投加羟胺原位恢复城市污水短程硝化-厌氧氨氧化工艺
李 佳,李夕耀,张 琼,彭永臻
投加羟胺原位恢复城市污水短程硝化-厌氧氨氧化工艺
李 佳,李夕耀,张 琼,彭永臻*
(北京工业大学,城镇污水深度处理与资源化利用国家工程实验室,北京市污水脱氮除磷处理与过程控制工程技术研究中心,北京 100124)
有效抑制或淘洗亚硝酸盐氧化菌(NOB)是短程硝化-厌氧氨氧化(PN/A)工艺应用于城市污水处理的关键.以因NOB大量增长受到破坏的城市污水PN/A系统为对象(硝酸盐(NO3--N)生成比例为0.90),考察了羟胺(NH2OH)投加浓度和投加方式对其恢复的效果.结果显示,当序批式反应器中初始NH2OH投加浓度为10mg/L时,每天投加1次,连续投加20d后,NO3--N生成量占NH4+-N消耗量的比例由0.90逐步降低至0.11.表明NH2OH(10mg/L)可原位恢复PN/A工艺.NH2OH停止投加59d后,出水NO3--N生成比例再次小幅度上升至0.15,此时继续投加5d NH2OH (10mg/L),PN/A工艺运行良好,因此间歇投加NH2OH可以维持PN/A工艺稳定运行.实时定量PCR结果表明,在投加NH2OH (10mg/L)后,NOB的丰度不断下降,从(4.52±0.44)×1010copies/g VSS(第6d)下降到(2.30±0.80)×109copies/g VSS(第157d),说明NH2OH的投加有利于抑制和淘洗NOB.
羟胺(NH2OH);短程硝化-厌氧氨氧化(PN/A);NOB;城市污水
短程硝化-厌氧氨氧化(PN/A)的脱氮过程为:好氧条件下,原水中部分氨氮(NH4+-N)被氨氧化菌(AOB)氧化为亚硝态氮(NO2--N),生成的NO2--N继而与NH4+-N在厌氧氨氧化菌(Anammox)的作用下转化为氮气(N2)[1-2].与传统硝化-反硝化脱氮工艺相比,此工艺理论上可节省60%的曝气量,100%的碳源[3-4].然而,在研究中发现,低氨氮城市污水PN/A工艺很容易出现亚硝酸盐氧化菌(NOB)的大量增长所导致的系统运行恶化[5-6].
NOB会与Anammox竞争底物NO2--N,从而导致出水硝态氮(NO3--N)的积累,以至PN/A工艺脱氮性能的下降.因此,PN/A工艺成功应用于污水处理的关键在于有效的抑制或淘洗NOB[7-8].目前,已经有许多在PN/A系统中解决NOB的增长的策略,如重新接种不含NOB的污泥、加大排泥量、降低系统溶解氧(DO)和采用间歇曝气方式等,然而这些策略均不能有效的抑制或淘洗NOB,恢复PN/A工艺[9-11].羟胺(NH2OH)是硝化和厌氧氨氧化2个过程的中间产物,可以成功启动短程硝化[12-15],提高Anammox的活性[16-17].但以NH2OH恢复一体化PN/A工艺鲜有报道.
本研究采用序批式反应器(SBR),以因NOB大量增长受到破坏的城市污水PN/A系统为对象,研究了投加NH2OH恢复PN/A工艺的适宜浓度及投加策略,通过测定SBR系统硝态氮生成比例及功能菌活性和丰度变化,分析了PN/A工艺原位恢复的微生物学机制.
1 材料与方法
1.1 反应装置及接种污泥
试验采用敞口圆柱体SBR反应器(图1),材质为有机玻璃,有效容积10L.反应器用黑色遮光材料包裹,避光运行.为保证反应阶段泥水充分混合及传质均匀,反应器设有搅拌装置;曝气采用曝气泵和曝气头实现,由转子流量计控制DO为0.2~0.5mg/L;反应器中配有便携式检测仪(WTW340i, Germany),监测反应过程中的DO、pH值和温度变化;设置有加热棒,以保证反应器内温度在30℃左右.
接种污泥为短程硝化絮体污泥和厌氧氨氧化颗粒污泥,接种前短程硝化絮体污泥的亚硝酸盐氮积累率平均为96.9%,厌氧氨氧化颗粒污泥总氮去除负荷平均为0.5kgN/(m3×d).短程硝化污泥与厌氧氨氧化颗粒污泥按质量比1:2的比例混合,接种后的混合液悬浮固体浓度为6000mg/L,混合液挥发性悬浮固体浓度为4980mg/L.
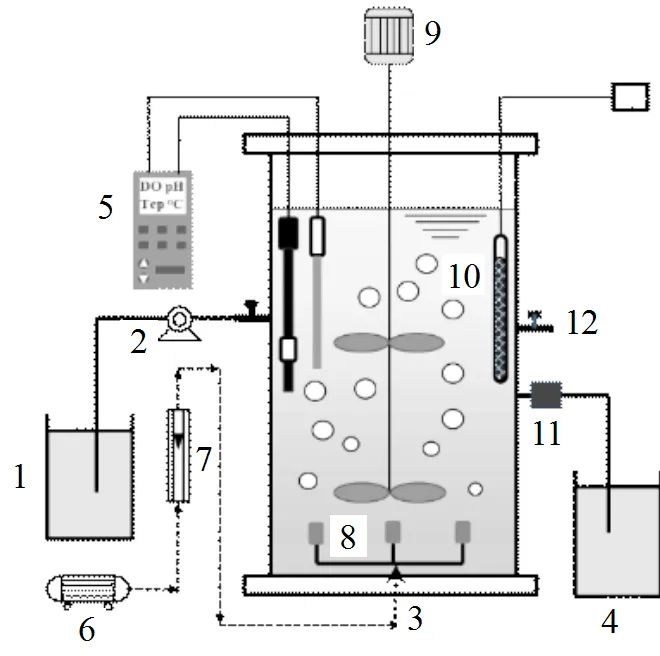
图1 SBR实验装置示意
1.进水箱;2.蠕动泵;3.SBR反应器;4.出水箱;5.DO、pH和温度在线监测仪;6.曝气泵;7.流量计;8.曝气头;9.搅拌器;10.加热棒;11.电动排水阀;12.取样口
1.2 进水水质及运行方式
试验用水为北京市某家属区化粪池实际生活污水,污水先经过前端预处理后再进入SBR反应器.具体水质:COD为55.7~128.2mg/L,NH4+-N浓度为25.8~83.5mgN/L,NO2--N和NO3--N浓度均小于1.0mgN/L,pH值7.1~7.6.
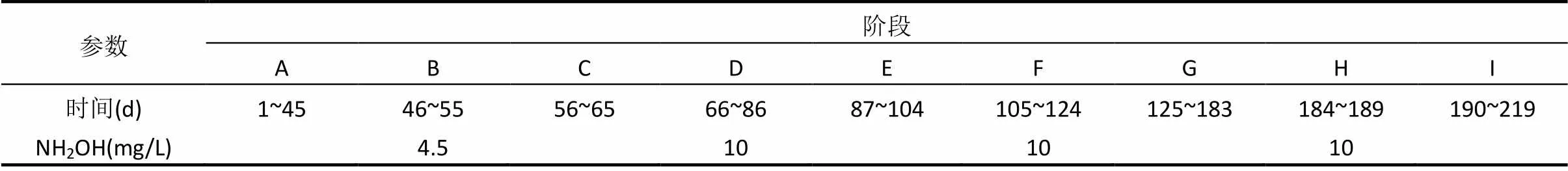
表1 系统运行阶段
反应器每周期进水5L,排水比为50%,污泥龄为10d(只排絮体污泥).每周期运行方式为:进水10min,缺氧搅拌30min,好氧曝气60~300min,沉淀30min,排水10min, 闲置40~100min.试验过程分为A~I9个阶段(表1),在阶段B、D、F和H时向SBR反应器中投加NH2OH,其中阶段B投加的NH2OH浓度为4.5mg/L,阶段D、F和H为10mg/L.NH2OH浓度均为SBR反应器好氧阶段初始时NH2OH浓度. NH2OH通过加药泵投加,每天投加1次.
1.3 检测指标及方法
水样经0.45μm中速滤纸过滤后测定各参数. NH4+-N、NO2--N和NO3--N采用Lachat Quikchem 8500型流动注射仪测定(Lachat Instrument, Milwaukee, Wiscosin),TN为NH4+-N、NO2--N和NO3--N三者浓度之和;NH2OH采用羟基喹啉分光光度法测定[18];MLSS与MLVSS采用标准方法测定[19]; DO、pH值和温度采用便携式检测仪(WTW340i, Germany)检测.
通过一系列的批次试验测定功能菌AOB、NOB和Anammox的活性[20],试验条件如表2所示.采用实时定量PCR(real-time qPCR)对微生物的种群结构进行分析.泥样DNA采用DNA快速提取试剂盒 (fast DNA spin kit for soil, Q BIOgene Inc., Carlsbad, USA) 提取,通过Nanodrop 分光光度计(NanoDrop Technologies,Wilmington, USA)测定DNA的质量和数量;real-time qPCR采用MX 3500p荧光实时定量PCR扩增仪测定(Agilent Technologies, USA).每个样品设立平行,取均值,最终以每克干污泥中菌的基因拷贝数表示菌的含量.扩增所用引物及其核苷酸序列见表3.
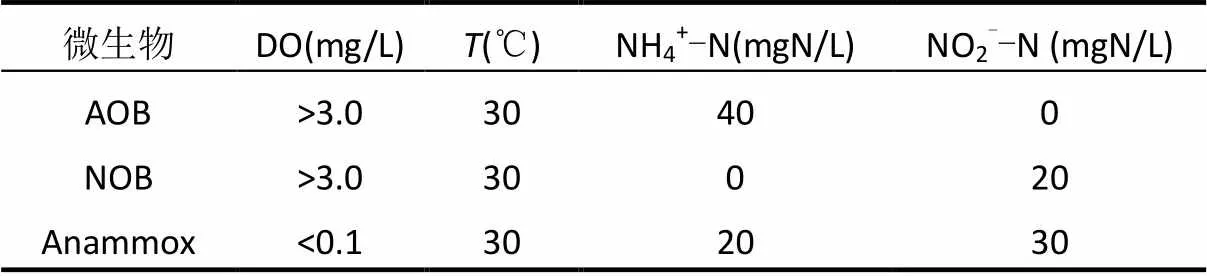
表2 微生物活性测定的批次实验条件
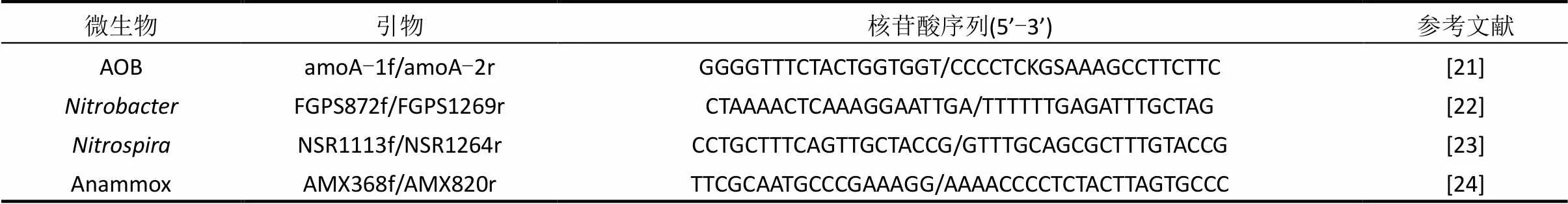
表3 real-time qPCR所用的引物及核苷酸序列
2 结果与讨论
2.1 NH2OH投加对PN/A工艺的恢复
如图2中阶段A所示,运行初期出水的NO3--N浓度逐渐升高,运行14d后,系统ΔNO3--N/ΔNH4+-N大于理论值0.11[25],说明PN/A工艺被破坏.在SBR反应器运行的第44d,ΔNO3--N/ΔNH4+-N升高至0.86(图2),PN/A工艺运行失稳.此时,如图3所示,NOB的活性由0.62mgN/(VSS·h)(第1d)升高为5.9mgN/(VSS·h)(第40d),说明PN/A工艺中短程硝化破坏;Anammox的活性由15.9mgN/(VSS·h)(第1d)下降为3.3mgN/(VSS·h)(第40d),Anammox活性的下降可能是由于NOB活性的提高使其底物NO2--N缺失,这表明Anammox与NOB在底物竞争上处于劣势地位.在阶段B(第46~55d),向反应器中投加NH2OH,使SBR中初始NH2OH浓度为4.5mg/L,每天投加1次,投加10d后系统中ΔNO3--N/ΔNH4+-N仅下降至0.63,PN/A工艺未恢复到较好状态.
在阶段B停止投加NH2OH后,ΔNO3--N/ ΔNH4+-N又上升为0.90(第65d),且NOB的活性在运行第64d升高至8.6mgN/(VSS·h)(图3),NH2OH的投加有助于PN/A工艺的恢复,但是NH2OH浓度为4.5mg/L对PN/A工艺的恢复效果较差.因此在阶段D(第66~85d)提高NH2OH的投加浓度到10mg/L.如图2所示,在提高NH2OH投加浓度后,SBR反应器出水NO3--N浓度明显降低,ΔNO3--N/ΔNH4+-N由0.90(第65d)下降到0.11(第85d),并且NOB的活性下降为3.4mgN/(VSS·h)(图3), NH2OH(10mg/L)的投加有效抑制了NOB的活性,使短程硝化过程在20d内快速恢复.
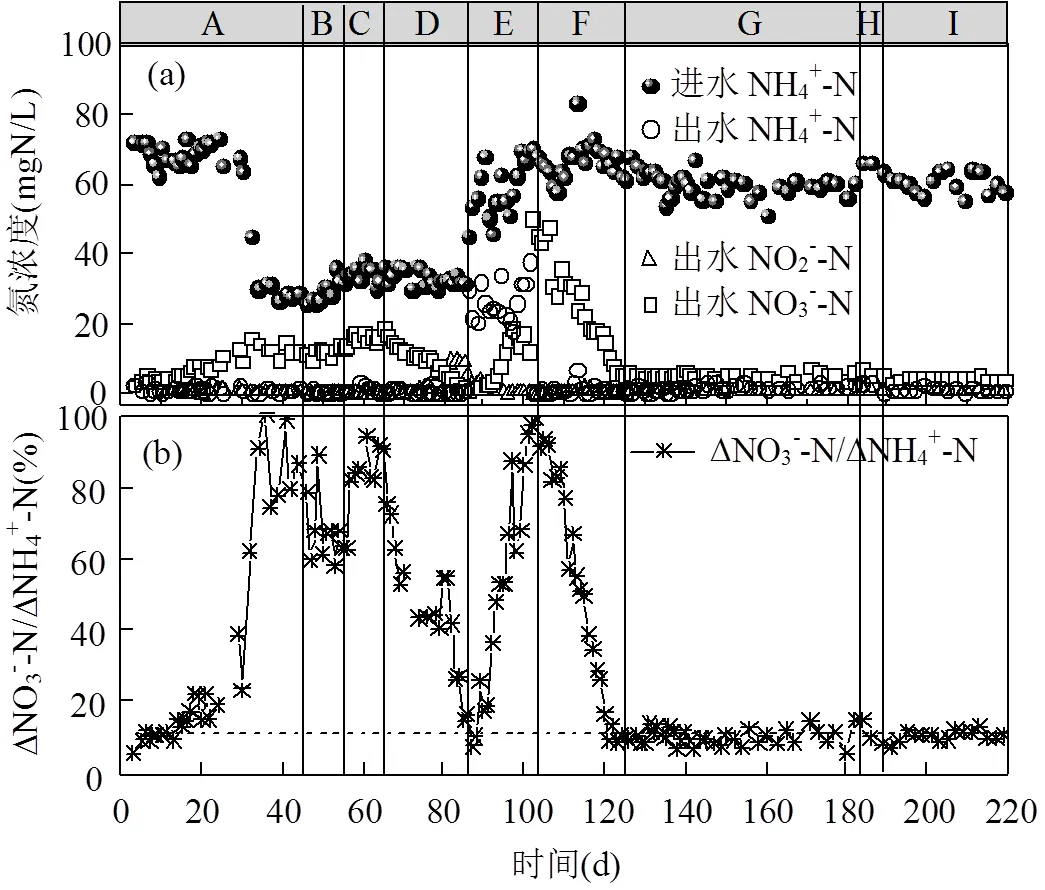
图2 SBR中氮素污染物浓度(a)和硝态氮生成比例(b)变化
由于阶段E(第86~104)进水NH4+-N的浓度突然大幅度升高(由(30.2±3.4) mgN/L上升为(60.3±7.3) mgN/L),AOB的活性受到进水波动的影响从14.5mgN/(VSS·h)(第74d)下降至6.8mgN/VSS/h(第101d),水质波动会影响硝化菌的活性[26].此时,即使适当延长曝气时间(从60min延长到150min),出水NH4+-N仍然由(1.1±0.8) mgN/L增长为(26.6±5.6) mgN/L.为了恢复AOB的活性,从第98d继续延长曝气时间到300min,运行5d后出水NH4+-N下降到0.6mgN/L.但是随着硝化效果的转好,刚刚恢复的短程硝化又因过度曝气而破环,系统ΔNO3--N/ ΔNH4+-N逐渐升高至0.95(第104d),NOB的活性上升至7.9mgN/(VSS·h)(第101d).可见过曝气对于短程硝化具有很强的破坏作用[27-28],尤其对于还未运行稳定的短程硝化.
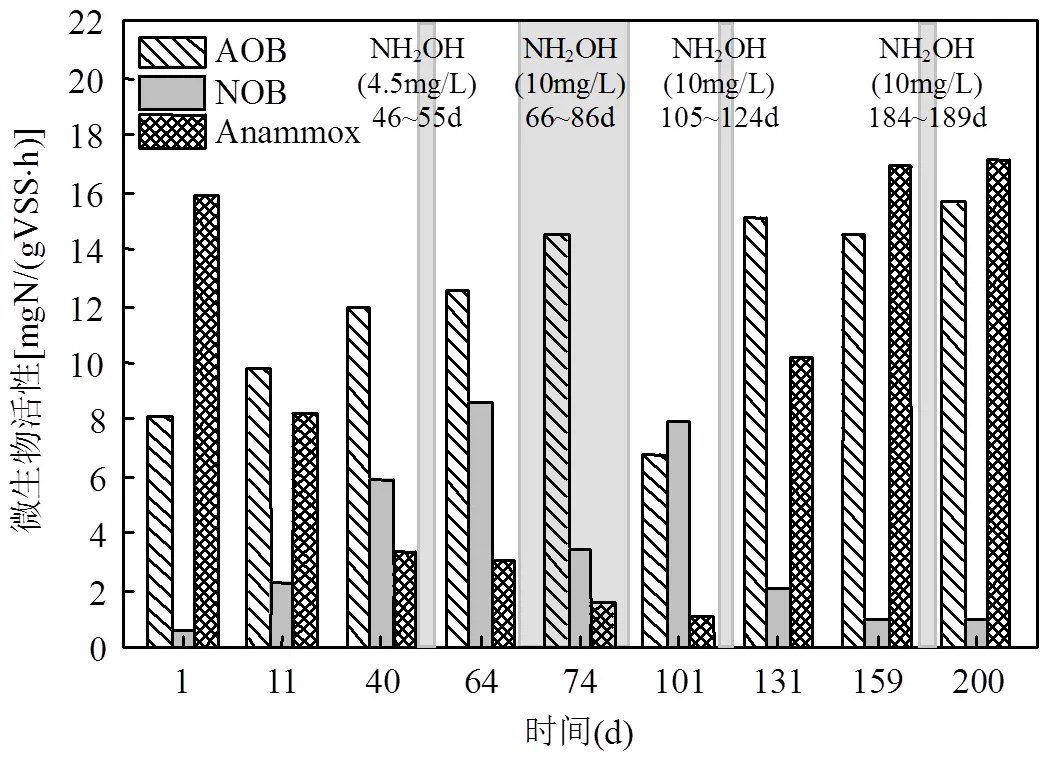
图3 反应器中功能菌活性变化
在阶段F(第105~124d)继续投加NH2OH (10mg/L)以恢复PN/A工艺, 投加20d后(第124d)出水中的NO3--N浓度大幅度下降至5.75mgN/L,此时ΔNO3--N/ΔNH4+-N为0.11,PN/A工艺恢复.此试验结果与阶段D相似,进一步验证了NH2OH对PN/A工艺恢复的有效性.在阶段G(第125~183d), PN/A工艺运行稳定,出水NH4+-N为(1.7±0.8)mgN/ L,NO3--N为(5.2±1.0) mgN/L,ΔNO3--N/ΔNH4+-N稳定在0.10±0.02.ΔNO3--N/ΔNH4+-N略低于理论值,表明系统中存在微弱的反硝化作用,将小部分NO3--N还原为N2,提高了总氮去除率[29].从图3中可以看出,Anammox活性随着NOB活性的降低而逐渐提高,到第131d升高为10.2mgN/(VSS·h).NH2OH的投加可能有利于Anammox在长期缺少底物而失活的情况下较快的恢复活性,有文献报道向在4℃条件下长期储存的Anammox系统中投加NH2OH,有利于血红素含量的提高,进而快速恢复Anammox的活性[17].在阶段G末期(第183d) ΔNO3--N/ΔNH4+-N又有小幅的增长(ΔNO3--N/ΔNH4+-N为0.15),故在阶段H(第184~189d)投加了5d的NH2OH(10mg/ L),PN/A工艺在阶段I(第190~219d)运行良好.因此,短期的NH2OH投加并不能维持PN/A工艺的长久稳定,其它研究也有相同的结论[30],但间歇投加NH2OH的策略可以使NOB持续受到抑制,从而维持PN/A工艺的长久稳定运行.
2.2 PN/A工艺恢复前后的典型周期分析
分析PN/A工艺破坏(第37d)和恢复(第150d)的典型周期内氮素污染物浓度、pH值、DO和温度变化.如图4所示,反应过程中pH值在6.6~7.4之间变化,反应初的pH值受进水水质的影响而不同.反应器中温度均逐渐升高并维持在30℃左右.在好氧阶段,2个典型周期的DO均在0.2~0.5mg/L.
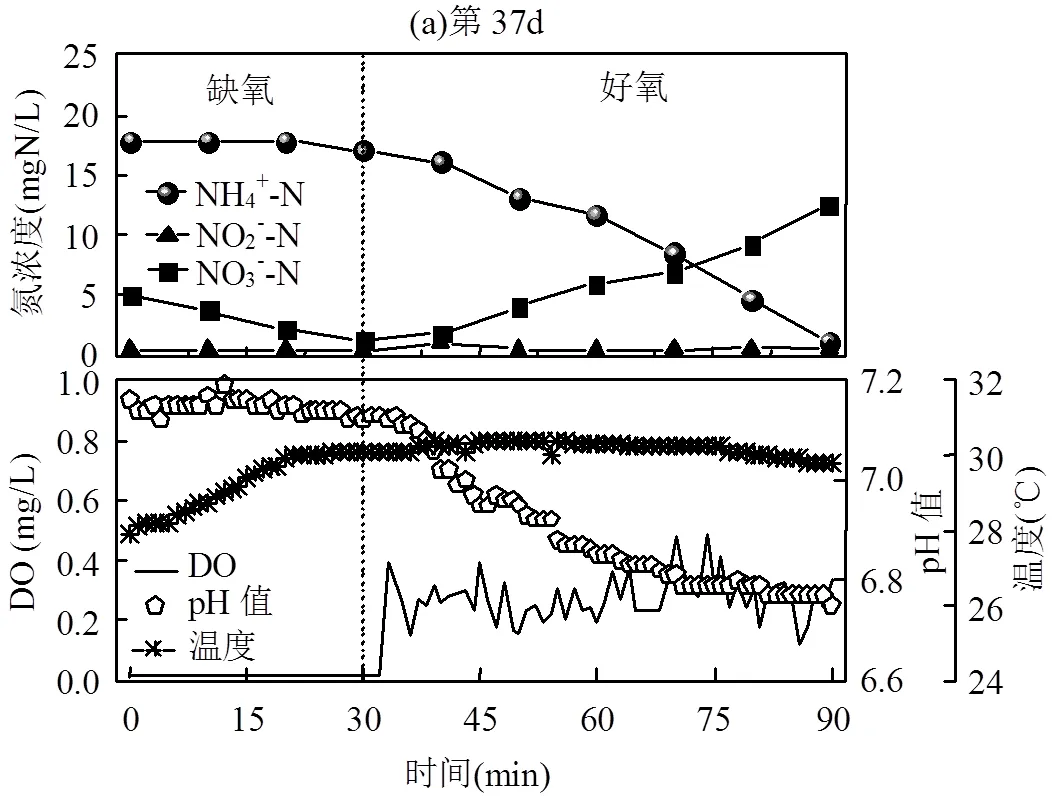
图4(a)为PN/A工艺破坏的典型周期,NH4+-N浓度因上周期剩余泥水混合液的稀释由34.07mgN/ L变为17.88mgN/L,NO2--N为0.42mgN/L, NO3--N因上周期反应末的剩余由0.51mgN/L变为4.95mgN/L.在缺氧搅拌阶段,反硝化菌利用进水中的有机物进行将NO3--N反硝化为N2,30min后NO3--N浓度由4.95mgN/L降低为1.18mgN/L.在好氧阶段,曝气60min后NH4+-N由17.00mgN/L降低为1.17mgN/L(ΔNH4+-N为15.83mgN/L),NO3--N由1.18mgN/L上升为12.56mgN/L(ΔNO3--N为11.83mgN/L).反应过程ΔNO3--N/ΔNH4+-N为0.74,远大于理论值0.11,TN去除率仅为23.0%,说明Anammox氮去除途径发挥的作用很小,短程硝化的破坏使PN/A工艺失稳而脱氮性能下降.
图4(b)为PN/A工艺通过投加NH2OH策略恢复后的典型周期.在好氧阶段,曝气120min后NH4+-N由29.45mgN/L降低为1.53mgN/L(ΔNH4+-N为27.92mgN/L),NO3--N由2.52mgN/L上升为5.49mgN/L(ΔNO3--N为2.97mgN/L),在反应过程中没有NO2--N的积累.ΔNO3--N/ΔNH4+-N为0.11,即PN/A工艺NO3--N生成比例的理论值,TN去除率为76.8%.此典型周期说明NH4+-N大部分氧化NO2--N而非NO3--N,且生成的NO2--N与进水中的NH4+-N进一步进行厌氧氨氧化反应,PN/A工艺呈现较好的运行状态.
2.3 NH2OH投加后系统微生物种群结构变化
为了进一步了解系统运行状态,对反应器中各功能菌AOB、NOB(和之和)及Anammox丰度进行了测定.
从图5中可以看出,NOB的丰度从第6d的(1.20±0.2)×107copies/g VSS上升到第44d的(2.65± 0.16)×108copies/g VSS,为原来的22倍左右, 此时ΔNO3--N/ΔNH4+-N大幅度上升(图2).因此NOB丰度的大量增长导致了PN/A工艺破坏.在阶段B(第46~50d)投加4.5mg/L的NH2OH后,NOB的丰度没有下降,反而上升为(4.06±0.80) ×109copies/g VSS(第60d),这与NOB活性升高相对应(图3).在阶段D(第66~86d)和F(第105~124d),提高NH2OH投加浓度到10mg/L后,NOB数量不断下降,最终降低为(3.03± 0.11)×107copies/g VSS(第157d).系统中NOB活性受到抑制或菌种被淘洗对于PN/A工艺的稳定性非常关键[31].本试验结果表明,NH2OH(10mg/L)的投加不仅抑制了NOB的活性,还抑制了NOB的增长,从而在排泥条件下实现菌种淘洗[13-14],以恢复PN/A工艺.在硝化过程中,AOB在氨单加氧酶(AMO)和羟胺氧化还原酶(HAO)的催化作用下完成NH4+-N的氧化; NOB在亚硝酸盐氧化还原酶(nitrite oxidoreductase enzyme, NXR)的催化作用下氧化NO2--N.当添加NH2OH后,AOB中HAO的存在可以分解NH2OH以减缓NH2OH的毒性,而在NOB的代谢过程中没有相应的缓解措施[32].有文献报道,在反应器中加入NH2OH后的拷贝数下降了一个数量级[33].因此,NH2OH的这种抑制作用可能是因为其对NOB中NXR的活性表达或合成过程的抑制[13].
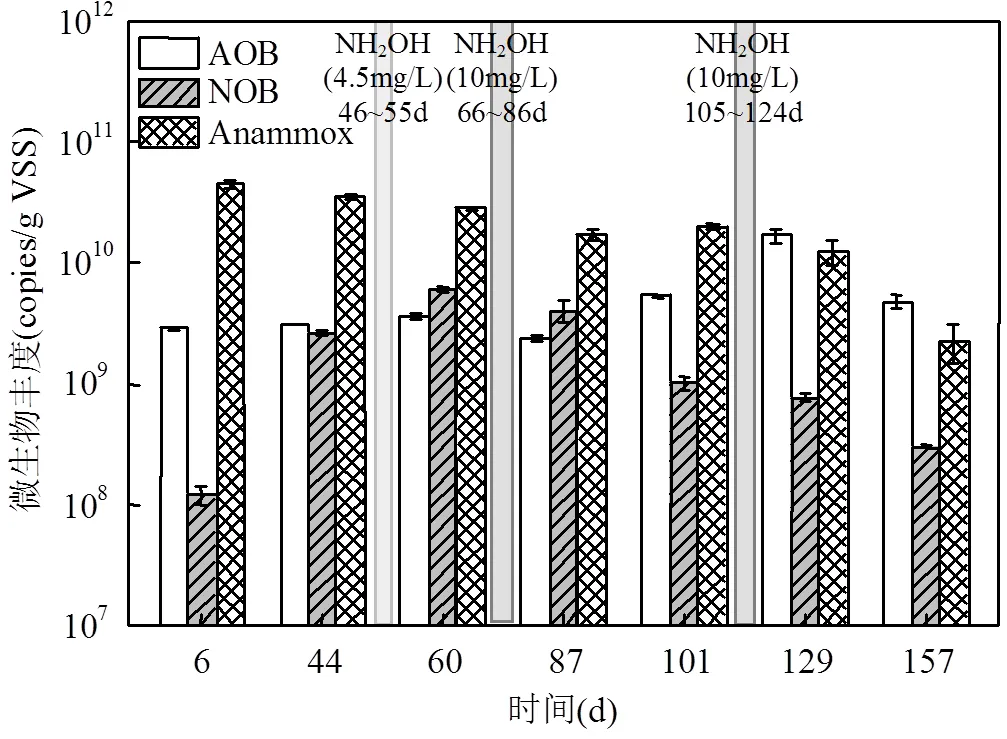
图5 反应器中功能菌丰度变化
在运行中AOB的丰度呈现小幅波动,但基本维持在(2.37±0.23)×109copies/g VSS之上,即使在AOB的活性显著下降时丰度也并没有明显降低,这说明在不利条件下微生物的活性和丰度变化并不相一致[34].Anammox丰度在运行过程中逐渐降低,从(4.52±0.44)×1010copies/g VSS(第6d)下降到(2.30± 0.80)×109copies/g VSS(第157d).Anammox丰度的降低可能是由于PN/A工艺运行的不稳定性,即短程硝化的不断破坏,使得Anammox底物缺失,长期处于饥饿状态,同时Anammox的有效持留也是PN/A工艺运行的关键点之一[35].
3 结论
3.1 向SBR中每天投加1次NH2OH并使其初始NH2OH浓度为10mg/L,20d后系统ΔNO3—N/ ΔNH4+-N逐渐降低至理论值0.11.表明NH2OH可快速原位恢复PN/A工艺.
3.2 NH2OH投加停止59d后,反应器出水NO3--N再次出现积累趋势,此时继续投加5dNH2OH,PN/A工艺运行良好,因此间歇投加NH2OH是一种维持PN/A工艺的稳定运行的有效策略.
3.3 实时定量PCR结果表明,在投加NH2OH (10mg/L)后NOB的丰度不断降低,从(4.52±0.44)× 1010copies/g VSS(第6d)下降到(2.30±0.80)× 109copies/g VSS(第157d),说明NH2OH的投加有利于抑制和淘洗NOB.
[1] 胡 石,甘一萍,张树军,等.一体化全程自养脱氮(CANON)工艺的效能及污泥特性[J]. 中国环境科学, 2014,34(1):111-117. Hu S, Gan Y P, Zhang S J, et al. Performance and sludge characteristics of the CANON process [J]. China Environmental Science, 2014,34(1):111-117.
[2] 杨延栋,黄 京,韩晓宇,等.一体式厌氧氨氧化工艺处理高氨氮污泥消化液的启动[J]. 中国环境科学, 2015,35(4):1082-1087. Yang Y D, Huang J, Han X Y. Start-up of one-stage partial nitrification/anammox process treating ammonium-rich reject water [J]. Chian Environmental Science, 2015,35(4):1082-1087.
[3] Mulder A. The quest for sustainable nitrogen removal technologies [J]. Water Science and Technology, 2003,48:67-75.
[4] Wang Z B, Zhang S J, Zhang L, et al. Restoration of real sewage partial nitritation-anammox process from nitrate accumulation using free nitrous acid treatment [J]. Bioresource Technology, 2018,251: 341-349.
[5] Joss A, Derlon N, Cyprien C, et al. Combined nitritation-anammox: advances in understanding process stability [J]. Environmental Science and Technology, 2011,45:9735–9742.
[6] Vlaeminck S E, De Clippeleir H, Verstraete W. Microbial resource management of one-stage partial nitritation/anammox [J]. Microbial Biotechnology, 2012,5:433-448.
[7] Lackner S, Gilbert E M, Vlaeminck S E, et al. Full-scale partial nitritation/anammox experiences-an application survey [J]. Water Research, 2014,55:292-303.
[8] Wett B, Omari A, Podmirseg S, et al. Going for mainstream deammonification from bench-to full-scale for maximized resource efficiency [J]. Water Science and Technology, 2013,68:283-289.
[9] Jardin N, Hennerkes J. Full-scale experience with the deammonification process to treat high strength sludge water-a case study [J]. Water Science and Technology, 2012,65:447-455.
[10] Strous M, Heijnen J J, Kuenen J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms [J]. Applied Microbiology and Biotechnology, 1998,50:589-596.
[11] Katsogiannis A N, Kornaros M, Lyberatos G. Enhanced nitrogen removal in SBRs bypassing nitrate generation accomplished by multiple aerobic/anoxic phase pairs [J]. Water Science and Technology, 2003,47:53-59.
[12] Okabe S, Oshiki M, Takahashi Y, et al. Development of long-term stable partial nitrification and subsequent anammox process [J]. Bioresource Technology, 2011,102:6801-6807.
[13] Xu G J, Xu X C, Yang F L, et al. 2012. Partial nitrification adjusted by hydroxylamine in aerobic granules under high DO and ambient temperature and subsequent anammox for low C/N wastewater treatment [J]. Chemical Engineering Journal, 2012,213:338-345.
[14] Kindaichi T, Okabe S, Satoh H, et al. Effects of hydroxylamine on microbial community structure and function of autotrophic nitrifying biofilms determined by in situ hybridization and the use of microelectrodes [J]. Water Science and Technology, 2004,49:61-68.
[15] Hao O J, Chen J M. Factors affecting nitrite buildup in submerged filter system [J]. Journal of Environmental Engineering, 1994,120: 1298-1307.
[16] Tian Z Y, Zhang J, Song Y H. 2015. Several key factors influencing nitrogen removal performance of anammox process in a bio-filter at ambient temperature [J]. Environmental Earth Sciences, 2015,73: 5019-5026.
[17] Wang G, Xu X C, Zhou L, et al. A pilot-scale study on the start-up of partial nitrification-anammox process for anaerobic sludge digester liquor treatment [J]. Bioresource Technology, 2017,241:181-189.
[18] Frear D S, Burrell R C. Spectrophotometric method for determining hydroxylamine reductase activity in higher plants [J]. Analytical Chemistry, 1995,27:1664-1665.
[19] APHA. Standard methods for the examination of water and wastewater [M]. American Public Health Association, 1976.
[20] Miao Y Y, Zhang L, Li B K, et al. Enhancing ammonium oxidizing bacteria activity was key to single-stage partial nitrification-anammox system treating low-strength sewage under intermittent aeration condition [J]. Bioresource Technology, 2017,231:36-44.
[21] Wang S Y, Wang Y, Feng X J, et al. Quantitative analyses of ammonia-oxidizing Archaea and bacteria in the sediments of four nitrogen-rich wetlands in China [J]. Applied Microbiology and Biotechnology, 2011,90:779-787.
[22] Degrange V, Bardin R. Detection and counting of nitrobacter populations in soil by PCR [J]. Applied and Environmental Microbiology, 1995,61:2093-2098.
[23] Geets J, de Cooman M, Wittebolle L, Het al. Real-time PCR assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge [J]. Applied Microbiology and Biotechnology, 2007,75:211-221.
[24] Schmid M C, Maas B, Dapena A, et al. Biomarkers for in situ detection of anaerobic ammonium-oxidizing (anammox) bacteria [J]. Applied and Environmental Microbiology, 2005,71:1677-1684.
[25] Sliekers A O, Derwort N, Gampos-Gomez J L, et al. Completely autotrophic nitrogen removal over nitrite in one single reactor [J]. Water Reseach, 2002,36:2475-2482.
[26] 程 军,张 亮,杨延栋,等.氨氮负荷波动对城市污水短程硝化-厌氧氨氧化工艺硝态氮的影响 [J]. 中国环境科学, 2017,37(2):520- 525. Cheng J, Zhang L, Yang Y D, et al. The effects of ammonium loading rate fluctuation on nitrate accumulation in municipal wastewater partial nitritation/anammox (PN/A) process [J]. China Environmental Science, 2017,37(2):520-525.
[27] Gao D W, Peng Y Z, Li B K, et al. Shortcut nitrification– denitrification by real-time control strategies [J]. Bioresource Technology, 2009,100:2298-2300.
[28] Qian W T, Peng Y Z, Li X Y, et al. The inhibitory effects of free ammonia on ammonia oxidizing bacteria and nitrite oxidizing bacteria under anaerobic condition [J]. Bioresource Technology, 2017,243: 1247-1250.
[29] 孙艳波,周少奇,李伙生,等.ANAMMOX与反硝化协同脱氮反应器启动及有机负荷对其运行性能的影响[J]. 化工学报, 2009,60(10): 2596-2602. Sun Y B, Zhou S Q, Li H S, et al. Start-up of ANAMMOX- denitrification reactor and effect of organic loading on its performance of synergistic interaction [J]. CIESC Joernal, 2009,60(10):2596-2602.
[30] Wang Y Y, Wang Y W, Wei Y S, et al. In-situ restoring nitrogen removal for the combined partial nitritation-anammox process deteriorated by nitrate build-up [J]. Biochemical Engineering Journal, 2015,98:127-136.
[31] Zeng W, Bai X L, Zhang L M, et al. Population dynamics of nitrifying bacteria for nitritation achieved in Johannesburg (JHB) process treating municipal wastewater [J]. Bioresource Technology, 2014,162: 30-37.
[32] Hooper A B, Vannelli T, Bergmann D J, et al. Enzymology of the oxidation of ammonia to nitrite by bacteria [J]. Antonie van Leeuwenhoek, 1997,71:59-67.
[33] Harper W F, Terada A, Poly F, et al. The Effect of hydroxylamine on the activity and aggregate structure of autotrophic nitrifying bioreactor cultures. Biotechnology and Bioengineering, 2009,102:714-724.
[34] Liu W, Yang Q, Ma B, et al. Rapid achievement of nitritation using aerobic starvation [J]. Environmental Science and Technology, 2017, 51:4001-4008.
[35] Li J W, Li J L, Gao R T, et al. A Critical Review of One-stage Anammox Processes for Treating Industrial Wastewater: Optimization Strategies Based on Key Functional Microorganisms [J]. Bioresource Technology, 2018,265:498-505.
In-situ restoring demostic wastewater partial nitritation/anammox (PN/A) process by addition of hydroxylamine.
LI Jia, LI Xi-yao, ZHANG Qiong, PENG Yong-zhen*
(National Engineering Laboratory for Advanced Municipal Wastewater Treatment and Reuse Technology, Engineering Research Center of Beijing, Beijing University of Technology, Beijing 100124, China)., 2019,39(7):2789~2795
The effective inhibition or wash-out of nitrite oxidizing bacteria (NOB) is the key challenge for the application of partial nitritation/anammox (PA/N) process in domestic wastewater treatment. The deteriorated domestic wastewater PN/A system was used to investigate the recovery effect of hydroxylamine (NH2OH) concentration and dosing mode on it. The nitrate (NO3--N) production ratio of the PN/A system was 0.90, which was due to the overgrowth of NOB. The results showed that when the initial NH2OH concentration was 10mg/L in the sequencing batch reactor (SBR) and NH2OH was added once a day, the ratio of NO3--Nproducted/NH4+-Nconsumedwas decreased from 0.90 to 0.11 after adding NH2OH for 20d. It indicated that NH2OH (10mg/L) addition could restore PN/A process-. The ratio of NO3--Nproducted/NH4+-Nconsumedincreased to 0.15 again after stopping NH2OH addition for 59d. When continued to add NH2OH into reactor for 5d, the PN/A process operated well. Therefore, the intermittent addition of NH2OH strategy could maintain the stable performance of the PN/A process. The real-time quantitative PCR results showed that the NOB abundance was decreased continuously from (4.52±0.44)×1010copies/g VSS (Day 6) to (2.30±0.80)×109copies/g VSS (Day 157) after NH2OH (10mg/L) addition. It indicated that NH2OH addition was beneficial to inhibit and wash out NOB.
hydroxylamin (NH2OH);partial nitritation/anammox (PN/A);NOB;demostic wastewater
X703
A
1000-6923(2019)07-2789-07
李 佳(1994-),女,河北保定人,北京工业大学硕士研究生,主要从事污水生物处理理论与应用研究.
2018-12-03
北京市科技计划(D171100001017001);北京市教委资助项目
* 责任作者, 教授, pyz@bjut.edu.cn
——李红,安明哲,苟梓希.CN 114180719A

