或20岁以前发病。目前,已知CPVT有6种亚型。CPVT一般好发于年轻人,且心电图基线正常。
CPVT高危患者须符合下列条件中至少一项:① 既往发生过心脏骤停;② 在年轻时首次出现CPVT;③ 在运动试验中出现复杂心律失常(图10);④ 未使用β受体阻滞剂的患者必定有更高的风险。此外,我们经研究得出,CPVT与(某些)智力低下存在相关性,具体研究结果尚未公布,但近期已向相关期刊投稿。
4 核纤层蛋白A/C基因突变致心肌病与遗传性原发性心律失常综合征的关系
核纤层蛋白A/C基因突变致心肌病不属于遗传性原发性心律失常综合征,却以房室阻滞和心房颤动为首发表现,且早期并没有明显的心肌病发作,到病程晚期才会出现左室功能不全。因此,这类患者起初可能会出现类似原发性心律失常综合征的症状。该病在神经系统的临床表现包括已知的肢带肌营养不良,如进行性肌营养不良和肌酸激酶升高;在心脏方面则以一度房室阻滞和心房颤动为最初临床表现,之后会发生左室功能不全。心电图的典型表现为低振幅P波、一度房室阻滞和正常的窄QRS波。
图11是一位32岁男性患者的心电图。该患者有猝死家族史,他的父亲42岁时死于心力衰竭,他的叔叔在35岁时接受心脏起搏器植入术,38岁离世。该患者的心电图表现为极小振幅的P波且有明显的一度房室阻滞,而QRS波群和复极波完全正常。这是典型的核纤层蛋白A/C基因突变致心肌病,其心电图提示猝死的高风险。
van Rijsingen等[4]开展的一项多中心研究纳入275例患者,研究指出危险因素为左室射血分数(LVEF)<50%,而非其他理论假设中提出的“LVEF<35%”。24 h动态心电图记录到的非持续性室速、男性和LVEF<50%,三者均为危险因素,大大增加了发生室性心律失常的风险。在20个月的随访期间,超过30%的患者最终发展为室速/室颤,故这类患者应该被视为高风险人群。
5 结语
遗传性心律失常综合征的风险评估可总结如下,① 长QT综合征:QTc≥500 ms,T波交替和遗传学异常;② Brugada综合征:自发性ST段抬高、多变的心电图图形形态和碎裂QRS波,且临床症状类似于长QT综合征;③ CPVT:所有患者均需要治疗。此外,其他患者,特别是在运动过程中出现复杂心律失常的患者亦处于高危状态。对于扩张型心肌病传导障碍,心电图的特异性有限,但如果同时存在LMNA作为致病基因,那么通常高度提示扩张型心肌病传导障碍的风险较高。

