Pulmonary tumor thrombotic microangiopathy of hepatocellular carcinoma: A case report and review of literature
Shinichi Morita, Kenya Kamimura, Hiroyuki Abe, Yukari Watanabe-Mori, Chiyumi Oda, Takamasa Kobayashi,Yoshihisa Arao, Yusuke Tani, Riuko Ohashi, Yoichi Ajioka, Shuji Terai
Abstract
Key words: Pulmonary tumor thrombotic microangiopathy; Hepatocellular carcinoma;Respiratory dysfunction; Prednisolone; Supportive care; Case report
INTRODUCTION
Various pathologic conditions, including diffuse alveolar lesions, lymphangiopathy,and pulmonary microembolism, are known causes of respiratory failure in cases of pulmonary malignancy[1]; however, these conditions are relatively rare in cases of hepatocellular carcinoma (HCC), possibly because HCC causes hepatic dysfunction and/or bleedings rather than respiratory dysfunction in the terminal stage. Therefore,pulmonary tumor microembolisms, including those of pulmonary tumor thrombotic microangiopathy (PTTM), are especially rare in HCC cases; and only a few cases have been reported, and the symptoms, imaging findings, therapeutic options, and prognoses have not been summarized to date. PTTM, first reported by von Herbayet al[2]in 1990, is a special cause of pulmonary tumor embolism in which tumor cells cause thickening of pulmonary arterial endothelium or form thrombi, which in turn cause narrowing and occlusion of the pulmonary arteries, resulting in pulmonary hypertension, dyspnea, and hypoxemia[3]. Our recent case with HCC who developed PTTM exhibited dyspnea with severe respiratory failure was diagnosed by minute histological analysis on autopsy and the information obtained was important to manage the symptoms in that stage. For a better understanding of the disease and management of symptoms, we have conducted a literature review of 18 reported cases[1,4-20]with our case.
CASE PRESENTATION
Chief complaints
A 72-year-old Japanese man was diagnosed with HCC and liver cirrhosis, caused by alcohol abuse, in 2011, and was referred to our hospital for therapeutic management.Since then, transcatheter arterial chemoembolization and radiofrequency ablation had been performed repeatedly, followed by the oral administration of sorafenib, 400 mg daily. After 1 year of sorafenib treatment, he was admitted to our hospital for dyspnea and low back pain. Computed tomographic (CT) scans revealed multiple HCC tumors in the liver (Figure 1A), as well as sacral bone metastases (Figure 1B) and multiple metastatic nodules in the lungs (Figure 1C) but no ascites.
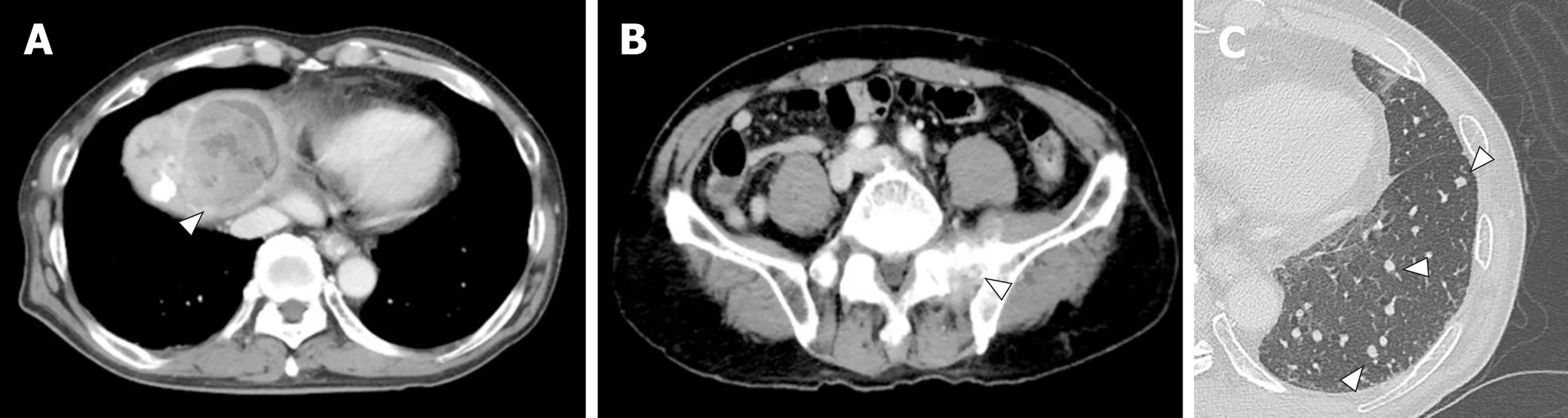
Figure 1 Computed tomographic scans of hepatocellular carcinoma. A: Computed tomographic scans of hepatocellular carcinoma (HCC) in the liver; B:Computed tomographic scans of HCC in the metastases to sacral bone; C: Computed tomographic scans of HCC in the lung. White arrowheads indicate the tumor.
Laboratory examinations
The laboratory examination showed a mild increase in aspartate aminotransferase (74 IU/L), blood urea nitrogen (31 IU/L), creatinine (1.0 mg/dL), and C-reactive protein(5.36 mg/dL); mild decrease of prothrombin time (76% of normal), and serum albumin (3.4 g/dL). The Levels of tumor markers—alpha-fetoprotein,Lens culinarisagglutinin-reactive alpha-fetoprotein isoform, and des-gamma-carboxy prothrombin—were significantly increased, to 67,183 ng/mL, 37.2%, and 75,000 milliarbitrary units per milliliter or higher, respectively (Table 1). No increase in white blood cell count, and other hepatobiliary enzymes were marked.
On the sixth day after hospital admission, the patient's respiratory condition worsened, and his blood gas analysis showed oxygen saturation (SpO2) of 91%, pH of 7.456, carbon dioxide tension of 35.2 mmHg, oxygen tension of 62.9 mmHg,bicarbonate level of 24.3 mmol/L, and BE of 0.7 mmol/L, with supplementation of 2 L/min of oxygen (Table 1, Figure 2). The chest radiograph showed a frosted glass-like shadow in the upper right lobe, middle lobe, and the lower left lobe (Figure 2). The blood and sputum cultures revealed no evidence of infectious pneumonia; however,respiratory distress and decreasing arterial blood oxygen saturation continued, and chest CT examination revealed worsening of the frosted glass-like shadow on day 8(Figure 2). On the basis of these findings, and because antibiotics produced no response, we diagnosed his condition as acute respiratory distress syndrome,potentially a result of the lung metastasis and involvement of the pulmonary vessels by tumor thrombus.
Chest radiographs showed worsening on day 14 (Figure 2). To alleviate the respiratory failure caused by the infiltration of the inflammatory cells and the reaction in the lung, we started oral administration of prednisolone, 80 mg daily, on day 16 after admission (Figure 2). The frosted glass-like shadow on chest radiographs and CT studies (Figure 2) showed temporary improvement and the symptom of dyspnea showed mild improvement; however, the patient's respiratory condition and the data from the blood gas analysis did not improve with oxygen supplementation. The patient's general condition worsened gradually and he died on the 37thday of hospitalization (Figure 2).
With the informed consent of the patient's family, autopsy was performed to assess the cause of the respiratory failure and the frosted glass-like shadow.Macroscopically, the lung appeared to be hard and yellowish, and the presence of multiple tumors in the area was confirmed (Figure 3A). Microscopically, these tumors were confirmed to be metastases of HCC (Figure 3B), and multiple pulmonary artery tumor emboli with diffuse alveolar damages of detachment of alveolar epithelial cells,edematous changes of alveolar wall, accumulation of macrophages, and exudation of fibrinous tissue were seen (Figure 3C) and in part with recanalization in the tumor thrombus and the fibrocellular intimal proliferation (Figure 3D). In addition, medial thickening of the arterioles (Figure 3E) were seen and the tumor emboli (Figure 3F)were accompanied by CD31-positive endothelial cell growth (Figure 3G) with fibrocellular intimal proliferation (Figure 3H) which are the characteristics of PTTM.
FINAL DIAGNOSIS
On the basis of these findings, the diagnosis was PTTM and diffuse pulmonary alveolar damage due to tumor emboli, which led to the cause of respiratory failure.
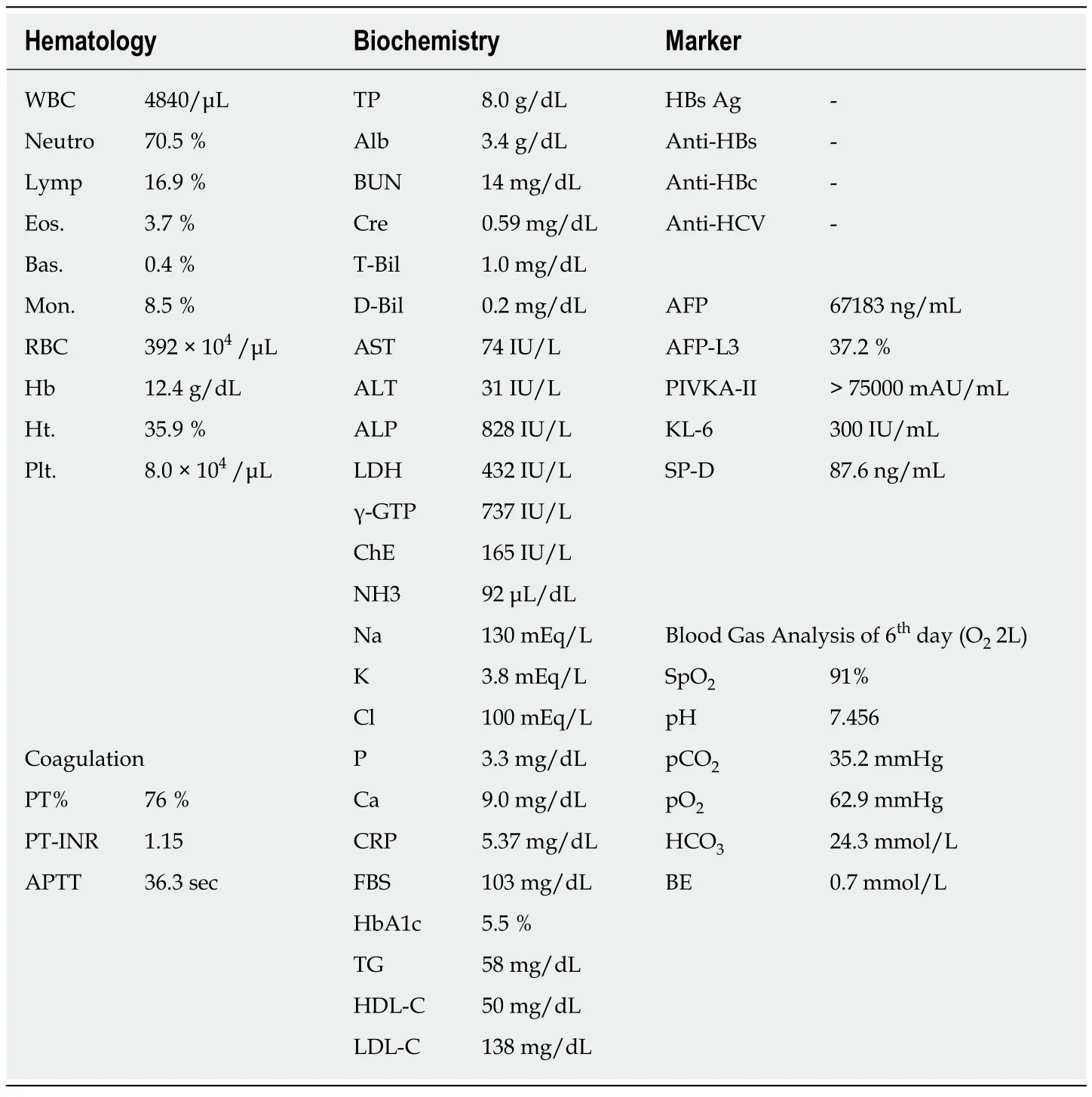
Table 1 Laboratory examination
TREATMENT
To alleviate the respiratory failure caused by the infiltration of the inflammatory cells and the reaction in the lung, we started oral administration of prednisolone, 80 mg daily, on day 16 after admission.
OUTCOME AND FOLLOW-UP
The patient's general condition worsened gradually and he died on the 37thday of hospitalization.
DISCUSSION
In the cases of pulmonary microembolism caused by tumor cells, the tumor cells move intravenously or lymphatically to pulmonary arteries that are smaller than muscular arteries, and cause embolism, which may in turn cause pulmonary hypertension or respiratory failure[1]. PTTM is a special cause of pulmonary tumor embolism, in which tumor cells cause thickening of the pulmonary arterial endothelium or form thrombi, that cause narrowing and occlusion of the pulmonary arteries[2].
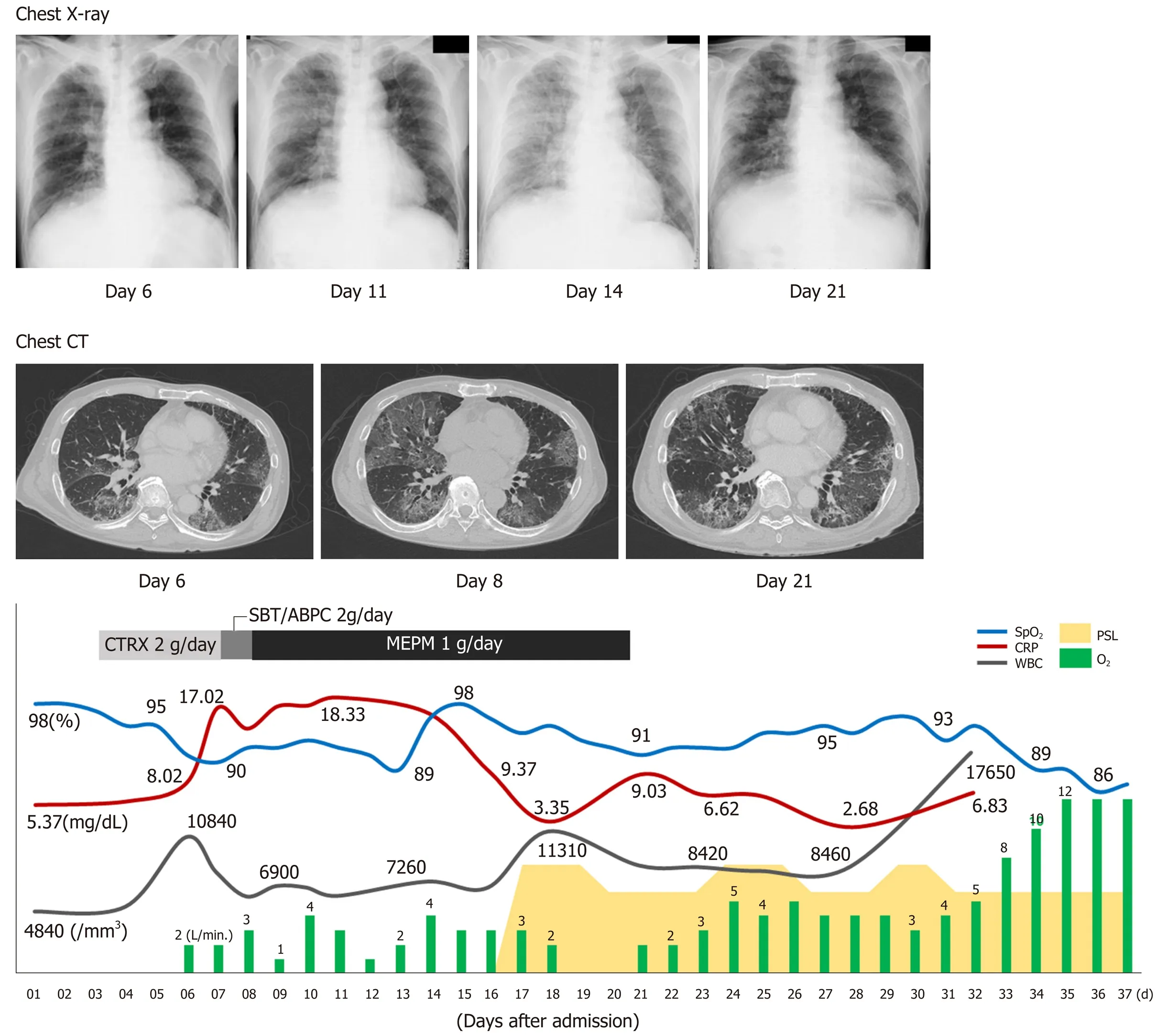
Figure 2 Clinical courses of physical and laboratory findings, chest radiograph, and computed tomographic scans. CT: Computed tomographic; BT: Body temperature; CRP: C-reactive protein; CTRX: Ceftriaxone sodium hydrate; MEPM: Meropenem; PSL: Prednisolone; SBT/ABPC: Sulbactam/ampicillin; SpO2: Oxygen saturation; WBC: White blood cell count.
In a report by Yamashitaet al[3]who surveyed findings from autopsies of 2215 cases of malignant tumors, 30 cases (1.4%) were diagnosed with PTTM, and 21 of those cases exhibited hypercoagulability. Eighteen cases (60%) were with gastric cancers;the others include the carcinomas of the breast, pulmonary system, prostate, ovary,and pancreas. The most common histological type was glandular carcinoma, which was observed in 28 cases (93%). With regard to HCC as the primary lesion in cases of PTTM, only a few reports have been published to date, and the symptoms, imaging findings, therapeutic options, and prognoses have not been summarized; we performed a literature review of 18 reported cases and summarized the information with that of our case[1,4-20](Table 2). According to our summary, the overall malefemale ratio for all PTTM cases was 2:1, and of the 17 patients with PTTM that started as HCC, 15 (89%) were male (Table 2).
For the symptoms, respiratory discomfort is the chief symptom recognized with PTTM. Of the 17 patients with HCC, 9 (53%) displayed symptoms of respiratory discomfort; in addition, 4 had chest pain, 2 had pyrexia, 2 had shortness of breath, and 1 each had cough, disturbance of consciousness, and ascites. Respiratory discomfort rapidly progresses to pulmonary hypertension and right-sided heart failure, and most cases result in death a short time after the appearance of respiratory discomfort.Respiratory discomfort ultimately occurred in 13 of the 17 cases (Table 2), and of those cases, 6 (46%) presented with more than two criteria for systemic inflammatory response syndrome.
For the imaging findings of PTTM, pulmonary CT scans show consolidation which means an increase in absorption by the pulmonary parenchyma that obscures the background of blood vessels and the bronchial wall and the appearance of groundglass attenuation, small nodules, and a tree-in-bud pattern. In our particular case,multiple small nodules appeared in the left inferior lobe; a decrease in SpO2coincided with the increase in systemic inflammatory response syndrome score; and a chestradiograph showed ground-glass opacity over the area from the right superior lobe to the inferior lobe and over to the left inferior lobe. A chest CT scan taken at the same time showed ground-glass attenuation over both lungs. The summary of the reported cases showed various imaging including tumor nodular shadows, air bronchograms,enlargement of both the heart shadow and pulmonary arterial shadows, and groundglass attenuation, therefore, there were no specific imaging findings directly suggesting the tumor embolism or pulmonary embolism. Because there is no typical pattern in imaging findings, it is difficult to diagnose PTTM while an affected patient is alive. As part of diagnosis, lung perfusion scintigraphy or cardiac ultrasonography is used to detect pulmonary hypertension[4]. In one report, cytodiagnosis was made with a specimen of pulmonary arterial blood taken with a Swan-Ganz's catheter[21];however, this method requires caution because the procedure is highly invasive and risky in patients with respiratory distress. In that report, the patient received a definitive diagnosis but died 4 d later. Among the cases in the literature, definitive diagnosis was obtained through autopsy in 13 cases, lung perfusion scintigraphy in 2 cases, cardiac ultrasonography in 2 cases and recovery of embolus in 1 case. The pathological findings have not been described minutely, and our patient showed not only the tumor embolisms, the thickening of vascular endothelium and fiber were confirmed which are suggesting the histological features of PTTM.
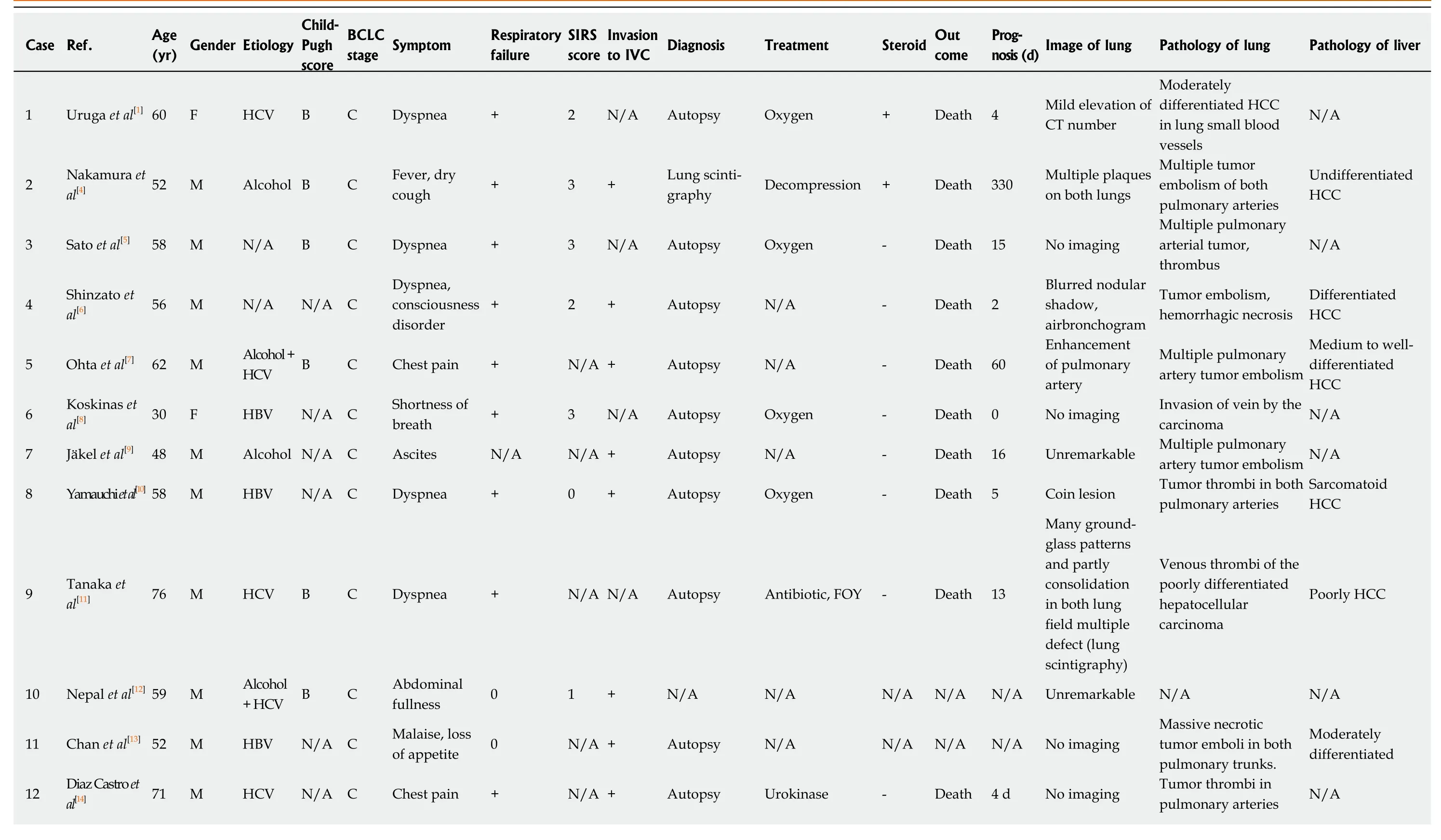
Table 2 Summary of the cases reported
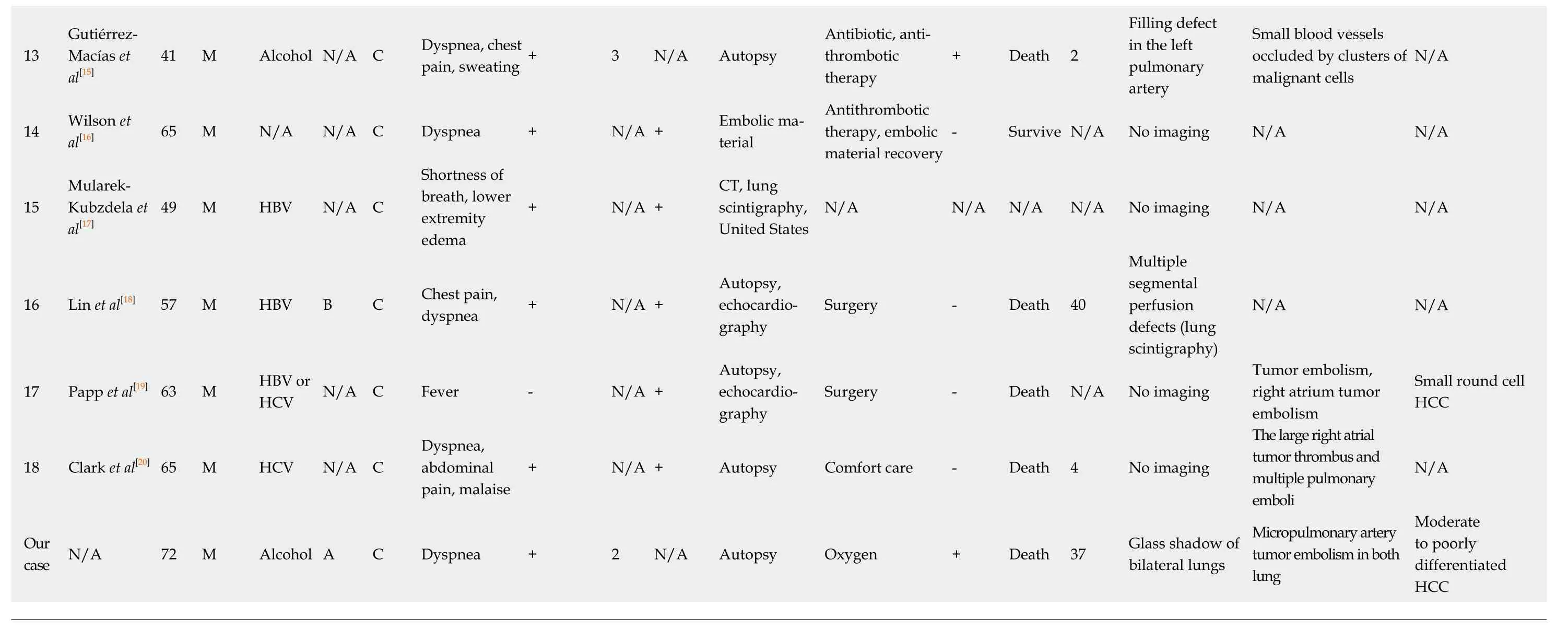
BCLC: Barcelona Clinic Liver Cancer; SIRS: Systemic inflammatory response syndrome; IVC: Inferior vena cava; HBV: Hepatitis B virus; HCV: Hepatitis C virus; N/A: Data not available; FOY: Gabexate mesylate; HCC:Hepatocellular carcinoma.

Figure 3 Histological analyses. A: Macroscopic findings of the lung; B: Hematoxylin and eosin staining of the tumor; C, D: Diffuse alveolar damages with multiple pulmonary artery tumor emboli. White arrows in cindicate the alveolar damage and arrowheads in Cindicate tumor cells. Black arrow in D indicates the recanalization and a white arrowhead indicates the fibrocellular intimal proliferation; E: Medial thickening of arterioles. A white arrowhead indicates the thickening; F: Tumor emboli(hematoxylin and eosin staining) were accompanied by the CD31-positive endothelial cell growth; G: CD31 staining, a white arrow head and fibrocellular intimal proliferation; H: Elastica van Gieson staining, a white arrow head.
For therapeutic options, as far as we could confirm, all patients with respiratory distress were administered oxygen, and additional treatments included antibiotics in two cases, one case of gabexate mesilate infusion in one case, and antithrombotic urokinase therapy in two cases. No effective therapeutic options have been established for PTTM at this stage. We used prednisolone infusion with the purpose of alleviating respiratory distress and improving the patient's deteriorating systemic condition, and the mild improvement of the symptom with the reduction of the ground-grass opacity in chest radiographs and CT scans were seen, however, no data of the respiratory distress and the necessary oxygen volume did not decrease. Based on the literature review, steroids were administered to three patients, and one of them showed the improvement of the images (Table 2). The prognosis for patients with PTTM is extremely poor; most of such patients die within 1 week of developing respiratory distress[22]. Among the cases featured in our literature review, only one patient survived. The shortest period between the commencement of treatment for respiratory distress and death was 0, the longest was 330 d, the average was 41 d, and the median was 9 d. PTTM is difficult to diagnose with general imaging tools, and a poor prognostic conditions with malignant tumors, therefore, the supportive care to reduce the symptoms by prednisolone, opioid, and etc. should be considered for the better terminal care.
CONCLUSION
Our summary demonstrated the poor prognosis of the PTTM of HCC and supportive care using oxygen, prednisolone, opioid, etc. might be effective to reduce the symptoms. Further accumulation of information from cases will be of great help for physicians diagnose, manage, and care the patients and their symptoms.
 World Journal of Gastroenterology2019年48期
World Journal of Gastroenterology2019年48期
- World Journal of Gastroenterology的其它文章
- Gastric electrical stimulation: An emerging therapy for children with intractable gastroparesis
- Comprehensive multi-omics analysis identified core molecular processes in esophageal cancer and revealed GNGT2 as a potential prognostic marker
- Diagnostic and prognostic value of lncRNA cancer susceptibility candidate 9 in hepatocellular carcinoma
- Operative complications and economic outcomes of cholecystectomy for acute cholecystitis
- Hepatitis C virus eradication with directly acting antivirals improves health-related quality of life and psychological symptoms
- Significance of postoperative follow-up of patients with metastatic colorectal cancer using circulating tumor DNA
