两个包含醚氧桥联三羧酸配体的锌Ⅱ配位聚合物的合成、晶体结构及荧光性质
黎 彧 邹训重 庄文柳 邱文达*, 顾金忠
(1广东轻工职业技术学院,佛山市特种功能性建筑材料及其绿色制备技术工程中心,广州 510300)
(2兰州大学化学化工学院,兰州 730000)
0 Introduction
Over the past decades,there has been a deal of great interest in constructing zinc coordination polymers (CPs)due to their novel structural features and promising functional properties in the areas of photochemistry,selective sensing,catalysis,antibiosis,or gas adsorption[1-8].However,the construction of coordination polymers in a predictable and controlled manner still remains a challenging task,since their structural and topological characteristics significantly depend on a lot of synthetic parameters[9-12],such as the metal atoms,the type of the main building blocks,templatesor auxiliary ligands[13-16].The preliminary work shows that different aromatic multicarboxylic acids have been extensively used as versatile building blocks toward the assembly of coordination polymers[1,10,13,16-17].
In order to extend our research in this field,we have selected two semi-rigid ether-bridged tricarboxylate ligands (Scheme 1),namely 2-(4-carboxylphenoxy)terephthalic (H3cpta)and 2-(3,5-dicarboxylatobenzyloxy)benzoic (H3dbba)acids,and explored them for the construction of novel zincⅡcoordination polymers.Both H3cpta and H3dbba blocks show several interesting features: (1)they can rotate or twist to generate different angles between the two phenyl planes via the ether-oxygen functionality to furnish a subtle conformational adaptation; (2)both blocks possess seven potential coordination sites (six carboxylate O atoms and one O-ether functionality),thus leading to a variety of potential coordination modes;(3)two blocks still remain largely unexplored in the design of coordination polymers.
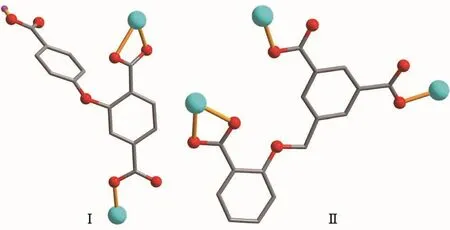
Scheme 1 Coordination modes of Hcpta2-and dbba3-ligands in complexes 1 and 2
Herein we report the syntheses, crystal structures,and luminescent properties of two ZincⅡcoordination polymers constructed from two etherbridged tricarboxylic acids.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis (TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃·min-1.Excitation and emission spectra were recorded for the solid samples on an Edinburgh FLS920 fluorescence spectrometer at room temperature.
1.2 Synthesis of[Zn(μ-Hcpta)(4,4′-bipy)(H 2O)]n (1)
A mixture of ZnCl2(0.041 g,0.30 mmol),H3cpta(0.091 g,0.30 mmol),4,4′-bipy (0.047 g,0.3 mmol),NaOH (0.024 g,0.60 mmol)and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃·h-1.Colorless block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:55%(based on H3cpta).Anal.Calcd.for C25H18ZnN2O8(%):C 55.62,H 3.36,N 5.19;Found(%):C 55.74,H 3.37,N 5.16.IR(KBr,cm-1):3 658w,3 060w,1 702w,1 576s,1 540w,1 492w,1 413s,1 366m,1 234m,1 167w,1 130w,1 071 w,1 040w,1 008w,957w,909w,857w,820w,783w,763w,720w,694w,641w.
1.3 Synthesis of{[Zn3( μ3-dbba)2(phen)3]·6H 2O}n(2)
Complex 2 was prepared by ZnCl2(0.041 g,0.30 mmol),H3dbba (0.063 g,0.2 mmol),phen (0.060 g,0.3 mmol),NaOH (0.024 g,0.60 mmol)and H2O (10 mL)using the same procedure for complex 1.Colorless block-shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:60%(based on H3dbba).Anal.Calcd.for C68H54Zn3N6O20(%):C 55.51,H 3.70,N 5.71;Found (%):C 55.37,H 3.68,N 5.74.IR (KBr,cm-1):3 440m,1 608s,1 586s,1 564s,1 520 w,1 487w,1 426m,1 364s,1 276w,1 251w,1 145w,1 105w,996w,908w,850m,809w,777m,726m,686w,646w.The complexes are insoluble in water and common organic solvents,such as methanol,ethanol,acetone and DMF.
1.4 Structure determinations
Two single crystals with dimensions of 0.25 mm×0.23 mm×0.22 mm (1)and 0.28 mm×0.25 mm×0.18 mm (2)were collected at 293(2)K on a Bruker SMART APEXⅡ CCD diffractometer with Mo Kαradiation(λ=0.071073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[18].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.Details of the crystal parameters,data collection and refinements for 1 and 2 are summarized in Table 1.The selected bond lengths and angles for complexes 1 and 2 are listed in Table 2.Hydrogen bond parameters of complexes 1 and 2 are given in Table 3 and 4.
CCDC:1866025,1;1866026,2.
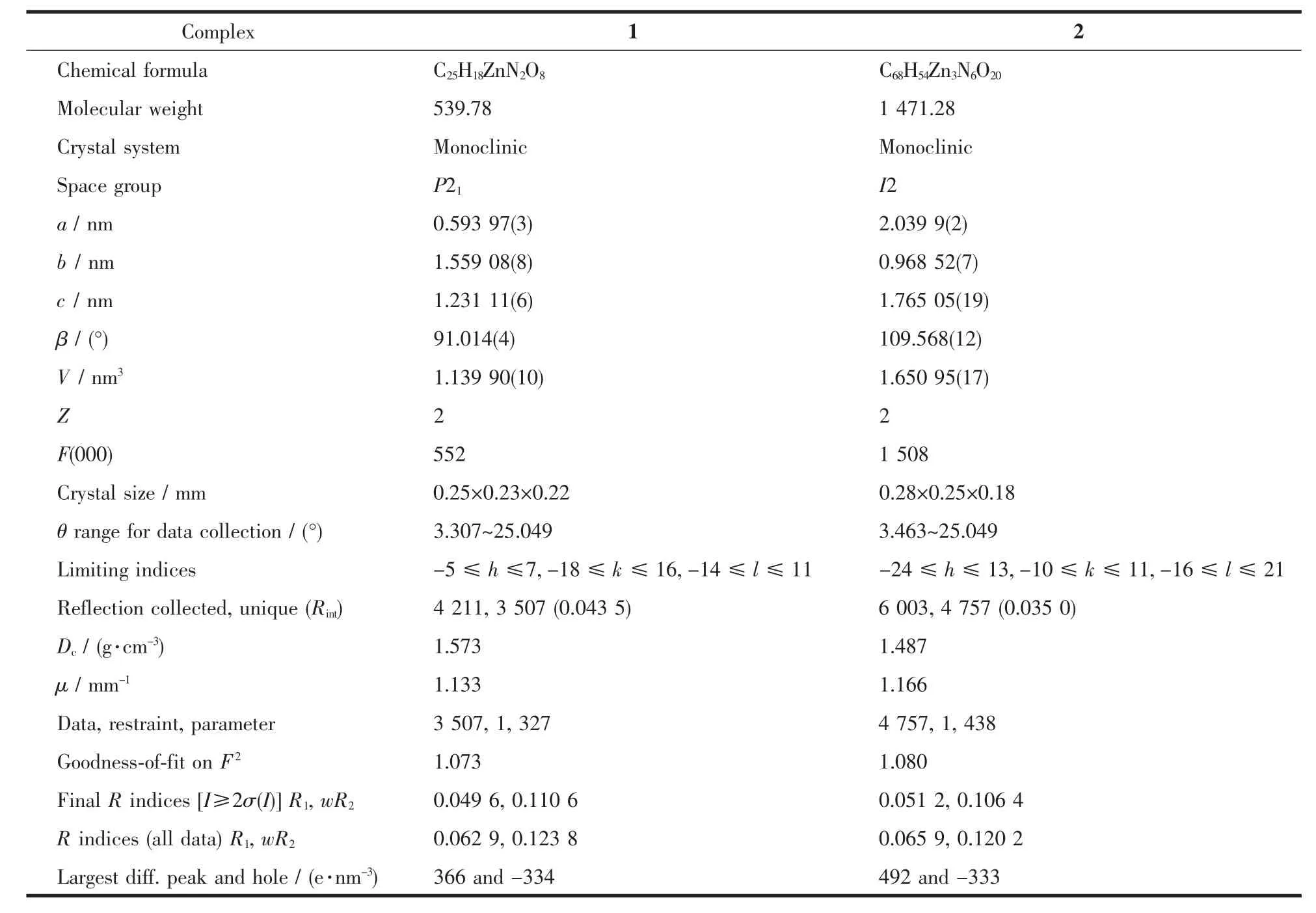
Table 1 Crystal data for complexes 1 and 2

Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2

Symmetry codes:A:-x+2,y-1/2,-z+1 for 1;A:-x+1,y,-z;B:-x+1,y,-z+1 for 2

Table 3 Hydrogen bond parameters for complex 1

Table 4 Hydrogen bond parameters for complex 2
2 Results and discussion
2.1 Description of the structures of complexes
2.1.1 [Zn(μ-Hcpta)(4,4′-bipy)(H2O)]n(1)
Complex 1 is a 1D coordination polymer and its asymmetric unit consists of a Zn1 atom,aμ-Hcpta2-block,one 4,4′-bipy ligand and one coordinated water molecule.As shown in Fig.1,penta-coordinate Zn1 atom adopts a distorted trigonal bipyramid{ZnNO4}geometry,which is taken by three carboxylate Oatoms from two differentμ-Hcpta2-linkers,one O atom from the coordinated water molecule and one N atom from the 4,4′-bipy moiety.Distances of the Zn-O bonds vary from 0.195 2(7)to 0.231 5(6)nm,while the Zn-N ones are 0.202 8(7)nm;all of them are in good agreement with those in related ZnⅡcomplexes[2,13-14].The Hcpta2-moiety acts as a μ-linker (Scheme 1,modeⅠ)with two COO-groups showing monodentate or bidentate modes;the COOH functionality remains uncoordinated.The 4,4′-bipy moiety adopts a terminal coordination fashion.In Hcpta2-,a dihedral angle(between two benzene rings)and a C-Oether-C angle are 67.27°and 118.46°,respectively.The μ-Hcpta2-blocks connect the Zn1 centers to form a 1D chain structure with the Zn1…Zn1 separation of 1.087 5(6)nm (Fig.2).
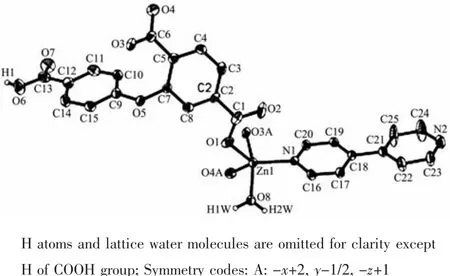
Fig.1 Drawing of the asymmetric unit of complex 1 with 30%probability thermal ellipsoids
2.1.2 {[Zn3(μ3-dbba)2(phen)3]·6H2O}n(2)
Polymer 2 also discloses a 1D chain structure and its asymmetric unit comprises two individual ZnⅡatoms (Zn1 with full occupancy;Zn2 with half occupancy),a μ3-dbba3-block,one and a half phen ligand and three lattice water molecules. As illustrated in Fig.3,the five-coordinate Zn1 atom is surrounded by three O atoms from two differentμ3-dbba3-blocks and two N atoms of phen,resulting in a distorted square pyramid{ZnN2O3}geometry.The Zn2 center is tetra-coordinate and reveals a distorted tetrahedral{ZnN2O2}environment,which is completed by two O atoms from two differentμ3-dbba3-blocks and two N atoms from phen.The lengths of the Zn-O and Zn-N bonds span in the 0.198 7(5)~0.215 9(7)nm and 0.209 2(7)~0.210 3(7)nm range;these are comparable to those observed in other zinc complexes[17,19-20].In 2,the dbba3-block adopts aμ3-coordination mode (Scheme 1,mode Ⅱ),wherein three COO-groups adopt a monodentate or a bidentate mode.Theμ3-dbba3-block shows a C-Oether-C angle of 118.54°,while a dihedral angle involving two aromatic rings attains 14.55°.The μ3-dbba3-linkers alternately connect the neighboring ZnⅡatoms to form a 1D metal-organic chain (Fig.4).The structural differences of two polymers may be attributed to the different ether-bridged tricarboxylate ligands and auxiliary ligands.
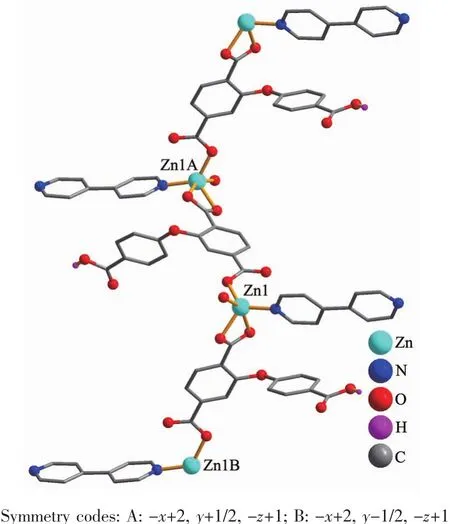
Fig.2 Perspective of 1D chain parallel to the bc plane in 1
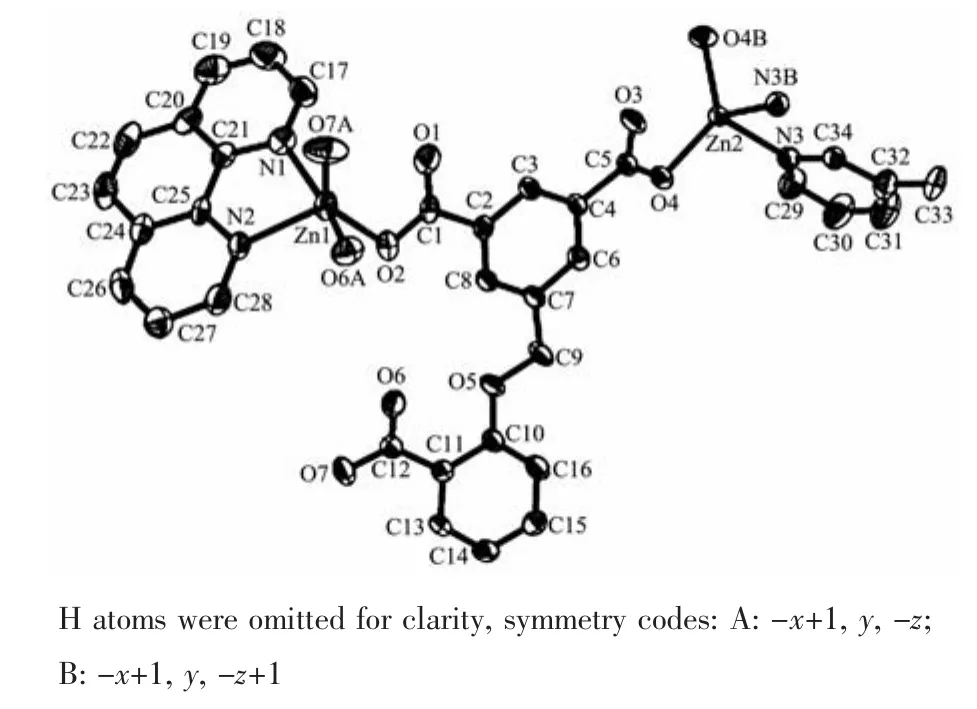
Fig.3 Drawing of the asymmetric unit of complex 2 with 30%probability thermal ellipsoids

Fig.4 One dimensional metal-organic chain viewed along the b axis in complex 2
2.2 TGA analysis
To determine the thermal stability of polymers 1 and 2,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.5,complex 1 lost its one coordinated water molecule in the range of 120~159℃ (Obsd.3.4%;Calcd.3.3%),followed by the decomposition at 222℃.The TGA curve of 2 shows that six lattice water molecules were released between 30 and 129 ℃ (Obsd.7.0%;Calcd.7.3%),and the dehydrated solid begins to decompose at 251℃.

Fig.5 TGA curves of complexes 1 and 2
2.3 Luminescence properties
The emission spectra of H3cpta,H3dbb a and complexes 1 and 2 were measured in the solid state at room temperature,as shown in Fig.6.The free H3cpta and H3dbba ligands showed two weak photoluminescence with emission maximum at 463 nm (λex=378 nm)and 456 nm (λex=325 nm),respectively.For polymers 1 and 2,the more intense emission bands were observed with maximum at 467 nm for 1 and 395 nm for 2 (λex=320 nm in both cases).All bands can be assigned to an intraligand (π*→n orπ*→π)emission[2,7,13]. The enhancement of luminescence of complexes 1 and 2 can be attributed to the binding of ligands to the metal atoms,which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay[1,14,16].

Fig.6 Solid state emission spectra of H3cpta,H3dbba and complexes 1 and 2
3 Conclusions
In summary,we have successfully synthesized and characterized two new zinc coordination polymers by using two ether-bridged tricarboxylic acids as bridging ligands under hydrothermal conditions.Two polymers feature two different 1D chain structures.Both the complexes also show promising luminescent properties. The obtained herein results should motivate further research aiming at the exploration of 2-(4-carboxylphenoxy)terephthalic acid and 2-(3,5-dicarboxylatobenzyloxy)benzoic acid as versatile semirigid tricarboxylate building blocks for the assembly of functional coordination polymers.

