三维镍ギ-镧バ混金属配位聚合物的合成、晶体结构及其荧光性质
林雨青 高 敏 张 辉 彭 雪 顾 文 刘 欣 廖圣云*,
(1天津理工大学有机太阳能电池和光化学转换重点实验室,天津 300384)
(2南开大学化学系,天津 300071)
0 Introduction
The photoluminescent properties of lanthanidebased coordination polymers with a large Stokes′shift and relatively long luminescence lifetimes make them appropriate for a wide range of applications such as luminescent probes,imaging and sensor applications[1-5].In order to improve the luminescent emission properties of coordination polymers,the antenna effect used to elude the disadvantage of the very low absorption coeffcients of lanthanide ions can′t be ignored during the course of construction of Ln-based coordination polymers.Design and synthesis of the novel ligands with fully allowed π-π*transitions in the UV region has been demonstrated to be the best way[6-11].At the same time,introducing the d-block metal nodes such as Crバ,Coギ,Znギ and Ruギ have been demonstrated to effectively transfer energy to Lnバions indirectly[12-16].So the 3d-4f heterometallic complexes pave us the way for finding some novel luminescence materials.
The main routine for constructing 3d-4f heterometallic complexes is the application of multiple N-and O-donor ligands,particularly the application of heterocyclic polycarboxylic ligands such as pyridine-2,4-dicarboxylic acid and pyrazine-2,3-dicarboxylic acid[17-20].That′s because lanthanide and transition metal ions possess different affinities for N and O atoms,owing to the hard-soft acid/base classification.It has been found that Zn-Ln,Co-Ln or Cd-Ln complexes with conjugated heterocyclic polycarboxylic ligands displayed enhancing luminescent properties[21-24].Especially,some heterodinuclear complexes containing Ndバ and Smバ exhibits emissions in NIR region[26-27].However,the competition among the different metal ions leads to the difficulty for synthesis of 3d-4f heterodinuclear complexes,especially for the complexes with high dimensions[13].
Herein,we selected multi-topic phenyl heterocyclic polycarboxyl acid H3ctia (H3ctia=5-(4-carboxy-1H-1,2,3-triazol-1-yl)isophthalic acid)as the starting material for assembling high dimension 3d-4f coordination polymers in consideration of the following reasons:(1)The linker modes of the multitopic ligand are controlled by O donors and N donors;(2)The aromatic triazol and benzene rings may be coplanar,which can enhance the conjugation degree of the system and influence the photoluminescent properties.
In this paper,we reported the synthetic details,the structuralinformation and the photophysical properities of a series of 3D Niギ-Lnバ heterometallic coordination polymers based on H3ctia.
1 Experimental
1.1 General procedure
The ligand H3ctia was prepared according to the reported methods[13].The reagents and solvents were obtained from commercial sources and used as received without further purification.Elemental analyses were determined on a Perkin-Elmer PE 2400 CHNS/O analyzer.The photoluminescence spectra were obtained using Edinburgh instruments FLS920 steady state spectrometer with a Xe-lamp source.
1.2 Synthesis of[Ln2Ni(tia)4(H2O)4]n(1~9)
The synthetic methods of complexes 1~9 are similar.Complex[La2Ni(tia)4(H2O)4]n(1)was set as an example to illustrate the detailed synthetic procedure.Ni(NO3)2·6H2O(0.058 3 g,0.2 mmol),and the aqueous solution of H3ctia(16 mL,pH=4.0,containing 0.2 mmol H3ctia,adding NaOH to adjust the pH value)was placed in a 20 mL Teflon-linear autoclave to result in the green transparent solution.After the solution was left standing for 12 h,La(NO3)3·6H2O(0.058 2 g,0.2 mmol)was added and the reaction mixture was placed in an oven.The reaction mixture was heated to 160℃within 2 h,and kept for 72 h,and then cooled down to room temperature at a rate of 2℃·h-1.Green block crystals suitable for X-ray structural analysis were obtained with a yield of 0.060 g(60%yield based on La(NO3)3·6H2O).Anal.Calcd.for C40H28La2N12NiO20(%):C,36.04;H,2.12;N,12.61.Found(%):C,36.06;H,2.13;N,12.62.
[Pr2Ni(tia)4(H2O)4]n(3) (33% yield based on Pr(NO3)3·6H2O)Anal.Calcd.for C40H28Pr2N12NiO20(%):C,35.93;H,2.11;N,12.57.Found(%):C,35.90;H,2.13;N,12.52.
[Nd2Ni(tia)4(H2O)4]n(4)(45% yield based on Nd(NO3)3·6H2O)Anal.Calcd.for C40H28Nd2N12NiO20(%):C,37.75;H,2.10;N,12.51.Found(%):C,37.80;H,2.11;N,12.52.
[Sm2Ni(tia)4(H2O)4]n(5) (21% yield based on Sm(NO3)3·6H2O)Anal.Calcd.for C40H28Sm2N12NiO20(%):C,35.43;H,2.08;N,12.39.Found(%):C,35.40;H,2.10;N,12.41.
[Eu2Ni(tia)4(H2O)4]n(6)(18% yield based on Eu(NO3)3·6H2O)Anal.Calcd.for C40H28Eu2N12NiO20(%):C,35.34;H,2.08;N,12.36.Found(%):C,35.38;H,2.11;N,12.39.
[Gd2Ni(tia)4(H2O)4]n(7)(14% yield based on Gd(NO3)3·6H2O)Anal.Calcd.for C40H28Gd2N12NiO20(%):C,35.07;H,2.06;N,12.27.Found(%):C,35.09;H,2.04;N,12.29.
[Tb2Ni(tia)4(H2O)4]n(8)(9%yeild based on Tb(NO3)3·6H2O)Anal.Calcd.for C40H28Tb2N12NiO20(%):C,34.98;H,2.06;N,12.24.Found(%):C,35.02;H,2.05;N,12.27.
[Dy2Ni(tia)4(H2O)4]n(9)(15% yield based on Dy(NO3)3·6H2O)Anal.Calcd.for C40H28Dy2N12NiO20(%):C,34.80;H,2.04;N,12.18.Found(%):C,34.85;H,2.05;N,12.20.
上边总是说,有一种看不见的硝烟到处弥漫着,弄得下边只好没完没了地开会。在这种时候,撞上一个不开会的夜晚,对我这个正为迟到的约会而闹心的人来说,就像饥饿难耐时天上掉下个肉馅饼,不禁暗自欢喜。于是,趁着月亮还没有爬上树梢,我悄悄地溜出了村庄。
1.3 Synthesis of([Yb2Ni(tia)4(H2O)2]·2H2O}n(10)
Ni(NO3)2·6H2O(0.058 3 g,0.2 mmol)and the mixture solution of H3ctia (16 mL,VH2O∶VCH3OH=5∶3,pH=2,containing 0.2 mmol H3ctia,adding NaOH to adjust the pH value)was placed in 20 mL Teflonlinear autoclave to result in the green transparent solution.After the solution was left standing for 12 h,Yb(NO3)3·6H2O(0.0918 g,0.02 mmol)was added and the reaction mixture was placed in an oven.The reaction mixture was heated to 140℃within 2 h,and kept for 72 h,and then cooled down to room temperature at a rate of 1.5 ℃·h-1.Green square crystals suitable for X-ray structural analysis were obtained with a yield of 0.005 0 g(4%yield based on Yb(NO3)3·6H2O),Anal.Calcd.for C40H28Yb2N12NiO20(%):C,34.28;H,2.01;N,11.99.Found(%):C,34.30;H,2.03;N,11.85.
1.4 X-ray structure determination
Diffraction data for complexes 1~10 were collected with a Bruker SMART APEX CCD instrument with graphite monochromatic Mo Kα radiation(λ=0.071 073 nm).The data were collected at 293(2)K.The absorption corrections were made by multi-scan methods.The structure was solved by XS direct methods with the program Olex2 and refined by full-matrix leastsquares methods on all F2data with Olex2.The nonhydrogen atoms were refined anisotropically.Hydrogen atoms of water molecules were located in a different Fourier map and refined isotropically in the final refinement cycles.Other hydrogen atoms were placed in calculated positions and refined by using a riding model.The final cycle of full-matrix least-squares refinement was based on observed reflections and variable parameters[28-29].Crystal data and structure refinement for the complexes are listed in Table 1~3.
CCDC:1822039,1;1822038,2;1822040,3;1822037,4;1822035,5;1822036,6;1822041,7;1822042,8;1822043,9;1821893,10.
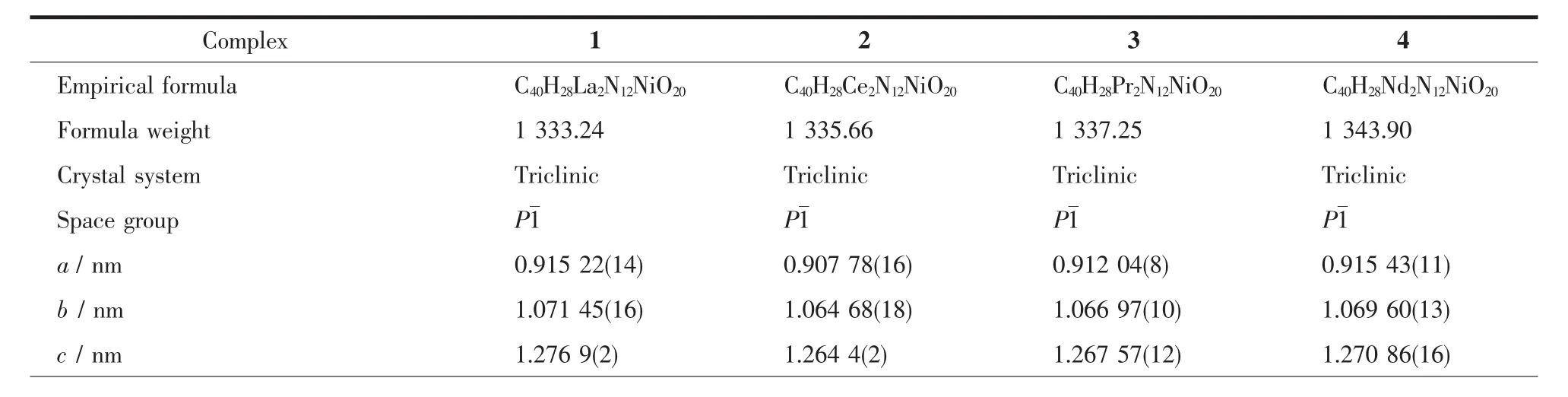
Table 1 Crystal data and structure refinement for 1~4
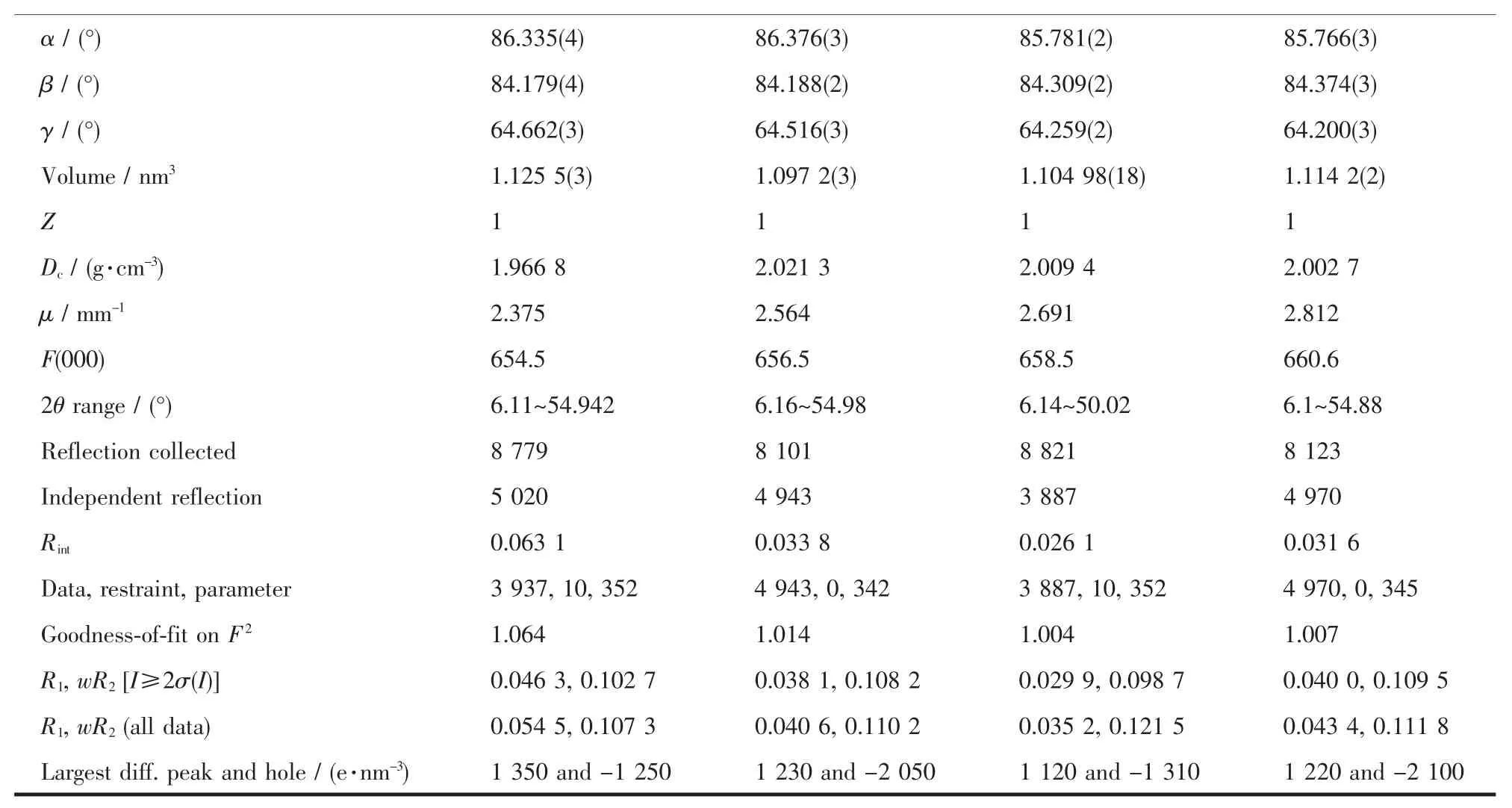
Continued Table 1
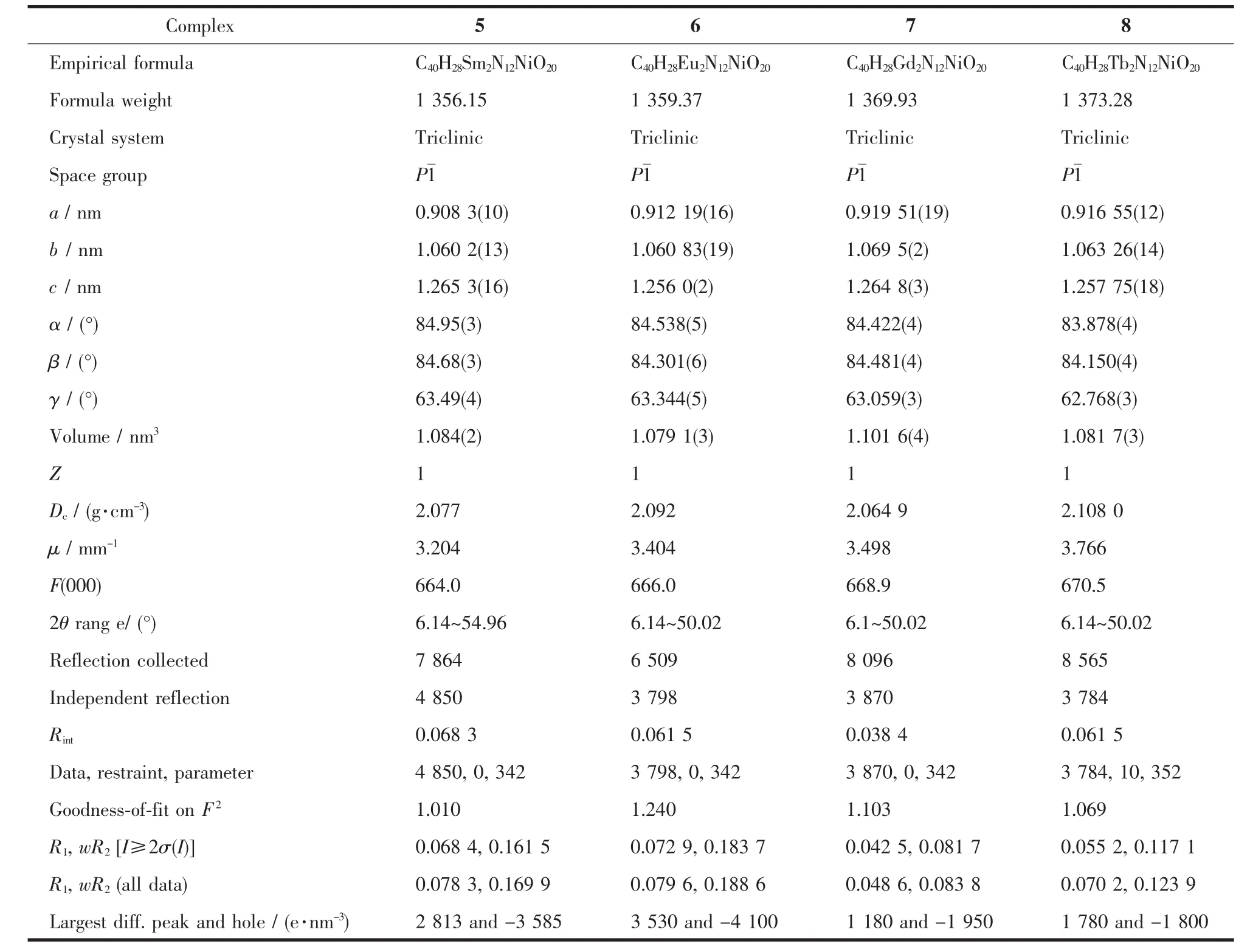
Table 2 Crystal data and structure refinement for 5~8
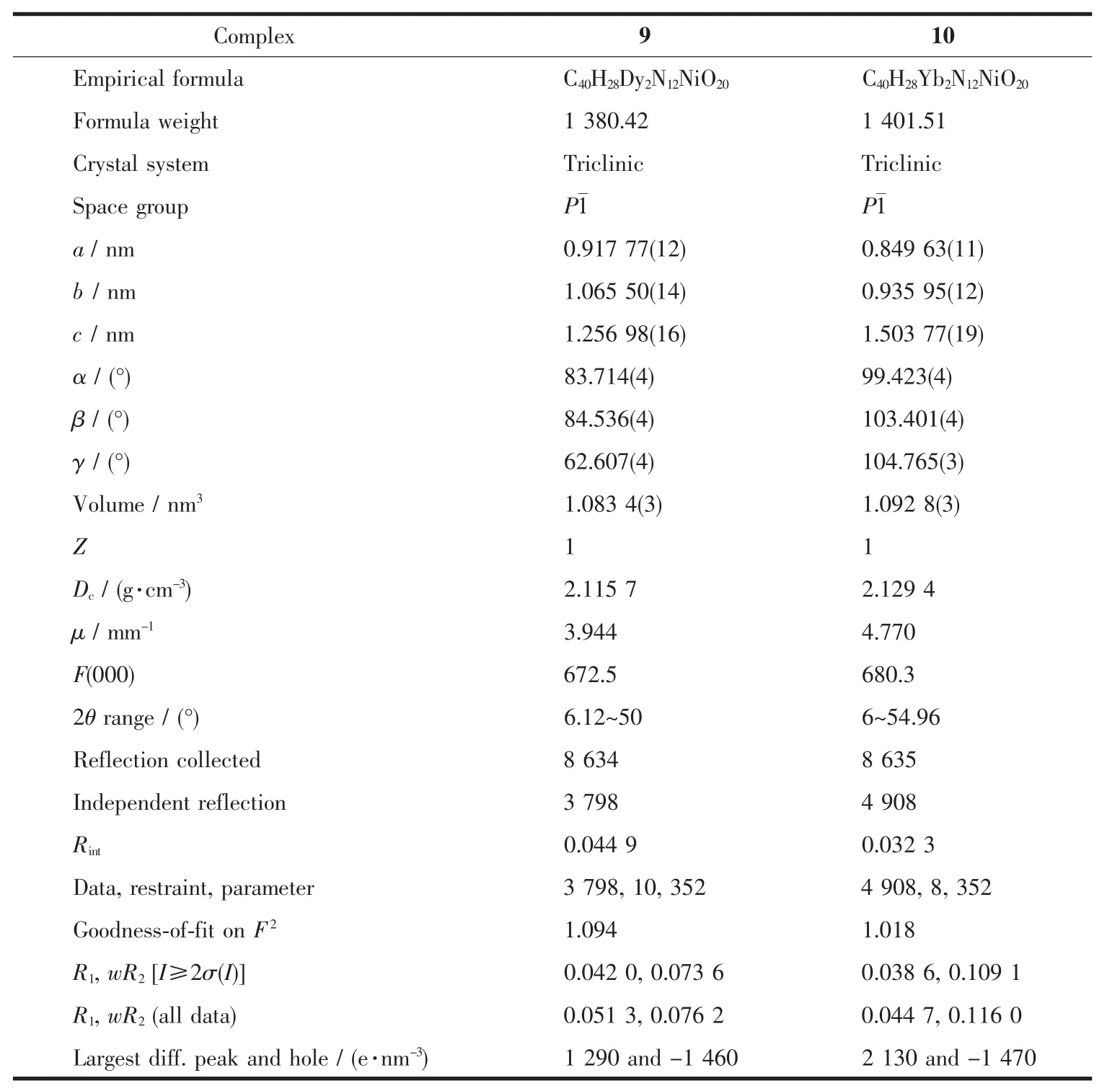
Table 3 Crystal data and structure refinement for 9 and 10
2 Results and discussion
2.1 Description of crystal structure
During the course of construction complexes 1~10,H3ctia underwent decarboxylation and doubly deprotonation to anionic tia2-(Scheme 1).
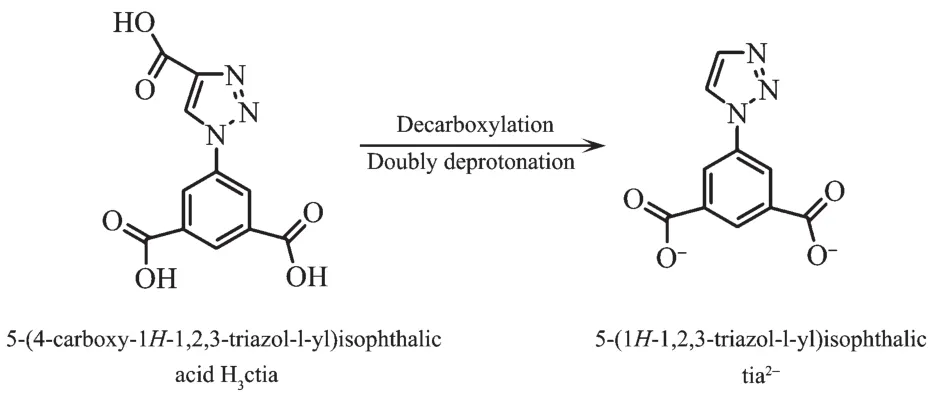
Scheme 1 Conversion of ligand H3ctia in construction of 1~10
Single-crystal X-ray analysis of 1~10 indicates complexes 1~9 crystalize in space group P1(Table 1~3).Their structure are very similar to our previous reported Co-Ln heterometallic complexes[13].The asymmetric unit of them contains one Lnバion,half a Niギion,two tia2-anions and two coordination water molecules (Fig.1a).The coordination modes of tia2-anions are μ3-bridging and μ5-bridging,designated as LAand LB,respectively (Fig.2a).The center Niギion is six-coordinated in an tetragonal bipyramid octahedral coordination geometry with four N atoms(two N1 and two N4)from four tia2-and two O3 from the relevant symmetrical tia2-occupied the equatorial position.The Lnバmetal center coordinate with O1,O2,O5,O6,O7vi,O8viiand two water molecules(O1 and O2 from the same ligand LA,while O5,O6,O7vi,and O8viifrom the four different ligand LB).Along the crystallographic a axis,ligand LBuse the carboxyl O atom to bridge Lnバto form the metal coordination chains.Along the b axis,the Lnバ coordination chains are connected by the carboxyl group on the meta-position of LBto afford the Lnバcoordination layers.The triazol ring of LAand LBis 56.7°and 69.4°deviated from the benzene ring,respectively.In ab plane,N1 and N4 from the triazol ring coordinated with Niギ to form the parallel arrangement Niギcoordination chains,which are sandwiched between two Lnバlayers[13].

Fig.1 (a)Coordination environments of Lnバ and Niギ in 1~9;(b)Coordination environments of Ybバ and Niギ in 10
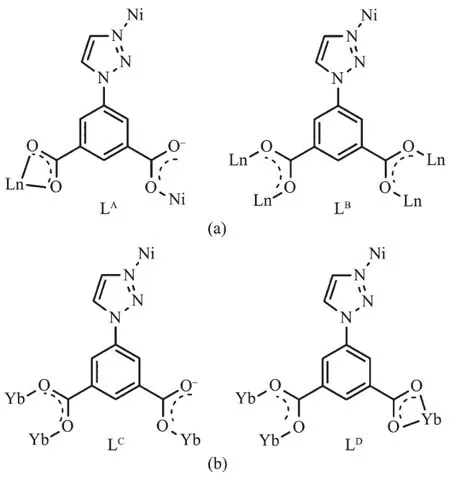
Fig.2 Bridging metal ions modes of the ligand tia2-in 1~10:(a)LAand LBin 1~9;(b)LCand LDin 10
Complex{[Yb2Ni(tia)4(H2O)2]·2H2O}n(10)crystallizes in triclinic system and space group P1.The chemical composition of 10 is same as complexes 1~9.The asymmetric unit of 10 also contains one Ybバion,half a Niギion,and two tia2-anions(Fig.1b).But the coordination modes of metal ions,the bridging patternsofthe ligand,and the spatialnetwork structure of 10 are totally different from 1~9.The dianionic ions bridge metal centers with two μ4-bridging modes(LCand LDin Fig.2b).In complex 10,the equatorial positions of the six-coordinated Niギin tetragonal pyramid geometry are occupied by two N1 from LDand two N4 from LC(Fig.1b).Two coordination water molecules occupy the aixs apical position(Fig.1b).The Ybバions coordinate with O1i,O2i,O3ii,O4iv,O5v,O7iiiand O8(O1iand O2ifrom the same LD;O3iiand O4ivfrom the other LD;O5v,O7iiiand O8 from three different LC,Fig.1b).In crystallographic ab plane,LCuse O7ivand O8iiito bridge two Ybバions to afford the dinuclear units.While in bc plane,LDuse O3iand O4iito bridge two Ybバions to form another dinuclear units (Fig.3 and 4).These Ybバdinuclear units are alternaterly arranged to form the laddershaped Lnバcoordination chains (Fig.3 and 4).The Niギcenters are bridged by LCand LD(Fig.5).These Lnバ coordination chains are connected by Niギcenters to form 3D network structure(Fig.6).
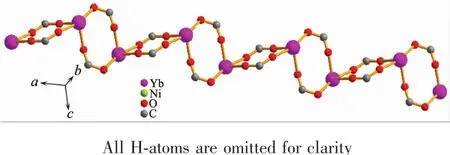
Fig.3 Ladder-shaped Ybバions coordination chains in 10

Fig.4 Linked modes of the ladder-shaped chains in 10
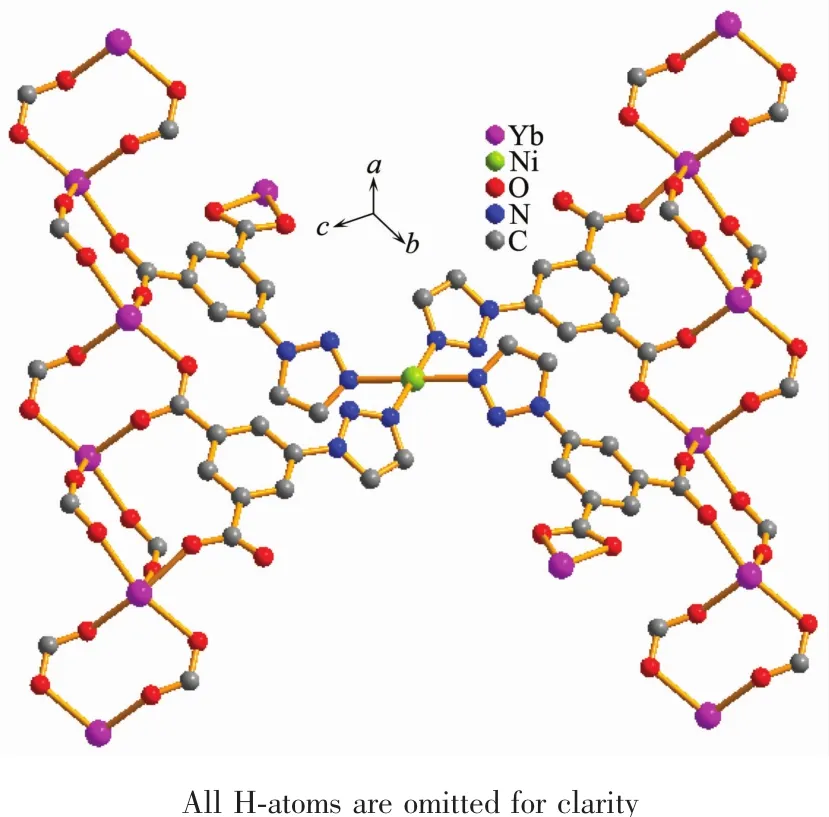
Fig.5 Ybバ coordination chains connected by Niギcenters to form the network in 10

Fig.6 View of the 3D network of 10
2.2 Luminescent properties
The luminescence properties of complexes 1~10 were investigated.Complexes 5,6,8 and 9 display the luminescence emissions with long luminescence lifetime in the visible region.As shown in Fig.7,the emission peaks at 487,544,584 and 620 nm should be assigned to the transitions from4G5/2→6Hj(j=5/2,7/2,9/2 and 11/2)of Smバ.
The characterstic transitions of Euバfrom5D0→7Fj(j=1,2,3 and 4)at 590,616,699 and 729 nm were observed from the visible emission spectra of complex 6(Fig.8a).The luminescence decay curves from the time-resolved luminescence experiments of complex 6 at the maximal excitation(λex=306 nm)and emission (λem=616 nm)wavelength in the solid at room temperature was fitted by multi-exponential Eq.(1).The best fitting parameter is τ1=92.01 μs(17.03%)and τ2=263.30 μs(82.97%)(Fig.8b).
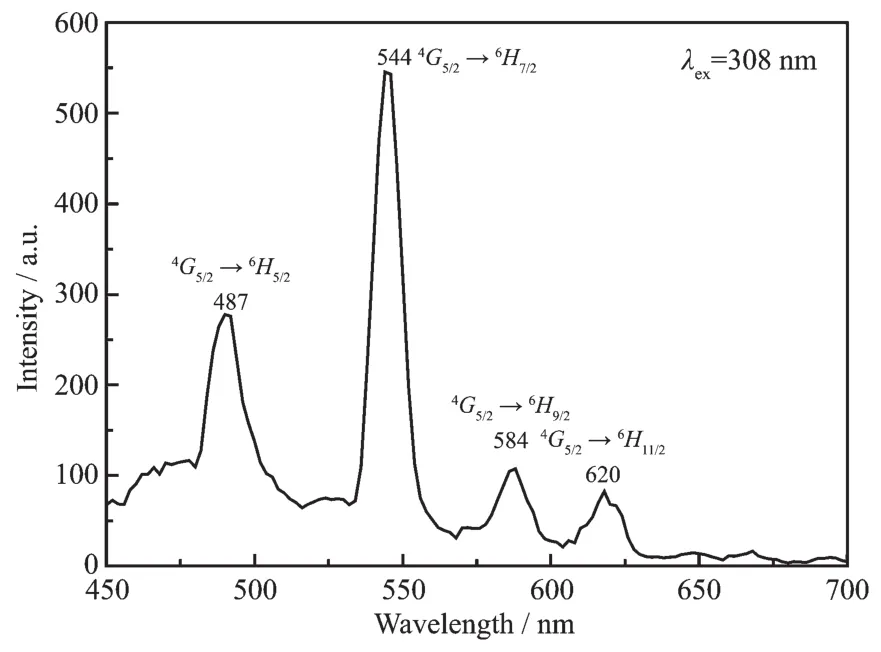
Fig.7 Emission spectrum of 4 in the solid state at room temperature

Fig.8 Emission spectrum and the decay curve of 6 in the solid state at room temperature

The emission peaks appeared at 487,544,584 and 620 nm could be ascribed to the characteristic transitions of Tbバ from5D4to7Fj(j=6,5,4,and 3)(Fig.9a).Excited at λ=310 nm,the luminescence decay curve of complex 8 was obtained and the best fitted results verify the luminescence lifetime of complex 8 display di-expotiential behavior with τ1=282.7 μs(22.88%)and τ2=690.7 μs(77.22%)(Fig.9b).
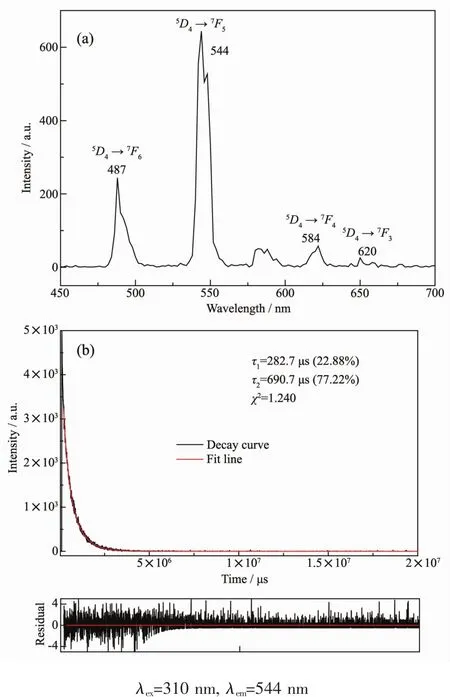
Fig.9 Emission spectrum and the decay curve of 8 in the solid state at room temperature
Excited at λ=306 nm,the characteristic emission peaks of Dyバin the visible region at 479 and 573 nm were observed(Fig.10a)and the transitions can be ascribed to4F9/2→6Hj(j=15/2 and 13/2).The fitted result of the luminescence decay curve excited at λ=306 nm indicates the luminescene lifetime of complex 9 possesses bi-exponential behavior with τ1=1.76 μs(72.39%)and τ2=6.08 μs(27.61%)(Fig.10b).

Fig.10 Emission spectrum and the decay curve of 9 in the solid state at room temperature
We also investigated the lunminescence properties of complexes 1~10 in the near infrared region.As shown in Fig.11a,the emission peaks at 898,1 063 and 1 330 nm should be assigned to the transitions of4F3/2→4Ij(j=9/2,11/2 and 13/2)of Ndバ.Excited at λmax=322 nm,the luminescence decay curve of complex 4 was measured and fitted with Eq.(1)(Fig.11b).The luminescence lifetime of complex 4 display di-expotiential behavior with τ1=1.06 μs(52.62%)and τ2=7.80 μs(47.38%).
For complex 8,the emission peak at 1 150 nm should be attributed the transition of4F9/2→6F5/2of Dyバ (Fig.12a).The transitions of4F9/2→6F7/2and4F9/2→6F3/2was not observed.The luminescence decay curve was obtained at λex=302 nm and the fitted result display the luminescence lifetime ofcomplex 8 possesses bi-expotiential behavior with τ1=1.764 μs(72.39%)and τ2=6.095 μs(27.61%)(Fig.12b).
The emission of spectrum of complex 10 in the near infrared was also obtained.The emission peaks at 996 nm should be assigned to the transition of2F5/2→2F7/2of Ybバ (Fig.13a).The fitted result of the luminescence decay curve excited at 372 nm display di-expotiential behavoir with τ1=1.53 μs(16.43%)and τ2=6.55 μs(82.57%)(Fig.13b).
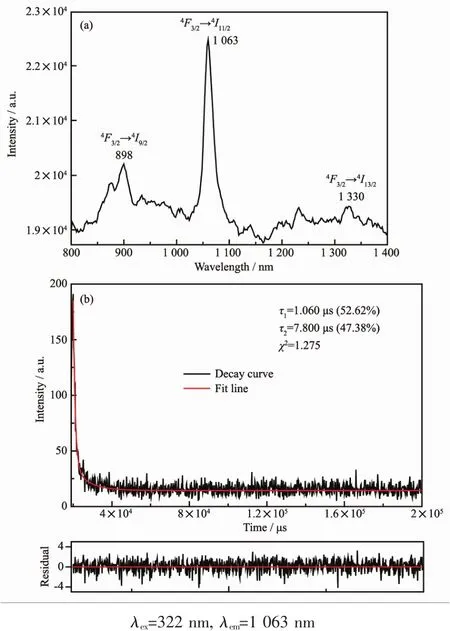
Fig.11 NIR emission spectrum and the decay curve of 4 in the solid state
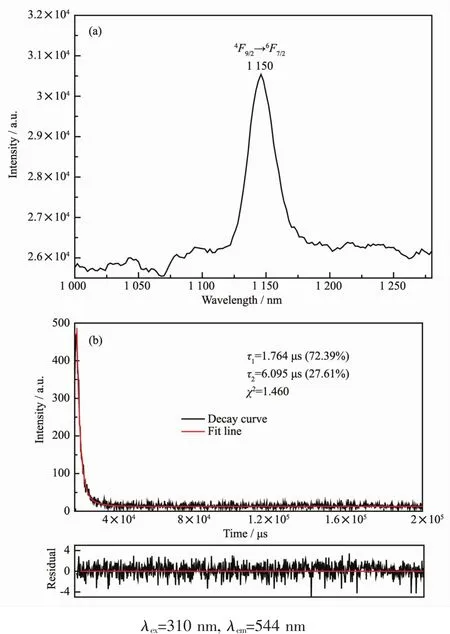
Fig.12 Emission spectrum and the decay curve of 9 in the solid state

Fig.13 Emission spectrum and the decay curve of 10 in the solid state
3 Conclusions
A series of 3D Niギ-Lnバ heterometallic complexes of 1~10(Ln=La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Dy and Yb)have been synthesized.The structural characterization from the single X-ray diffraction data show complexes 1~9 are isostructural,in which the 3D frameworks are composed of the parallel arrangement Niギ coordination chains and Lnバcoordination layers.While in complex 10,the ladder-shaped Lnバcoordination chains are connected by Niギcenters to form 3D network structure.Investigation to the photoluminescence of these 3D coordination polymers verify that Niギ-Smバ,Niギ-Euバ,Niギ-Tbバ and Niギ-Dyバcomplexes exhibit luminescent emissions with the long luminescentlifetime in the visible region.Furthermore,the characteristic emission bands of Niギ-Ndバ,Niギ-Dyバ and Niギ-Ybバ complexes in NIR regions are observed.So this work might paint new picture for expanding the crystal engineering strategy and enriching luminescent materials.

