Efficacy of epalrestat plus α-lipoic acid combination therapy versus monotherapy in patients with diabetic peripheral neuropathy: a meta-analysis of 20 randomized controlled trials
Ming Zhao , Jia-Yi Chen , Yu-Dong Chu, Ya-Bin Zhu, Lin Luo, , Shi-Zhong Bu ,
1 Runliang Diabetes Laboratory, Diabetes Research Center, Ningbo University, Ningbo, Zhejiang Province, China
2 Department of Public Health, Longsai Hospital, Ningbo, Zhejiang Province, China
3 Department of Nephrology, Ningbo Medical Center Lihuili Eastern Hospital, Ningbo, Zhejiang Province, China
4 Medical School, Ningbo University, Ningbo, Zhejiang Province, China
Introduction
Diabetic peripheral neuropathy (DPN) is a common microvascular complication of diabetes, and approximately 60% to 70% of people with diabetes have different forms of neuropathy (Tesfaye, 2011; Alam et al., 2017). A recent epidemiological survey showed that 30−40% of diabetic patients experienced DPN. Ninety percent of these patients suffered sensory neuropathy, the main clinical symptoms of which are distal limb sensory abnormalities, hypoesthesia or anesthesia (Boulton et al., 2004; Ogbera et al., 2015). Half of patients with DPN are asymptomatic, so the disease is often neglected, leading to ulceration and serious infections that,in some cases, results in amputation; the quality of life is significantly lower when the disease becomes severe (Won and Park, 2016). DPN is not only an important cause of disability and death in diabetic patients, but also promotes other complications of diabetes. Therefore, prevention and treatment of DPN are of great clinical significance, but effective prevention and treatment measures are still lacking. The pathogenesis of DPN is complicated and involves diverse mechanisms (Zhang et al., 2007). Although strict control of glucose is still the most important approach for the treatment of DPN, several studies have suggested oxidative stress as a mechanism of DPN and that antioxidant treatment can significantly improve the long-term quality of life of patients with DPN and effectively prevent the progression of DPN(Obrosova et al., 2002; Yang et al., 2015).
α-Lipoic acid (ALA), a powerful antioxidant, inhibits oxidative stress by reducing the formation of free radicals(Packer, 1998). ALA can improve the clinical symptoms of DPN and enhance nerve conduction velocity (NCV) (Gu et al., 2010). Epalrestat is a specific inhibitor of aldose reductase. Activation of the polyol pathway leads to the production of large amounts of free radicals, resulting in enhanced oxidative stress. Epalrestat reduces oxidative stress by blocking the polyol pathway (Li et al., 2016). Epalrestat is mainly used to treat diabetic neuropathy and it is effective for DPN and autonomic neuropathy. It also has a therapeutic effect on diabetic macroangiopathy and diabetic nephropathy.The efficacy of ALA plus epalrestat combination therapy in patients with DPN compared with ALA or epalrestat monotherapy has been evaluated by many researchers from mainland China. We conducted a meta-analysis of relevant randomized controlled trials to comprehensively understand the efficacy of ALA and epalrestat combination therapy for DPN. We evaluated primary outcomes, including therapeutic efficacy, median motor nerve conduction velocity (MNCV), median sensory nerve conduction velocity(SNCV), peroneal MNCV and peroneal SNCV. In addition,adverse events were recorded as secondary outcomes.
Data and Methods
Search strategy
The electronic databases of PubMed, Medline, Embase, the Cochrane Library, the Chinese National Knowledge Infrastructure, the Wanfang Database and the Chinese Biomedical Database were used to retrieve relevant studies without language restrictions. We combined MeSH and free terms to identify all relevant articles. The search terms were (diabetic peripheral neuropathy, diabetic neuropathies or DPN) AND(alpha-lipoic acid, thioctic acid or lipoic acid) AND epalrestat. The search was conducted from the inception of each database to 7 October 2016.
Study selection
All of the eligible studies in this meta-analysis met the following inclusion criteria: (a) Randomized controlled trials that compared efficacy and safety of epalrestat plus ALA combination therapy versus epalrestat or ALA monotherapy in patients with DPN. (b) The minimum duration of treatment was 2 weeks. (c) Patients with DPN were diagnosed using the World Health Organization standardized type 2 diabetes mellitus and DPN criteria. (d) The studies contained at least one measure that could reflect the efficacy of the drug and nerve conduction velocities (NCVs).
The exclusion criteria were as follows: The control group used epalrestat or ALA combined with other drugs.
Data extraction and quality assessment
Studies were reviewed in detail if they met the inclusion and exclusion criteria when we screened the titles and abstracts.The following information was extracted by two independent investigators (Ming Zhao and Jia-Yi Chen): the first author’s name, sample size of the intervention and control groups, baseline characteristics (age, the number of males and females, the duration of diabetes and DPN), duration of treatment, daily dose of epalrestat and ALA, and primary outcomes including therapeutic efficacy, median MNCV,median SNCV, peroneal MNCV and peroneal SNCV. In addition, adverse events were recorded as secondary outcomes.Discrepancy was resolved by consensus or adjudication by a third investigator.
The methodological quality of included studies was assessed using the parameters proposed by Jadad et al. (1996).Four items, random sequence generation, allocation concealment, double blinding and withdrawals and dropouts,were evaluated, and the score ranged from 0 to 2 for each item. Studies scoring 4–7 points were regarded as high quality, while 0–3 points indicated low quality (Moher et al.,1998).
Outcome measures
In our meta-analysis, the primary outcomes were therapeutic efficacy (valid or invalid), median MNCV, median SNCV,peroneal MNCV and peroneal SNCV. Adverse events were recorded as secondary outcomes.
Statistical analysis
Meta-analysis was performed using STATA, version 12.0(Stata corporation, College Station, TX, USA). The efficacy and NCV data were dichotomous and continuous, respectively, and they were expressed as relative risk (RR) and weighted mean difference (WMD) with 95% confidence intervals (CIs), respectively. Heterogeneity was evaluated using Cochran’sQtest withP< 0.1 considered statistically significant. TheI2statistic was also used to assess the magnitude of heterogeneity across studies. Values ofI2less than 25%, 50% and 75% represented low, medium and high heterogeneity, respectively. IfP> 0.1, indicating no significant heterogeneity, a fixed effect model was selected; otherwise, a random effect model was applied (Higgins et al., 2003).
To explore the source of heterogeneity, subgroup analysis was conducted based on sample size, study duration or study quality. Sensitivity analysis was performed to evaluate the stability of results using different statistical models ( fixed effect modelvs. random effect model) or different effect measures (relative riskvs. odds ratio). Moreover, funnel plots and Begg’s and Egger’s tests were used to assess publication bias, with aPvalue ≤ 0.1 considered statistically significant (Begg and Mazumdar, 1994; Egger et al., 1997). We also undertook the nonparametric “trim and fill” procedure to further assess the possible effect of publication bias in our meta-analysis. The possibility of hypothetical “missing”studies (negative or unpublished studies) was considered;the “trim and fill” method was used to impute theirRRs and recalculate a pooledRRthat incorporated the hypothetical missing studies as though they actually existed (Duval and Tweedie, 2000).

Figure 1 Flow chart of the study selection process.
Results
Study description
We identified 168 relevant studies from the electronic databases, but only 20 met the inclusion and exclusion criteria for selection (Qu and Zeng, 2009; Deng, 2011; Chang and Zhang, 2012; Liang et al., 2012; Gao et al., 2013; He et al.,2013; Luo et al., 2013; Wang et al., 2013; Fang, 2014; Liu,2014; Wang, 2014; Wang and Chen, 2014; Xiong, 2014;Yang, 2014; Zhang et al., 2014; Yan, 2015; Zhao et al., 2015;Hu et al., 2016; Huang, 2016; Liu, 2016).
Among 41 studies that were excluded after being reviewed in detail, 31 were removed because the treatment group did not receive epalrestat plus ALA combination therapy; eight studies were removed because the control group did not receive epalrestat or ALA monotherapy; one did not report the outcomes of the efficacy of drugs or NCVs and one had a trial duration of less than 2 weeks.
The control groups of studies identified in PubMed, Medline, Embase, and the Cochrane Library were almost all placebo or blank controls, which did not satisfy the inclusion criteria; therefore, the studies included in this meta-analysis were all from mainland China. The study selection process is shown in Figure 1.
A total of 1894 DPN patients were included in this meta-analysis, with 864 in the ALA plus epalrestat group, 473 in the ALA group and 557 in the epalrestat group. The treatment duration among studies ranged from 14 to 84 days.The daily dose of ALA was 300, 450 or 600 mg administeredviaintravenous infusion, and the dose of epalrestat was 150 mg through oral administration. Four studies did not report the age of patients (He et al., 2013; Fang, 2014; Zhang et al.,2014; Yan, 2015). In addition, eight studies did not report the duration of diabetes (Deng, 2011; He et al., 2013; Fang,2014; Liu, 2014, 2016; Zhang et al., 2014; Yan, 2015; Hu et al., 2016) and eleven studies did not report the duration of DPN (Qu and Zeng, 2009; Chang and Zhang, 2012; Liang et al., 2012; Gao et al., 2013; He et al., 2013; Luo et al., 2013;Fang, 2014; Liu, 2014; Yang, 2014; Yan, 2015; Liu, 2016).Tables 1 and 2 show the characteristics of the included studies. The quality assessment of the 20 studies is summarized in Table 3; most studies had a quality score less than 4, and only two studies had a score of 4 (Liu, 2014; Wang, 2014).
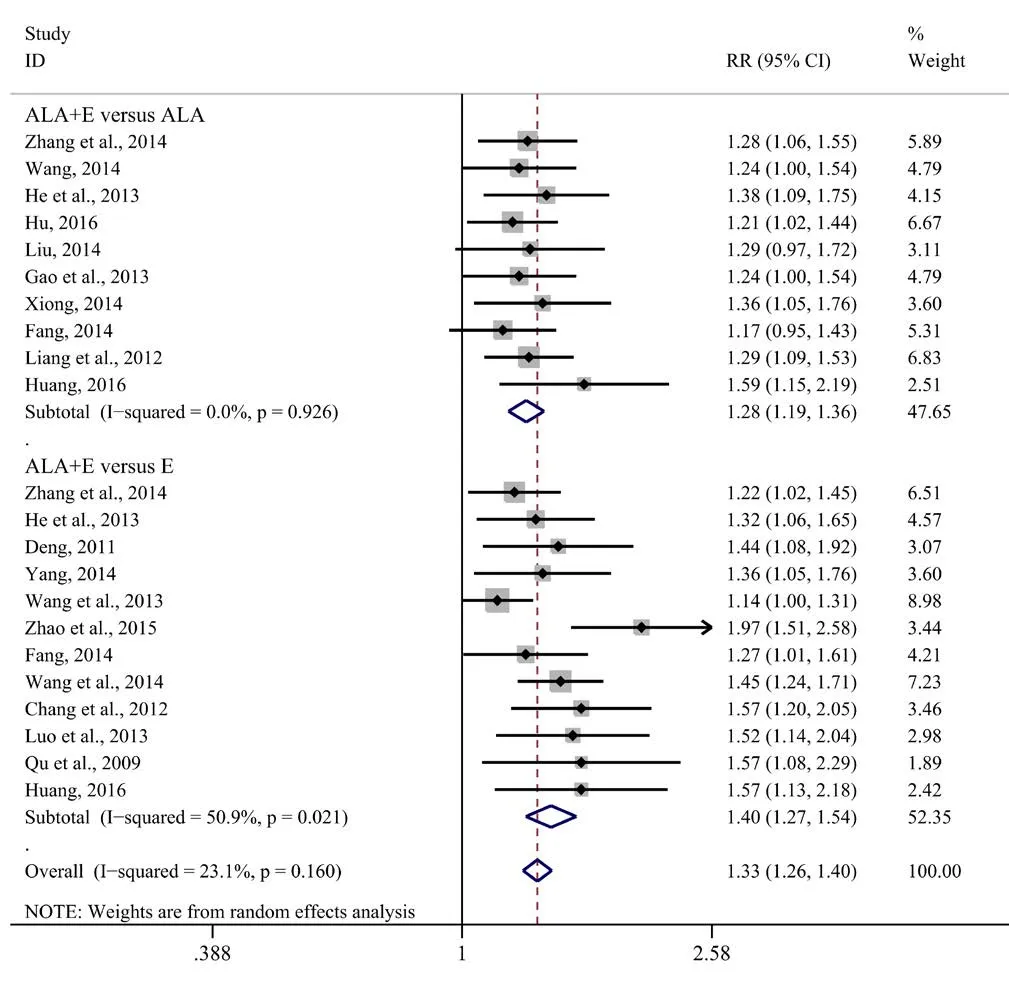
Figure 2 Comparison of efficacy of ALA plus epalrestat combination therapy and monotherapies for diabetic peripheral neuropathy.
Efficacy
Analysis of 18 studies with 1754 DPN patients indicated that the efficacy of ALA plus epalrestat combination therapy was remarkably better than that of epalrestat or ALA monotherapy (Qu and Zeng, 2009; Deng, 2011; Chang and Zhang,2012; Liang et al., 2012; Gao et al., 2013; He et al., 2013; Luo et al., 2013; Wang et al., 2013; Fang, 2014; Liu, 2014; Wang,2014; Wang and Chen, 2014; Xiong, 2014; Yang, 2014; Zhang et al., 2014; Zhao et al., 2015; Hu et al., 2016; Huang, 2016).A fixed effect model was applied because the heterogeneity among studies was insignificant (I2= 23.1%,P= 0.160). The efficacy of ALA plus epalrestat combination therapy was better than that of ALA alone (RR= 1.28, 95%CI: 1.19–1.36,P<0.001), and also better than that of epalrestat alone (RR= 1.40,95%CI: 1.27–1.54,P< 0.001) (Figure 2).

Figure 3 Comparison of efficacies of ALA, epalrestat, and their combination in nerve conduction velocities for diabetic peripheral neuropathy.
Median MNCV
Sixteen studies with 1555 DPN patients measured median MNCV (Qu and Zeng, 2009; Deng, 2011; Liang et al., 2012;Gao et al., 2013; He et al., 2013; Luo et al., 2013; Wang et al.,2013; Fang, 2014; Liu, 2014; Wang, 2014; Wang and Chen,2014; Xiong, 2014; Yang, 2014; Zhang et al., 2014; Yan, 2015;Zhao et al., 2015). Significant heterogeneity between studies was observed (I2= 98.0%,P< 0.001), so the random effect model was applied. Median MNCV in the ALA plus epalrestat group was significantly higher than that in the ALA group (WMD= 5.41, 95%CI: 2.07–8.75,P= 0.002), and also higher than that in the epalrestat group (WMD= 4.77, 95%CI: 1.71–7.83,P= 0.002) (Figure 3A).
Median SNCV
Sixteen studies with 1555 DPN patients measured median SNCV (Qu and Zeng, 2009; Deng, 2011; Liang et al., 2012;Gao et al., 2013; He et al., 2013; Luo et al., 2013; Wang et al.,2013; Fang, 2014; Liu, 2014; Wang, 2014; Wang and Chen,2014; Xiong, 2014; Yang, 2014; Zhang et al., 2014; Yan, 2015;Zhao et al., 2015). Heterogeneity was statistically significant among the studies (I2= 98.6%,P< 0.001), so the random effect model was applied. Median MNCV in the ALA plus epalrestat group was significantly higher than that in the ALAgroup (WMD= 5.87, 95%CI: 1.52–10.22,P= 0.008), and also higher than that in the epalrestat group (WMD= 4.71,95%CI: 1.93–7.48,P= 0.001) (Figure 3B).

Table 1 Characteristics of included studies in the meta-analysis of ALA plus epalrestat combination therapy versus ALA monotherapy in patients with DPN

Table 2 Characteristics of included studies in the meta-analysis of ALA plus epalrestat combination therapy versus epalrestat monotherapy in patients with DPN
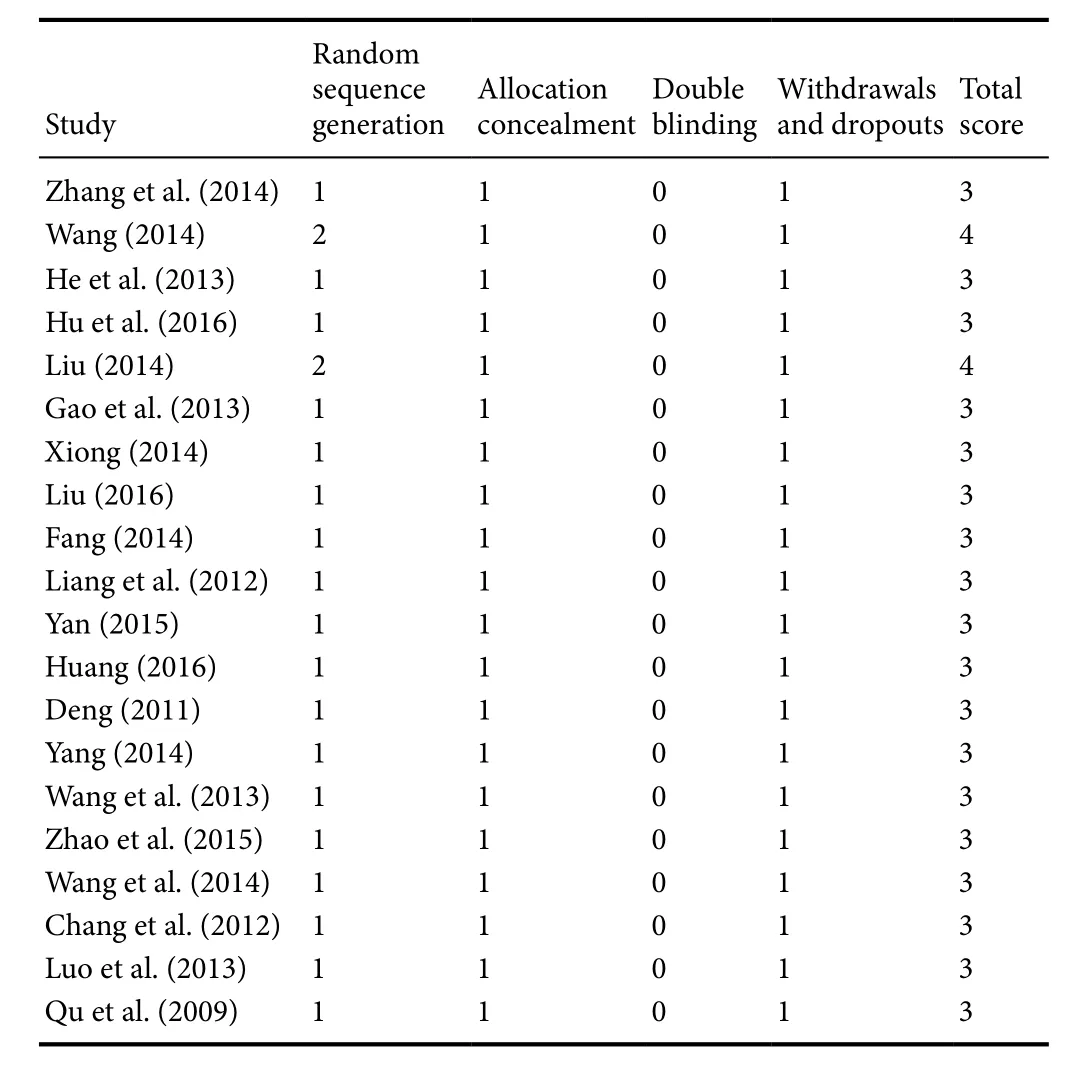
Table 3 Quality assessment of the studies included in the meta-analysis

Figure 4 Funnel plot of publication bias in the analysis of efficacy of ALA plus epalrestat combination therapy versus ALA or epalrestat monotherapy in patients with diabetic peripheral neuropathy.
Peroneal MNCV
Seventeen studies with 1642 DPN patients measured peroneal MNCV (Qu and Zeng, 2009; Deng, 2011; Liang et al.,2012; Gao et al., 2013; He et al., 2013; Luo et al., 2013; Wang et al., 2013; Fang, 2014; Liu, 2014; Wang, 2014; Wang and Chen, 2014; Xiong, 2014; Yang, 2014; Zhang et al., 2014;Yan, 2015; Zhao et al., 2015; Huang, 2016). Heterogeneity was statistically significant among the studies (I2= 98.9%,P= 0.000). Peroneal MNCV in the ALA plus epalrestat group was significantly higher than that in the ALA group (WMD= 5.59, 95%CI:2.70–8.47,P< 0.001), and also higher than that in the epalrestat group (WMD= 6.05, 95%CI:2.63–9.46,P< 0.001) (Figure 3C).
Peroneal SNCV
Eighteen studies with 1710 DPN patients measured peroneal SNCV (Qu and Zeng, 2009; Deng, 2011; Liang et al., 2012;Gao et al., 2013; He et al., 2013; Luo et al., 2013; Wang et al.,2013; Fang, 2014; Liu, 2014; Wang, 2014; Wang and Chen,2014; Xiong, 2014; Yang, 2014; Zhang et al., 2014; Yan, 2015;Zhao et al., 2015; Huang, 2016; Liu, 2016). Heterogeneity was statistically significant among the studies (I2= 95.9%,P< 0.001). Peroneal SNCV in the ALA plus epalrestat group was significantly higher than that in the ALA group (WMD= 4.57, 95%CI: 2.46–6.68,P< 0.001), and also higher than that in the epalrestat group (WMD= 3.77, 95%CI:2.06–5.47,P< 0.001) (Figure 3D).
Subgroup analysis
To explore the source of heterogeneity among the studies,subgroup analyses based on sample size and trial duration were performed. The results are shown in Tables 4 and 5. In some cases, for instance, the sample size was less than 100 or the trial duration was more than 28 days, NCVs were not always higher in the ALA plus epalrestat treatment group compared with the ALA or epalrestat group, because we found that some confidence intervals forRRcontain 1. This indicated no significant difference between the ALA plus epalrestat group and the ALA or epalrestat groups. In addition,significant heterogeneities regarding NCV outcomes still existed in every subgroup.
Safety
There were no serious adverse events during the treatments.Only a few mild adverse events were observed, such as nausea (Xiong, 2014; Zhao et al., 2015; Hu et al., 2016) and stomach upset (Liang et al., 2012; Gao et al., 2013) in the combination therapy group, pain at the injection site (Fang,2014; Xiong, 2014) in the ALA therapy group, and dizziness(Liang et al., 2012) in the epalrestat group. However, the studies did not report these events in detail, so no further analysis was performed.
Sensitivity analysis and publication bias

Table 4 Subgroup analysis for outcomes with ALA plus epalrestat combination therapy versus ALA monotherapy in patients with DPN

Table 5 Subgroup analysis for outcomes with ALA plus epalrestat combination therapy versus epalrestat monotherapy in patients with DPN
Discussion
The prevalence of diabetes has increased rapidly in the past decades, and the number of diabetic patients is estimated to approach 642 million worldwide in 2040 (Zhao et al., 2016).In the life-time of a diabetic patient, the possibility of DPN occurrence is more than 60%, and 36% of patients with DPN suffer from severe and refractory pain (Mehra et al., 2014).The pathogenesis of DPN is a result of multiple factors that are not fully understood. The mechanisms by which high blood glucose leads to DPN include mitochondrial dysfunction, oxidative stress, polyol pathway activation, microvascular dysfunction and altered protein kinase C activity(Caballero et al., 1999; Yagihashi et al., 2007). High blood glucose acts as an initiating factor, generating a large number of reactive oxygen species through the mitochondrial transmission chain, consuming free radical scavengers, and leading to weakened antioxidant ability in nerve tissue (Calcutt et al., 2008). Catanaro et al. (2013) found bradykinin B1 receptor (BKB1-R) over-expression in the sciatic nerve of streptozotocin-induced diabetic rats. They also found that the BKB1-R antagonist, R-954, inhibited oxidative stress,promoted the recovery of Na (+)/K (+)-ATPase and to some extent alleviated diabetic neuropathy. It is generally believed that DPN results from interactions among multiple factors;therefore, DPN treatment should not only focus on lowering the level of blood glucose, but should also take a multi-directional comprehensive approach, including protection of microcirculation.
ALA, as an antioxidant, can directly eliminate free radicals, inhibit peroxidation, increase blood flow in neurocutaneous vessels, raise the reduced glutathione content of peripheral nerves, and improve microcirculation in patients with DPN (Nickander et al., 1996; Haak et al., 2000). Several large-scale randomized controlled trials have shown that ALA is an efficient medication in treating DPN, leading to significant improvement of patients’ symptoms, subjective sensation and, therefore, better quality of life (Ziegler et al.,1995, 1999, 2006; Ametov et al., 2003).
Epalrestat is a noncompetitive and reversible inhibitor of aldose reductase, which is the rate-limiting enzyme in the polyol pathway. Epalrestat is important for protection against oxidative injuries and is therefore used for the treatment of DPN (Sato et al., 2013). Epalrestat is easily absorbed into neural tissues and inhibits aldose reductase with few adverse effects (Yama et al., 2016). In a long-term clinical trial conducted by Hotta et al. (2006), patients with DPN were treated with epalrestat for over three years, and epalrestat effectively delayed the progression of DPN and ameliorated the associated clinical symptoms of this disease.
In our meta-analysis, 20 trials with 1894 DPN patients were included to assess the efficacy and safety of the combination therapy of ALA plus epalrestat in comparison with the monotherapies. The results demonstrated that ALA plus epalrestat combination therapy had a better efficacy and led to higher NCVs than ALA or epalrestat monotherapy.Moreover, no serious adverse events were observed during any of these treatments. However, in subgroup analysis based on sample size or trial duration, no statistical signi ficance was found between ALA plus epalrestat combination therapy and either monotherapy regarding NCVs. The reason for this may be that the sample size was small and that the methodological quality was poor in the included studies.
To the best of our knowledge, this meta-analysis is the first to evaluate the efficacy of ALA plus epalrestat combination therapy in DPN patients. We conducted this meta-analysis using rigorous search and statistical analysis methods to ensure accuracy of the results. However, several potential limitations of our meta-analysis should be fully recognized. First, most studies did not report the blinding of participants and personnel or the concealment of randomization allocation, resulting in low-quality scores for these studies. Second, the sample sizes were small and patient withdrawal or dropout was not described. Third, the studies included were all published, which may cause potential bias because data without statistical significance may have not been published.
In conclusion, the results of this meta-analysis show that compared with ALA or epalrestat monotherapy, the ALA plus epalrestat combination therapy dramatically improves the clinical efficacy and accelerates nerve conduction. However, additional large-scale randomized controlled trials still need to be conducted to confirm these findings.
Author contributions:LL and SZB designed the study. JYC and YDC conducted the experiments. MZ and YDC obtained the data. MZ and JYC analyzed the data. MZ drafted the paper. YBZ and SZB made critical revisions to the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no competing financial interests.
采用SPSS19.0软件处理,以n(%)表示计数资料,行χ2检验;以(± s)表示计量资料,行t检验。P<0.05为差异有统计学意义。
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81370165; a grant from the Public Benefit Technology and Society Development Program of Zhejiang Province of China, No. 2015C33309; a grant from the Ningbo Science and Technology Innovation Team Program in China, No. 2014B82002,2015B11050; a grant from the Ningbo Science and Technology Project in China, No. 2015A610217; the Fang Runhua Fund of Hong Kong, K. C.Wong Magna Fund in Ningbo University. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Reporting statement:This study follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Ningbo University, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Abu Rafee Malik, Indian Veterinary Research Institute, India; Valerio Magnaghi, Università degli Studi di Milano dept.Pharmacological and Biomolecular Sciences, Italy; Huiyin Tu, Zhengzhou University, China.
Alam U, Riley DR, Jugdey RS, Azmi S, Rajbhandari S, D’Aout K, Malik RA (2017) Diabetic neuropathy and gait: a review. Diabetes Ther 8:1253-1264.
Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ,Low PA, Nehrdich D, Novosadova M, O’Brien PC, Reljanovic M,Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D, Group STS (2003) The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care 26:770-776.
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088-1101.
Boulton AJ, Malik RA, Arezzo JC, Sosenko JM (2004) Diabetic somatic neuropathies. Diabetes Care 27:1458-1486.
Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A (1999) Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes 48:1856-1862.
Calcutt NA, Jolivalt CG, Fernyhough P (2008) Growth factors as therapeutics for diabetic neuropathy. Curr Drug Targets 9:47-59.
Catanzaro O, Capponi JA, Michieli J, Labal E, Di Martino I, Sirois P(2013) Bradykinin B(1) antagonism inhibits oxidative stress and restores Na+K+ATPase activity in diabetic rat peripheral nervous system. Peptides 44:100-104.
Chang ZY, Zhang YH (2012) Alpha lipoic acid combined with epalrestat in treatment of diabetic peripheral neuropathy clinical observation of 50 cases. World Latest Medicine Inform 12:65-66.
Deng XZ (2011) Clinical observation of epalrestat combined with α-lipoic acid in the treatment of diabetic neuropathy. Contemp Med 17:135.
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455-463.
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629-634.
Fang MF (2014) Clinical observation of thioctic acid combined with epalrestat for diabetic peripheral neuropathy. J Mod Med Health 30:483-487.
Gao L, Yang Y, Tian Y (2013) Efficacy and safety of epalrestat combined with α-lipoic acid in the treatment of diabetic peripheral neuropathy. Practical Pharm Clin Remedies 16:684-685.
Gu XM, Zhang SS, Wu JC, Tang ZY, Lu ZQ, Li H, Liu C, Chen L, Ning G (2010) Efficacy and safety of high-dose alpha-lipoic acid in the treatment of diabetic polyneuropathy. Zhonghua Yi Xue Za Zhi 90:2473-2476.
Haak E, Usadel KH, Kusterer K, Amini P, Frommeyer R, Tritschler HJ,Haak T (2000) Effects of alpha-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp Clin Endocrinol Diabetes 108:168-174.
He YM, Li XH, Yin Z (2013) Efficacy and safety of epalrestat combined with lipoic acid in the treatment of senile diabetic peripheral neuropathy. Zhongguo Laonianxue Zazhi 11:51-52.
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring in-consistency in meta-analyses. BMJ 327:557-560.
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M,Toyota T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y (2006)Long-term clinical effects of epalrestat, an aldose reductase inhibitor,on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care 29:1538-1544.
Hu XJ, Ren JG, Luo H, Yang Y (2016) Comparative analysis of vibration pereption thresholds in patients with diabetic peripheral neuropathy treated by epalrestat combined with antioxidants. Zhongguo Yaoxue Zazhi 36:1022-1024.
Huang AH (2016) Effect of α-lipoic acid and epalrestat in treating type 2 diabetes mellitus peripheral neuropathy. Linchuang Heli Yongyao Zazhi 9:3-5.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1-12.
Li QR, Wang Z, Zhou W, Fan SR, Ma R, Xue L, Yang L, Li YS, Tan HL, Shao QH, Yang HY (2016) Epalrestat protects against diabetic peripheral neuropathy by alleviating oxidative stress and inhibiting polyol pathway. Neural Regen Res 11:345-351.
Liang KY, Ou XJ, Lu YQ, Zhang YW, Zhou Y (2012) Therapeutic effect of lipoic acid combined with epalrestat on diabetic peripheral neuropathy. Shiyong Tangniaobing Zazhi 8:46-47.
Liu HM (2014) Efficacy of epalrestat combined with α-lipoic acid in the treatment of diabetic peripheral neuropathy. Tangniaobing Xinshijie 34:17-19.
Liu J (2016) To explore the clinical effect of lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy. Jin Ri Jian Kang 15:156.
Luo F, Hu JP, Li QC (2013) Clinical observation of α-lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy. Zhongguo Shiyong Yixue Zazhi 40:61-62.
Mehra M, Merchant S, Gupta S, Potluri RC (2014) Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ 17:637-645.
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P,Klassen TP (1998) Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352:609-613.
Nickander KK, McPhee BR, Low PA, Tritschler H (1996) Alpha-lipoic acid: antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy. Free Radical Biol Med 21:631-639.
Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ (2002) An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J 16:123-125.
Ogbera AO, Adeleye O, Solagberu B, Azenabor A (2015) Screening for peripheral neuropathy and peripheral arterial disease in persons with diabetes mellitus in a Nigerian University Teaching Hospital.BMC Res Notes 8:533.
Packer L (1998) alpha-Lipoic acid: a metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev 30:245-275.
Qu P, Zeng JE (2009) Treatment of diabetic peripheral neuropathy by α-lipoic acid combined with epalrestat in 25 cases. Changjiang Daxue Xuebao 6:33-34.
Sato K, Yama K, Murao Y, Tatsunami R, Tampo Y (2013) Epalrestat increases intracellular glutathione levels in Schwann cells through transcription regulation. Redox Biol 2:15-21.
Tesfaye S (2011) Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2:33-42.
Wang HX (2014) Efficacy of epalrestat and lipoic acid in the treatment of diabetic peripheral neuropathy. Chin J Prim Med Pharm 21:396-397.
Wang J, Chen JD (2014) Clinical observation of lipoic acid combined with epalrestat in the treatment of diabetic peripheral neuropathy.Med Inform 27:222.
Wang WL, Zhu H, Wang SX, Wang JY (2013) Efficacy of epalrestat combined with α-lipoic acid in the treatment of senile diabetic peripheral neuropathy. Chin J Gerontol 33:4854-4856.
Won JC, Park TS (2016) Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab 31:230-238.
Xiong WH (2014) Epalrestat combined with alpha lipoic acid in the treatment of diabetic peripheral neuropathy. Zhongguo Shiyong Yiyao 9:109-110.
Yagihashi S, Yamagishi S, Wada R (2007) Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Prac 1:S184-S189.
Yama K, Sato K, Murao Y, Tatsunami R, Tampo Y (2016) Epalrestat upregulates heme oxygenase-1, superoxide dismutase, and catalase in cells of the nervous system. Biol Pharm Bull 39:1523-1530.
Yan B (2015) Clinical efficacy of α-lipoic acid combined with epalrestat in the treatment of type 2 diabetic peripheral neuropathy. Zhongnan Yixue Kexue Zazhi 43:169-171, 216.
Yang XM (2014) Clinical observation of epalrestat combined with alpha lipoic acid in the treatment of diabetic peripheral neuropathy.Zhongguo Shequ Yishi 30:21-22.
Yang XW, Liu FQ, Guo JJ, Yao WJ, Li QQ, Liu TH, Xu LP (2015) Antioxidation and anti-inflammatory activity of Tang Bi Kang in rats with diabetic peripheral neuropathy. BMC Complement Altern Med 15:66.
Zhang QJ, Shi Y, Zhang YY, Dong HS, Zheng M (2007) Effect of electroacupuncture on the conduction velocity and microstructure of the sciatic nerve in rats with experimental diabetic peripheral neuropathy. Zhongguo Zuzhi Gongcheng Yanjiu 11:3069-3073.
Zhang T, Wang XM, Li X, Tang T (2014) Clinical observation of epalrestat combined with lipoic acid in the treatment of diabetic peripheral neuropathy. Zhongguo Xiandai Yaowu Yingyong 8:138-139.
Zhao HJ, Meng SH, Zhang XJ (2015) Curative effect of thioctic acid in the treatment of 66 cases of diabetic peripheral neuropathy. Shanghai Yixue 36:30-32.
Zhao M, Lin H, Yuan Y, Wang F, Xi Y, Wen LM, Shen P, Bu S (2016)Prevalence of Pre-Diabetes and Its Associated Risk Factors in Rural Areas of Ningbo, China. Int J Environ Res Public health 13:808.
Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA (1995) Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 38:1425-1433.
Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R (1999) Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-lipoic acid in diabetic neuropathy. Diabetes Care 22:1296-1301.
Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R (2006)Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 29:2365-2370.
- 中国神经再生研究(英文版)的其它文章
- Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease

