Risk factors,clinical features,and short-term prognosis of spontaneous fungal peritonitis in cirrhosis:A matched case-control study
Chun-Hong Huang,Lan-Tian Pang,Li-Chen Xu,Tian-Tian Ge,Qiao-Mai Xu,Zhi Chen
Abstract
Key words: Spontaneous fungal peritonitis;Risk factor;Cirrhosis
INTRODUCTION
Spontaneous peritonitis is one of the most common infectious complications,with a prevalence ranging from 7% to 27% and mortality from 10% to 46% at 1 year in cirrhotic patients with ascites[1-3].In advanced cirrhosis,damage to the reticuloendothelial system and gut barrier compromises the immune surveillance function of the organ and hinders the bactericidal ability of phagocytic cells,increasing the opportunity for bacteria to translocate from the gut to the abdominal cavity[4].Additionally,ascites is transudative and contains agents with a low immune activity,providing a good environment for pathogen growth.The risk factors for the incidence and mortality of patients with spontaneous bacterial peritonitis (SBP) have been comprehensively described.A high bilirubin level,a low ascitic fluid protein concentration,and an episode of variceal haemorrhage are strongly associated with SBP occurrence[5].Renal injury is a reliable predicting factor for mortality and develops in 30%-40% of patients with SBP[6].
Spontaneous fungal peritonitis (SFP) is a type of spontaneous peritonitis,and it is a less recognized but devastating complication in end-stage cirrhosis.Previous studies have shown that fungi can be responsible for 3.6% of spontaneous peritonitis cases in cirrhotic patients,while the 28-d mortality can be as high as 56%-73.3%[7-10].Although high mortality was previously noted,scant data are available to fully define the factors responsible for the occurrence of SFP and its mortality.
To improve the survival rate,it is crucial to fully recognize the risk factors responsible for the occurrence and mortality of SFP and further optimize stratification.Additionally,recognizing the clinical features is critical for the early diagnosis of SFP.The aims of the present case-control study were to:(1) Compare the difference in the clinical manifestations of SFP and SBP;(2) Examine the risk factors for the occurrence and short-term mortality of SFP;and (3) Evaluate the predictive ability of different prognostic scoring systems.
MATERIALS AND METHODS
Patients and settings
Patients were initially identified from the hospital’s microbiology database.In total,3119 patients with culture-positive ascites fluid were screened between January 1,2007 and December 31,2018 at the First Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou,China).Cirrhosis was diagnosed by (1) Liver biopsy;(2) Radiological evidence of liver nodularity in patients with chronic liver diseases;and (3) Clinical evidence of the signs of portal hypertension or hepatic decompensation.The following diagnostic criteria were determined for spontaneous peritonitis:(1) Ascitic fluid polymorphonuclear neutrophil (PMN) count (≥250 cells/mm3;and (2)Exclusion of abdominal surgical procedures or endoscopic biliary intervention during the past 4 wk.Patients with spontaneous peritonitis with fungal growth from ascites culture were identified as SFP and were included in a case group.Patients with fungiascites were identified as those with an ascitic PMN count lower than 250 cells/mm3but showed fungal growth from ascites culture.Control subjects were matched for age,sex,and time and were randomly selected from the same database.Forty-four patients (1:2) with bacterial culture-positive spontaneous peritonitis were divided into control-1 group,and 72 patients (1:3) with culture-negative spontaneous peritonitis were divided into control-2 group.
Data collection
The data of each patient were collected retrospectively from inpatient records and included sex,age,alcohol abuse,smoking status,antibiotic treatment,aetiology of cirrhosis,clinical presentation,cirrhotic complications,co-morbidity,laboratory examination,microbiological data,intensive care unit (ICU) admission,and prognosis.All the data were collected at the diagnosis of spontaneous peritonitis.Nosocomial infections were infections confirmed after 48 h of admission.Cirrhotic complications were identified as previously described[11].A systemic inflammatory response (SIRS) was confirmed when at least two of the following symptoms appeared:a heart rate higher than 90 per minute;a temperature > 38 °C or < 36 °C;a respiratory rate > 20/min;and a white blood cell (WBC) count > 12000/mm3or <4000/mm3[12].Prognostic models,including the Child-Turcotte-Pugh (CTP),sequential organ failure assessment (SOFA),model for end-stage liver disease (MELD),integrated MELD (iMELD),MELD-Na,chronic liver failure-SOFA (CLIF-SOFA),and acute physiology and chronic health evaluation (APACHE) II score,were used to predict the 15-d mortality.CTP,MELD,iMELD,and MELD-Na were applied to assess disease severity and prognosis.The calculation of MELD is as follows:3.8 × loge (total bilirubin,mg/dL) + 9.6 × loge (creatinine,mg/dL) + 11.2 × loge (international normalized rate) + 6.4 × (aetiology of 0 for alcoholic or cholestatic cirrhosis;1 for others).MELD-Na is 1.59 × (135 - Na) + MELD.The ACLF-SOFA score was proposed to assess organ failure in patients with acute-on-chronic liver failure by evaluating functional failures of the liver,kidney,brain,coagulation,circulation,and respiration[13].The APACHE II score was mainly evaluated in the first 24 h in ICU but was calculated at the time of the diagnosis of spontaneous peritonitis in this study.
Statistical analysis
The data were statistically analysed using SPSS software version 20 (IBM Inc.,Chicago,IL,United States).We defined statistical significance whenP< 0.05.The data are expressed as the mean ± standard deviation (SD) if they were normally distributed or as the median ± interquartile range (IQR) if they were not.Student’st-test or the Wilcoxon test was used to analyse the difference between continuous variables depending on whether the data were in normality,and the χ2-test or Fisher’s exact probability test was used for categorical variables.Univariate logistic regression analysis was used to recognize risk factors for SFP occurrence.The Cox regression model was used to examine the relationship between independent variables and the 15-d mortality in patients with SFP or fungiascites.The cumulative incidence of death was described by Kaplan-Meier analysis and was compared in each group by the logrank test.The predictive ability of the different prognostic scoring systems was compared using the receiver operating characteristic (ROC) curve.The value of different prognostic models was compared using DeLong's test.
RESULTS
Comparing the clinical manifestations between SFP and SBP
In this study,we screened 410 (13.1%) patients with fungal positivity in ascitic fluid culture from 3119 patients with positive ascites culture from January 1,2007 to December 30,2018.Twenty-two (0.71%) cirrhotic patients were consistent with the diagnosis of SFP,and 13 (0.42%) were consistent with the diagnosis of fungiascites.
The clinical characteristics and outcomes of the case cohort and control cohort are depicted in Table 1.The control group was further divided into the SBP with positive bacterial culture group (control-1,n= 44) and SBP with negative bacterial culture group (control-2,n= 72).Of the 22 patients with SFP,the mean age was 60 ± 11.6 years and 17 (77.3%) were male.The case and control cohorts did not show differences in baseline characteristics,including current smoking,alcohol abuse,and cause of cirrhosis.Regarding the complications of cirrhosis,SFP patients showed a higher frequency of variceal bleeding (SFPvscontrol-1P= 0.050) and hepatorenal syndrome (SFPvscontrol-2P= 0.023).However,the Charlson comorbidity index showed no difference between the cohorts.Patients with SFP showed a more serious inflammatory response.The C-reaction protein (CRP) levels (SFPvscontrol-1:P<0.001;SFPvscontrol-2P< 0.001),WBC (SFPvscontrol-1:P= 0.001;SFPvscontrol-2:P< 0.001),ascitic PMNs (SFPvscontrol-2:P= 0.004),SIRS (SFPvscontrol-2:P< 0.001),and fever (SFPvscontrol-2:P= 0.02) were higher in the SFP cohort.Additionally,a significant difference was also observed in the length of antibiotic treatment before peritonitis (SFPvscontrol-1:P< 0.001;SFPvscontrol-2:P< 0.001).Notably,coinfection with bacteria was much common in SFP (SFPvscontrol-2:P< 0.001) with a higher frequency of pneumonia and blood infection.
SFP and fungiascites were also compared in our study and are shown in Table 2.Candida spp.was the most commonly isolated fungus in SFP or fungiascites.Comparing the baseline characteristics of SFP with fungiascites,the length of antibiotic treatment before peritonitis and antifungal therapy were similar,but WBC(P= 0.046) and CRP (P= 0.010) were higher in the SPF group.
Risk factors for the occurrence of SFP
The risk factors analysed for patients with SFP and the matched control group are shown in Table 3.The factors that may be related to SFP were compared between the case and control groups by univariate logistic regression analysis.The length of antibiotic administration was closely related to SFP compared with the control-1 group (OR = 1.063,95%CI:1.012-1.115,P= 0.014) or control-2 group (OR = 1.054,95%CI:1.014-1.095,P= 0.008).The median length of antibiotic administration before the occurrence of SFP or fungiascites was 12 d in our study (Figure 1).Additionally,variceal bleeding (SFPvscontrol-1:OR = 3.619,95%CI:1.065-12.296,P= 0.039) and hepatorenal syndrome (SFPvscontrol-2:OR = 4.000,95%CI:1.312-12.194,P= 0.015)were also significantly associated with SFP.However,in univariate analysis,high CTP and MELD scores were not shown to be risk factors for SFP.
Risk factors associated with early mortality
SFP is a devastating complication of cirrhosis and has the most undesirable consequence in the short term.The 15-d survival or death events were recorded in all patients.As shown in Table 1,the case group (10/22) exhibited a significantly higher mortality than the control-1 group (9/44P= 0.046) and control-2 group (5/72P<0.001).However,the mortality between SFP and fungiascites (3/13P= 0.28) did not show a statistically significant difference (Table 2,Figure 2A).Furthermore,the risk factors that may be related to the 15-d mortality in patients with SFP or fungiascites were analysed using the univariate Cox regression model (Table 4).The parameters hepatorenal syndrome,WBC,total bilirubin,creatinine,serum sodium,and severity scores (MELD,SOFA,CTP,and APACHE II) were identified as significant risk factors for SFP and fungiascites mortality within 15 d.The significant risk factors were applied to the multivariate Cox regression model.Hepatorenal syndrome (HR = 5.328,95%CI:1.050-18.900,P= 0.010) and total bilirubin (HR = 1.005,95%CI:1.002-1.008,P=0.002) emerged as independent risk factors for 15-d mortality in SFP and fungiascites.
Based on the identified risk factors for 15-d mortality,the optimal cut-offs were used to stratify patients,and their survival was compared by the Kaplan-Meier curve(Figure 2).Patients with SOFA ≥ 8 (AUROC:0.850,95%CI:0.707-0.993),MELD ≥ 19(AUROC:0.724,95%CI:0.514-0.934),WBC ≥ 10 × 109/L (AUROC:0.733,95%CI:0.560-0.905),total bilirubin ≥ 150 mg/dL (AUROC:0.860,95%CI:0.708-1.000),and creatinine≥ 101 μmol/L (AUROC:0.753,95%CI:0.575-0.932) at the time of SFP and fungiascites diagnosis presented higher risks for short-term mortality.Additionally,patients who were complicated with hepatorenal syndrome further increased the mortality and presented a poor prognosis (Figure 2D).
Value of prognostic models in predicting the outcome of SFP and fungiascites
Six models were tested to predict the 15-d mortality in patients with SFP or fungiascites (Table 5).Among the models,SOFA exhibited a higher AUROC (0.85,95%CI:0.707-0.993) in predicting 15-d mortality.The Delong test was used to comparethe value between SOFA and five other predicting models.The remaining five models did not show a statistical difference compared with SOFA.

Table1 Comparison of clinical manifestations between spontaneous fungal peritonitis (case) and spontaneous bacteria peritonitis with a positive (control-1) or negative bacterial culture (control-2)

Table2 Comparison of clinical manifestations between spontaneous fungal peritonitis and fungiascites
DISCUSSION
SFP is a rare but devastating complication in end-stage cirrhosis,and its clinical characteristics as well as the risk factors for its occurrence and prognosis were less depicted previously.In the current case-control study,we compared the clinical features and susceptible factors between SFP patients and SBP patients with bacterial culture-positive (Control-1) or bacterial culture-negative (Control-2) results.To the best of our knowledge,this is the largest retrospective study of patients with SFP and fungiascites.We found the following:(1) SFP patients exhibited more severe systemic inflammation,including elevated WBC and CRP levels,higher frequency of SIRS,and higher short-term mortality;(2) Long-term antibiotic administration remarkably increased the occurrence of SFP or fungiascites,and the median length of antibiotic administration before the SFP diagnosis was 12 d in our study;(3) Patients with SFP displayed a worse prognosis than patients with SBP but showed no significant difference with fungiascites;and (4) Hepatorenal syndrome,total bilirubin (≥150 mg/dL),and creatinine (≥101 μmol/L) represented independent predictors of SFPrelated early mortality.
Spontaneous peritonitis is one of the most common infectious complications of endstage cirrhosis and often leads to the rapid deterioration of liver function[14].SFP is a subtype of spontaneous peritonitis.Because fungi are much larger in size than bacteria,their translocation across the gut mucosa seems more difficult than bacterial translocation.Thus,the incidence rate of SFP is much lower than that of SBP.As reported previously,SFP accounted for 15% of invasive fungal infections in acute-onchronic liver failure and 3.6% of patients with liver cirrhosis and spontaneous peritonitis[15].We found that SFP was much stronger in inducing an inflammatory response than SBP or fungiascites.Systemic inflammation is closely associated with a worse outcome[16].High levels of pro-inflammatory cytokines disrupt hemodynamic stability and facilitate portal hypertension-related complications[17].Systemic inflammation has been shown to favour serious complications such as variceal bleeding,encephalopathy,and acute renal failure[11,18,19].An intense inflammatory response in patients with SFP may be a key factor in initiating fast deterioration of cirrhosis and resulting in undesirable prognosis.
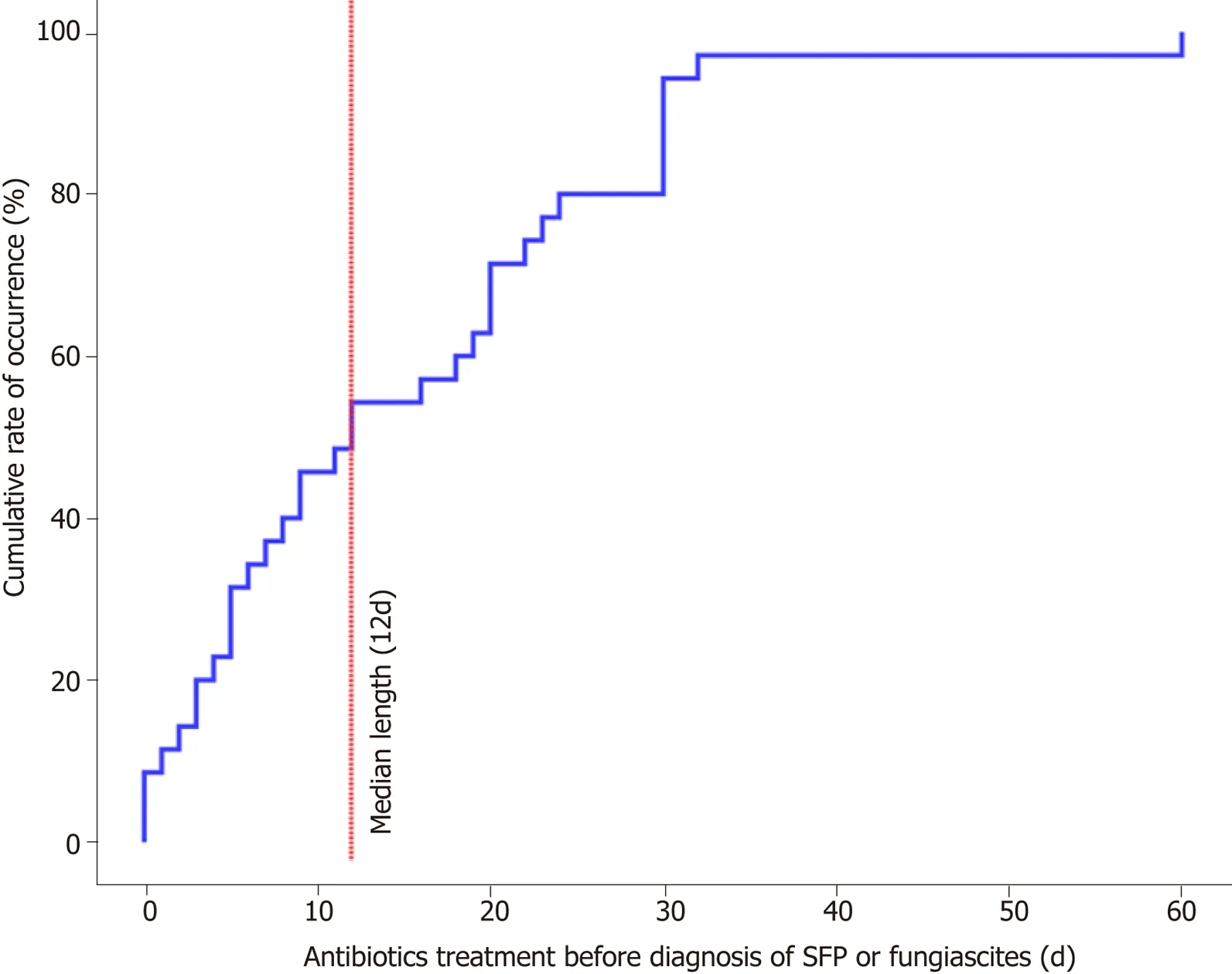
Figure1 Length of antibiotic administration before occurrence of SFP.
Clarifying risk factors is useful to identify candidates for early treatment.Previous studies have indicated that high CTP or MELD scores,invasive procedures,and length of hospital stay increased the risk of SFP in cirrhosis[20].A recent meta-analysis indicated that hospitalization is related to a significant increase in SFP risk[21].However,we found no significant difference between SFP and SBP in the length of hospital stay.We found that long-term antibiotic administration could increase the occurrence of SFP.This result is in agreement with the data of previous reports related to fungal infection in patients with end-stage liver disease[15,22].Additionally,a case reported by Mark D Berzsenyi cued the link between the long-term administration of antibiotics and SFP[23].Antibiotic administration disturbs the balance between bacteria and fungi and leads to excessive growth of fungi,making the opportunity for increased fungal translocation[9].Compared with the control groups,variceal bleeding and hepatorenal syndrome were also associated with the occurrence of SFP.The complications of cirrhosis that refer to hemodynamic instability may further impair intestinal barrier function and promote fungal translocation.In contrast to previous reports,the severity of disease (MELD or CTP scores) showed no difference between the SFP and control groups[9].
Considering the fast course of SFP,death mainly occurred within 2 wk after diagnosis.The risk factors for 15-d mortality were also discussed.Consistent with previous reports[7],we found that the 15-d mortality of SFP showed no significant difference compared with that of fungiascites but was higher than that of SBP.Notably,mortality was not influenced by antifungal therapy in our study.Because traditional empirical antibiotic therapy was used to identify fungi and a long period was applied for the remaining culture,many patients died prior to obtaining the culture result.Only 36.4% of patients with SFP received antifungal therapy in our study.However,it was reported that adequate antifungal treatment improved survival of cirrhotic patients with candidemia and intra-abdominal candidiasis[24].Therefore,timely and adequate antifungal treatment may be important to improve prognosis.Because of the low incidence of SFP,the risk factors for mortality were only discussed in a small-sized cohort.The multivariate Cox regression analysis performed in our study indicated that hepatorenal syndrome,total bilirubin,and creatinine were three independent risk factors for short-term mortality.Bremmeret al.showed that the APACHE II score was a predictor of 28-d mortality[7].Renal dysfunction was a main prognostic factor for 15-d mortality of SFP in the current study.Creatinine ≥ 101 μmol/L was an optimal cut-off point to predict a poor prognosis.These findings agree with previous findings for SBP.Tandonet al[25]reported that renal dysfunction was the most important independent predictor of mortality in cirrhotic patients with SBP.Additionally,liver function was another important prognostic factor.Total bilirubin ≥ 150 mg/dL and MELD ≥ 19 acted as good discriminators of subsequent SFP 15-d mortality.Notably,the cut-off point of MELD to predict SFP 15-d mortality was slightly lower than that in a previous report on SBP-related 30-d mortality(MELD ≥ 22)[26,27].The predictive value of the six prognostic models was compared.SOFA exhibited the highest AUROC among the selected models,while DeLong's test indicated no significant difference between SOFA and the remaining five models.However,low sample size may conceal the difference.The value of prognosis shouldbe tested in a large cohort.

Table3 Risk factors for spontaneous fungal peritonitis as determined by univariate logistic regression analysis
Our study had some limitations.Considering the low sample size of the current study,more samples are required to fully determine the risk factors for occurrence and mortality.As a one-centre and retrospective study,a potential bias in data collection may result in a statistical bias.Additionally,it was difficult to distinguish between SFP and SBP using fungal colonization,which may lead to inaccurate results.
Nevertheless,our investigation provides a comprehensive study that characterizes the clinical features of SFP.We found that long-term antibiotic administration increases the risk of SFP occurrence.Hepatorenal syndrome and total bilirubin are closely related to short-term mortality.
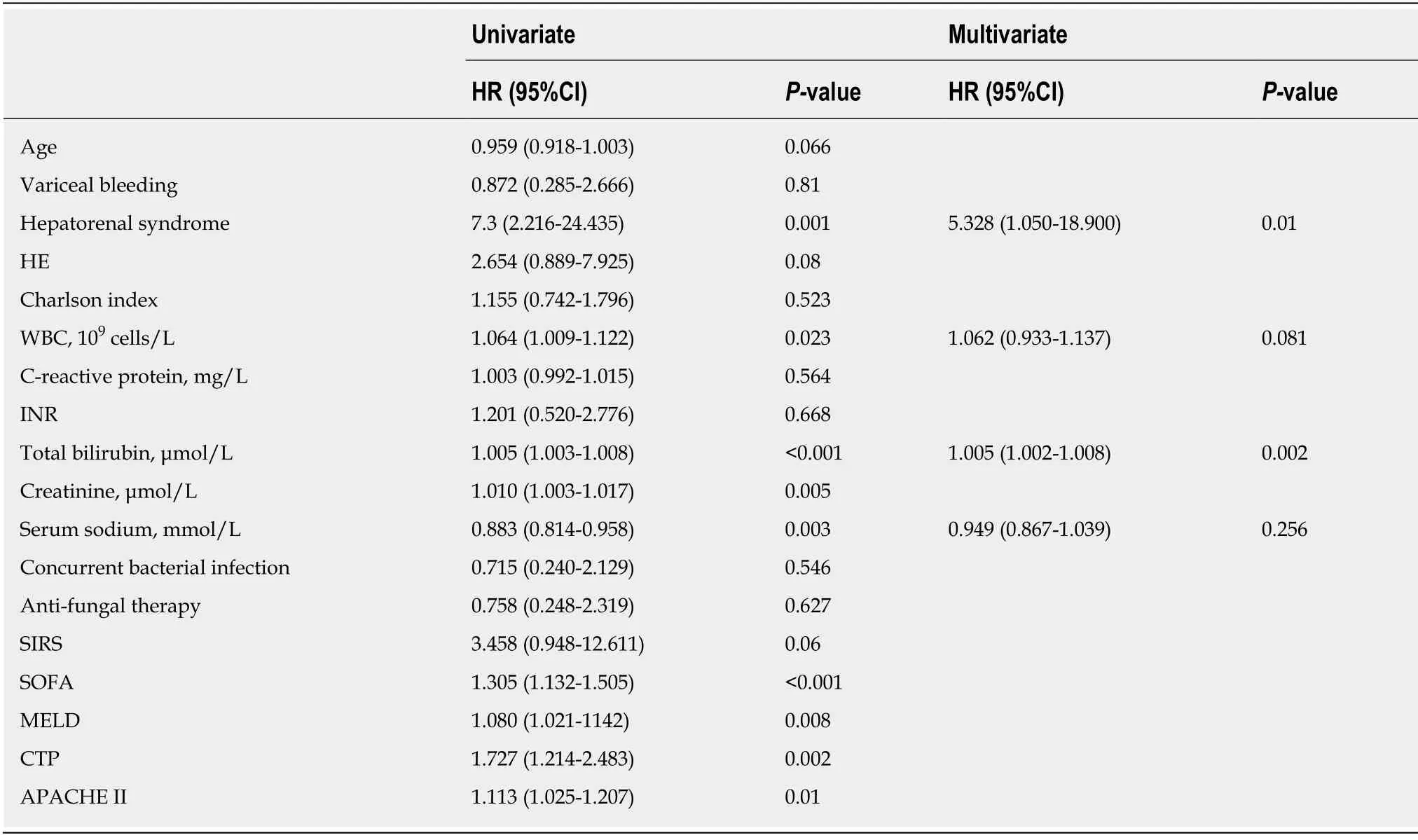
Table4 Cox regression analyses of risk factors associated with 15-d mortality (dead:n = 13;alive:n = 22) in patients with spontaneous fungal peritonitis or fungiascites
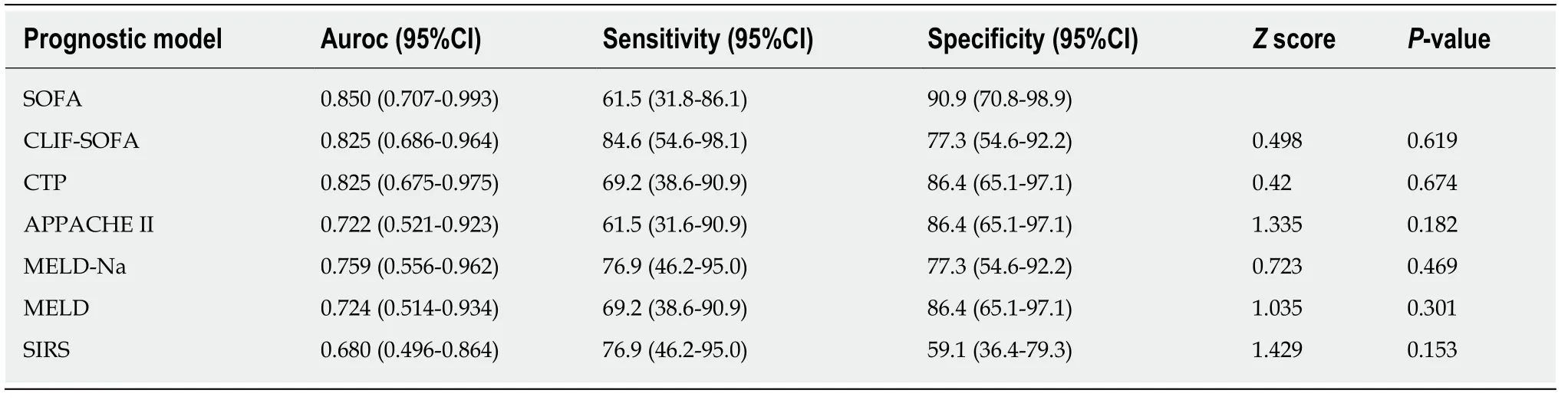
Table5 Performance of six prognostic scoring systems in predicting mortality in patients with spontaneous fungal peritonitis /fungiascites

Figure2 Kaplan-Meier diagrams.
ARTICLE HIGHLIGHTS
Research background
Spontaneous peritonitis is one of the most common infectious complications in cirrhotic patients with ascites.However,spontaneous fungal peritonitis is a less recognized but devastating complication in end-stage cirrhosis.
Research motivation
To improve the survival rate,it is crucial to identify the risk factors for occurrence and mortality and to optimize stratification.Additionally,recognizing the clinical features is critical for the early diagnosis of spontaneous fungal peritonitis (SFP).
Research objectives
We aimed to illustrate the differences between SFP and spontaneous bacterial peritonitis (SBP)and discuss the risk factors for occurrence and short-term mortality of SFP.
Research methods
In this case-control study,138 cirrhotic patients with spontaneous peritonitis were recruited.Patients with SFP were included in a case group.Sex-,age-,and time-matched SBP patients were included in a control group.Furthermore,the control group was divided into control-1 group(positive bacterial culture) and control-2 group (negative bacterial culture),according to the bacterial culture result.Differences in the clinical features,laboratory examinations,severity models,and prognosis were compared between the case and control groups.The risk factors for the occurrence of SFP were identified by the logistic regression model.The short-term mortality of SFP was determined by the Cox regression model.Additionally,the predictive ability of different prognostic scoring systems was evaluated.
Research results
Patients with SFP had severe systemic inflammation,including higher white blood cell counts and C-reaction protein levels,and exhibited poor short-term mortality.However,no significant difference was found regarding the short-term mortality between patients with SFP and fungiascites.Long-term antibiotic administration dramatically increased the occurrence of SFP or fungiascites.The median length of antibiotic administration before the occurrence of SFP or fungiascites was 12 d in our study.Hepatorenal syndrome (HR = 5.328,95%CI:1.050-18.900) and total bilirubin (μmol/L,HR = 1.005,95%CI:1.002-1.008) represented independent predictors of SFP-related early mortality.
Research conclusion
The present study found that long-term antibiotic administration increases the incidence of SFP and that hepatorenal syndrome and total bilirubin are closely related to short-term mortality.
Research prospective
Although a large sample size is required for further evaluation,our investigation provides a comprehensive study on characterizing the clinical features of SFP.
 World Journal of Clinical Cases2019年17期
World Journal of Clinical Cases2019年17期
- World Journal of Clinical Cases的其它文章
- Multifocal G1-G2 gastric neuroendocrine tumors:Differentiating between Type I,II and III,a clinicopathologic review
- Attention deficit hyperactivity disorder and comorbidity:A review of literature
- Dietary manipulation and testosterone replacement therapy may explain changes in body composition after spinal cord injury:A retrospective case report
- Incidence of portal vein thrombosis after splenectomy and its influence on transjugular intrahepatic portosystemic shunt stent patency
- Multiplex gene expression profile in inflamed mucosa of patients with Crohn’s disease ileal localization:A pilot study
- Analysis of the postoperative hemostatic profile of colorectal cancer patients subjected to liver metastasis resection surgery
