Reporting and methodological quality of systematic reviews or meta-analyses in nasogastric and nasojejunal enteral nutrition for severe acute pancreatitis
Si-Yuan Yang, Li-Min Guo, Yuan Jia, Fan-Jie Meng
Abstract Objective: To evaluate the methodological and reporting quality of systematic reviews and meta-analyses (SRs/MAs) in the nasogastric and nasojejunal enteral nutrition for severe acute pancreatitis (SAP).Methods: The SRs/MAs of nasogastric and nasojejunal enteral nutrition for SAP published in the journal before July.1st 2018 were identified by searching the databases of OVID, Cochrane Library,Wiley, PubMed, CNKI, Wanfang Data and VIP. The methodological and reporting quality of included studies was respectively assessed by AMSTAR 2 scale and PRISMA statement.Results: A total of 6 articles included at last. (1) Several issues of methodological quality in 6 studies were reviewed, such as: no prior design, comprehensive searching strategy, assessment of publication bias, the reason for incorporating the type of research design, or the statement of conflict of interest. (2) The average PRISMA score is 18.83 ± 1.97 (17-22). Results on the quality of reporting evaluation showed that 5 SRs/MAs were rated as moderate and 1 as high. What's more, we assessed the quality of indicators of the result by GRADE, but the grade was low.Conclusion: The quality of the involved studies published at home and abroad is uneven, so it is necessary to seek for further improvement of methodology and standardization of reporting to provide high-quality support for evidenced-based decision.
Key words: Enteral nutrition, Severe acute pancreatiti, Systematic review, Meta-analysis, AMSTAR 2, PRISMA
Abbreviations
Assessing the methodological quality of systematic review (AMSTAR); Enteral nutrition (EN); Meta-analysis (MA);Nasogastric enteral nutrition (NGEN); Nasojejunal enteral nutrition (NJEN); Preferred reporting items for systematic reviews and meta-analyses (PRISMA); Randomized controlled trial (RCT); Severe acute pancreatitis (SAP); System review (SR); Total parenteral nutrition (TPN)
Introduction
S evere acute pancreatitis (SAP),also known as acute hemorrhagic necrotizing pancreatitis, is initiated by abnormally activated pancreatin,which lead to chemical inflammation of pancreatin and surrounding tissues.SAP will give rise to severe diseases and malnutrition complications, such as high energy consumption, high catabolism and negative nitrogen balance. As a result, the nutrition support becomes a critical part in the treatment of SAP [1]. Recent reports [2-4] found that enteral nutrition (EN) is effective in preventing intestinal flora migration, reducing co-infection and mortality of SAP. Nasogastric enteral nutrition (NGEN) and nasojejunal enteral nutrition (NJEN) are the main methods for EN. When the food passes through the stomach and duodenum, the irritation of the pancreas will be minimized, NJEN can not only provide nutrition for patients, but also improve the body resistance and avoid stimulating the pancreas to secrete pancreatic juice, Ragins [5] suggested. While they ignored some key points, the theory of “pancreatic rest” was considered as the most ideal EN in the past years. NJEN has such shortcomings: (1) Its catheterization is difficult and requires the assistance of endoscope and X-ray, which needs the high experience and cost for operators; (2) Due to the different technical level of the operator and the equipment used,the success rate of catheterization is affected to a certain extent. Some patients may delay the best time for nutrition because of catheterization; (3) The diameter of the nasojejunal tube is relatively small, which is prone to tube occlusion and requires high nursing requirements [6].With many disadvantages of the nasojejunal tube, the researchers began to explore the nasogastric tube for nutrition support in recent years. NJEN and NGEN have their own characteristics. For example, the former can increase up to 30 minutes to achieve a small feeding tube [6]. Instead, NG tube placement is more convenient and lower cost. Traditionally, it was believed that NGEN could increase the chances of aspiration pneumonitis and stimulate pancreatic secretion, whereas NGEN did not.Many of Meta-analyses[7-12] showed that there is no significant difference between NGEN and NJEN, and the NGEN regarding safety and tolerance for SAP patients. Though there are many of SRs and Mas, their level of quality are uneven. So, NGEN can be established as a standard of care after sufficiently comprehensive and well MA or SR on NG versus NJ required in SAP.AMSTAR checklist is a scale for researchers to evaluate the methodological quality of SR/MA, including 16 items,which is recommended as the preferred tool for evaluating the methodological quality. In order to improve the reporting quality of SR/MA, in 2009 the PRISMA work group formulated the referring reporting standards—a revised version of PRISMA [13], which consists of 27 items in 7 parts, and a four-stage flow chart. The purpose of this study is to evaluate the quality of methodology and report of studies referring to SR or MS of statement and AMSTAR work group [14].
1 Materials and methods
1.1 The Criteria of Inclusion and Exclusion
1.1.1 Inclusion Criteria:
(1) Chinese or English Systematic Reviews or Meta-Analysis based on randomized controlled trial (RCT);
(2) SAP inpatients diagnosed by SAP diagnostic criteria made by the Atlanta Meeting in 1992, or Grade C above Balthazar CT, or the criteria of Ranson ≥3 and/or APACHE II ≥8 within 48 hours;
(3) Comparison between NGEN and NJEN;
(4) Outcomes mainly include: mortality; surgery intervention; diarrhea; rate of tube displacement; exacerbation of pain; infectious complications; tracheal aspiration; energy balance; length of hospital stays and pancreatic necrosis.
1.1.2 Exclusion criteria
Duplicate records, case reports, thesis of master and doctor, protocol, comments, or failing to get full-text.
1.2 Search Strategy
We searched literatures published in the OVID, Cochrane Library, Wiley, PubMed, CNKI, WanFang Data, VIP with the combination of subject term and free word till July 1, 2018. Retrieval strategies among Chinese: CNKI for example, the strategy is as follows: #1 (Title/ Abstract)=胰腺炎OR急性胰腺炎OR重症胰腺炎; #2 (Title/Abstract)=鼻胃管OR 鼻空肠管OR鼻饲OR肠内营养; #3 (Title/ Abstract)=Meta分析OR系统评价OR系统综述OR荟萃分析; #4 “#1 AND #2 #3”. While the English: PubMed for example, #1 “Pancreatitis'' (Title/Abstract) OR “Sever acute pancreatitis” (Title/Abstract) OR“SAP” (Title/ Abstract); #2 “Nasogastric enteral nutrition”(Title/ Abstract) OR “Nasojejunal enteral nutrition” (Title/Abstract) OR “enteral nutrition” (Title/ Abstract) OR “EN”(Title/ Abstract); #3 “Meta-analysis” Mesh OR “Systematic review” Mesh; #4 “#1 AND #2 AND #3”.
1.3 Study Selection
2 researchers conducted the criteria of inclusion and exclusion strictly by reading the title, abstract and full text separately and independently.
1.4 Data Extraction
We extracted the data independently from the studies satisfying the criteria of inclusion, which includes the name of authors, titles, published year, intervention and control measures, course of treatment, outcome indicators and results. Then we used Excel 2003 to establish a table of extractions named “Reporting and Methodological Quality of Systematic and Meta-Analysis of NGEN and NJEN in SAP”, which contains the name of the research, published date, study types, design types of studies, whether integrating the former related systematic reviews and Meta-analysis, PRISMA statement and other information required by AMSTAR 2. If difference occurs, the third researcher will be invited for discussion if necessary.
1.5 Quality Assessment and Grade Classification
The latest AMSTAR 2 was applied to evaluate the methodological quality of SR/ MA, which includes 16 indictors,covering the whole procedure of systematic review such as selection, design, registration, data extraction, data analysis and discussion. The latest edition remains 10 indictors in former ones, and reformulates the description. Moreover, “Not applicable” and “Cannot answer” were deleted in the latest edition, and 1 score of 1 was given to each item when the study satisfied the criteria. “Yes” means correct and sufficient, a score of 1 was given; “Part” means correct but not sufficient, 0.5 was given; “No” means no related measurement or inappropriate, 0 was given.According to the latest version of AMSTAR, it has no longer graded total score of studies [14]. So, the total score of AMSTAR2 is 16. PRSIMA contains 7 aspects, 27 checklist items about reporting quality of SR/MA. In order to ensure the normalization, clarity and integrity. A score of 1 was given to each item if the study satisfied the criteria of the item. If the study satisfied only part of the criteria 0.5 was given.The total scores are 27. Score <15 means serious information missing. When the score is 15 to 21, the report is considered to have certain defects; when the score is 21 to 27 points, the report is considered relatively complete[13]. Two reviewers independently assessed the articles,if a difference occurred, the third one was invited for discussion if necessary. The system of GRADE [15] was used to assess the quality of evidences, which includes the type of research, methodological quality, consistency of result,substantivity, accuracy and bias.
2 Results
2.1 Search Results
1724 articles were retrieved, including 988 records from OVID, 74 records from Cochrane Library, 97 records from Wiley, 468 records from PubMed, 32 records from CNKI,37 records Wanfang Data, 27 records from VIP, 1 record from the other sources. After removing 1403 duplicate records. After that, we read the titles and abstracts, 305 pieces were excluded for protocols only, failing to get fulltext, ineligible intervention, ineligible outcomes or recurrent literatures. In the end, 6 pieces of literatures met the requirements of the criteria of inclusion, showing in Figure 1.
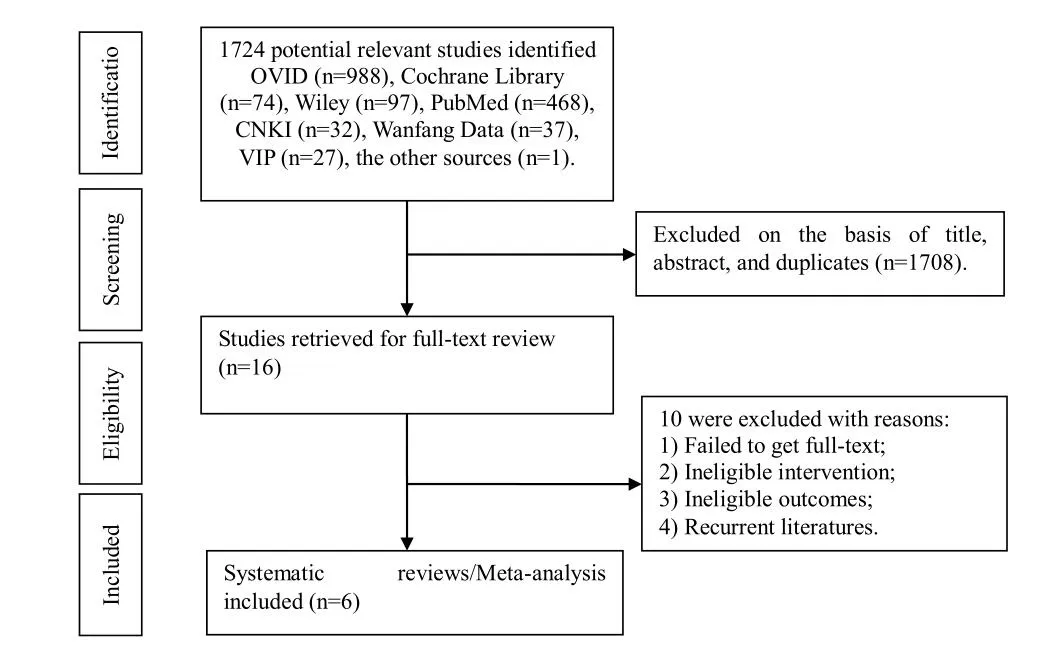
Figure1 PRISMA Flow Chart for the Study Selection Process
2.2 Characteristics of Included Articles
6 articles [7-12] were included at last. The lahnguage of 2 articles [8,9] is English, the other [7,10-12] is Chinese. All of them [7-12] are from China, object of which is NGEN and NJEN of SAP inpatients was applied in the trial. Referring to the assessment of the methodological quality,1 studie [10] was measured with Cochrane Handbook for the risk of bias, 3 [8,11,12] by Jadad score, and 2 [7,9]by both.The summarization of the articles included is stated in Table 1.
2.3 The Assessment of Methodological Quality for the Literature Included (AMSTAR 2)
Methodological quality evaluation was conducted among 6 literatures according to 16criteria in AMSTAR2. The basic results were summarized in Table 2. Participant, Intervention, Comparison, Outcome (PCIO) were reported in all 6 literatures, but no protocols. Only 1 article has already reported the reason why SR was included in designing. 5 of them partially reported the completed search strategy. 5 of them reported that 2 researchers searched the literatures independently. 4 reported that 2 researchers extracted the data independently. 4 reported the list of exclusion and stated the reason.6 described all the primary features of the studies.Appropriate tools were selected to measure the risk of bias in all 6 literatures. 3 of them reported the fund support. An appropriate method was chosen to analyze the result in those 6 literatures. v3considered the po tential influence of risk of bias on Meta-analysis and other
evidence.4 considered the risk of bias. There is no significant heterogeneity in 4 of them. The bias was assessed by figure, graph or statistical method, and the influence of which was also discussed. 3 of them has reported the fund support and stated that there is no competing interest. The total score ranges from 6 to 14 and the average of which is 9.75 ± 3.42. All the results were shown in Table 2.
2.4 The Assessment of Reporting Quality for the Literature Included (PRSIMA)
We assessed the completion of 6 literatures included, 1 stood for completed, 0.5 part and 0 for the rest. Title, Abstract and Introduction were contained in all 6 studies but no protocol and registration. The criteria of inclusion and resources were sufficiently reported in all 6 studies. 5 partially reported the search strategy and 1 completely reported. 4 of them completely reported the study selection, 1 partially. 4 completely reported the method of data extraction, while the rest 2 no. 5partially reported the data used to extract the literatures. 1 reported the risk of bias across study. At the part of result, 6 reported the results. 3 reported the bias of risk across study. 6 did not report additional analyses, the risk of bias may exist in all 6 literatures. At the discussion part, only 2 reported the conclusion sufficiently. All of the 6 reported the limitation and the conclusion.About the fund part, 3 of them reported the fund support and/or competing interest. The PRSIMA score of those 6 literatures is respectively 18, 22, 20.5, 18, 17 and 17.5, the average of which is 18.83 ± 1.97. As shown in Table 3.

Table 3 The Results of Assessment in PRISMA Statement
2.5 Quality of Evidence in the Included SRs Assessed by GRADE
6 studies included were assessed by GRADE subject to 10 indicators, the result of which was stated in the Table 4.On account of the poor quality, consistency and accuracy of methodology etc., the result was degraded.
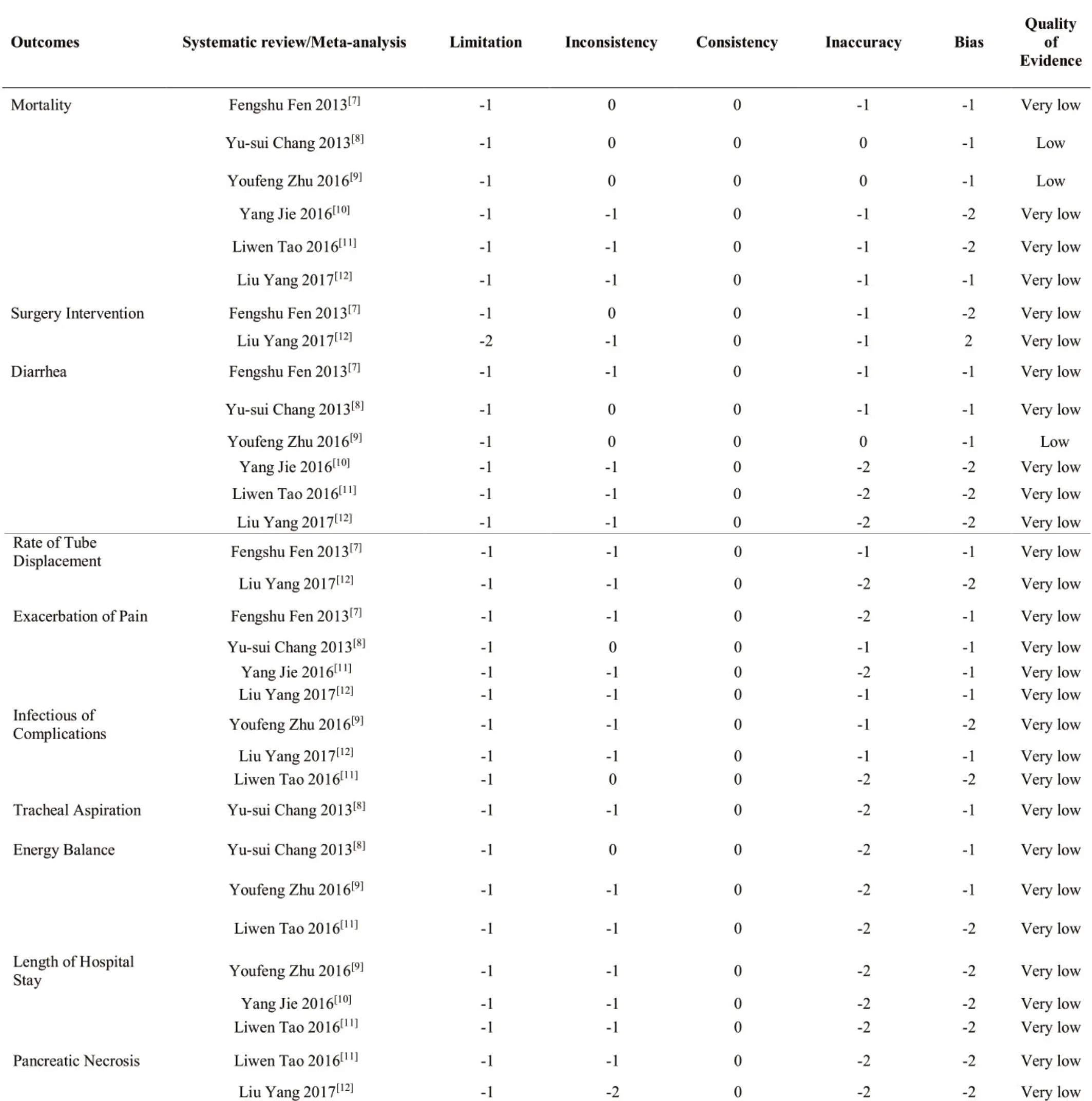
Table 4 GRADE classification of outcomes
3 Discussion
The major function of evaluating the methodological quality is to assess the authenticity of the literatures, which will benefit the difference between the truth and literatures published, so as to satisfy the standard and minimize the risk of bias [16]. There is some insufficiency in these 6 studies, 1) There is no pre-design plan; 2) There is no reason why SRs/MAs are selected in the design of the research, 3) k5 of them did not report the comprehensive search strategy. Though the comprehensive retrieval costs time and is challenging, it is the premise for the overwhelming, objective and repeatable SR [17]. 4) Given the risk of bias and related potential influence on the result,a statistical table or graph is not used to measure the bias
risk and potential influence, nor is the statistical method,
which indicates that the author did not realize the importance of stating the risk of bias. In the system of GRADE,stating the risk of bias matters much. If the bias risk is not stated in the SR/MA, the persuasiveness of the conclusion will be cut down, leading to the unreliability for the readers to assess the quality of the evidence [18]. 5) Fund support and competing interest: 3 of them did not report the fund support, nor the competing interest, leading to the unawareness of impact of potential competing interest on design, implementing and report of the study.
Not all 27 indicators are satisfied when the literatures are assessed by PRSIMA, normally 10 of them are missing.More clearly, the protocol and registration, the part of Method (such as sensitivity analysis, subgroup analysis,Meta-regression analysis), and the inner bias at the Result part, are not stated in all 6 literatures. Recently, the registration is only required by Cochrane, this may be the main reason why that indicator is not mentioned so often in Chinese literatures [19]. At the Result part, 5 of them did not state the retrieval strategy, extraction term, reasoning procedure or other analysis terms (such as sensitivity analysis,subgroup analysis, Meta-regression analysis), potentially misleading the methodology of selecting the literatures,furtherly influence the clinical decisions. At the Discussion part, the persuasiveness is only stated in 2 of them, causing to degrade the persuasiveness. Last but not least, at the fund part, only 3 of them had already reported the fund support or the competing interest. It is necessary to declare the fund support and competing interest, because it assists the reader to judge the influence of fund support and competing interest on the result of the study.
The result of GRADE shows that the quality of evidences is low or extremely low. Only 2 outcomes [8,9] got Low,because they had selection bias and performance bias of limitation. Moreover, these trails included did not conduct allocation concealment and blind method. Most of studies suggested that NGEN had positive efficacy, while their outcomes were short of objective indicators, low evidence and reference about the SRs/ MAs included. 2 low outcomes show that NGEN is better than NJEN in mortality and diarrhea. Consequently, the poor quality of literatures implied the bias risk. All studies addressed the quality of clinical research in order to cumulate the valid data for approving the effectiveness and safety of treatment.
The following limitations may be existing potentially. 1)Only the published records are considered in this study,which means the number of the literatures is not sufficient, leading to the bias of selection. 2) The AMSTAR 2 checklist and PRSIMA statement are quite subjective.Even though 2 researchers participated in the evaluation andcollation, the third one was invited for discussion if necessary,while we could not get rid of the impact of subjectivity on the result of study.
More attention should be paid to the researches of SAP treatment by NGEN/ NJEN, so as to advance the quality of literatures; 2) The pre-design protocol, fund support and competing interest are supposed to be stated in the study, in order to improve the quality furthermore; 3) The researchers are better to get the professional training such as evidence-based medicine and systematic review. If possible, forming a group will be encouraged to study with the guidance of AMSTAR 2 and PRSIMA. We believe that after these efforts, the result of research can be repeated and the accuracy will be improved.
At present, the literatures about the treatment of SAP by NGEN/ NJEN are not efficient published home and abroad, and the quality of which is not at the level of high,so is the reporting quality. We wish high-quality of research can be made in the future, in order to give a better guideline to the clinical practice.
- Food and Health的其它文章
- Progress of Bai He Di Huang decoction on intestinal flora of mouse with depression
- Effects of medicinal diets on patients with non-small cell lung cancer undergoing chemotherapy
- Green tea and its active compounds in cancer prevention andtreatment
- Intestinal microflora in patients with liver disease

