Effects of the Fhb1 gene on Fusarium head blight resistance and agronomic traits of winter wheat
Tng Li, Hongjun Zhng,Yiwn Hung, Zhnqi Su, Yun Dng, Hongwi Liu,Chunyn Mi,Gungjun Yu,Huili Li,Liqing Yu,Tongqun Zhu,Li Yng,Hongji Li,*,Yng Zhou,*
aNational Engineering Laboratory for Crop Molecular Breeding, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081,China
bInstitute of Cereal and Oil Crops,Hebei Academy of Agriculture and Forestry Sciences,Shijiazhuang 050035,Hebei,China
cNanping Institute of Agricultural Sciences of Fujian Province,Jianyang 354200,Fujian,China
dXinxiang Innovation Center for Breeding Technology of Dwarf-Male-Sterile Wheat,Xinxiang 453731, Henan,China
eZhaoxian Experiment Station,Shijiazhuang Academy of Agricultural and Forestry Sciences,Zhaoxian 051530,Hebei,China
fWheat Research Institute,Zhumadian Academy of Agricultural Sciences,Zhumadian 463000,Henan,China
Keywords:Triticum aestivum L.Fhb1 gene Marker-assisted selection Fusarium head blight resistance Agronomic traits
ABSTRACT The gene Fhb1 has been used in many countries to improve wheat Fusarium head blight(FHB) resistance. To make better use of this gene in the Yellow-Huai River Valleys Winter Wheat Zone(YHWZ),the most important wheat-producing region of China,it is desirable to elucidate its effects on FHB resistance and agronomic traits in different genetic backgrounds. Based on a diagnostic marker for Fhb1, six BC2 populations were developed by crossing dwarf-male-sterile (DMS)-Zhoumai 16 to three Fhb1 donors(Ningmai 9,Ningmai 13,and Jianyang 84)and backcrossing to Zhoumai 16 and Zhoumai 16's derivative cultivars(Lunxuan 136 and Lunxuan 13)using marker-assisted backcross breeding. The progenies were assessed for FHB resistance and major agronomic traits.The Fhb1 alleles were identified using the gene-specific molecular marker. The plants with the Fhb1-resistant genotype (Fhb1-R) in these populations showed significantly fewer infected spikelets than those with the Fhb1-susceptible genotype (Fhb1-S). When Lunxuan 136 was used as the recurrent parent, Fhb1-R plants showed significantly fewer infected spikelets per spike than Fhb1-R plants produced using Lunxuan 13 as the recurrent parent, indicating that the genetic backgrounds of Fhb1 influence the expression of FHB resistance. Fhb1-R plants from the DMS-Zhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 136 population showed the highest FHB resistance among the six populations and a significantly higher level of FHB resistance than the moderately susceptible control Huaimai 20. No significant phenotypic differences between Fhb1-R and Fhb1-S plants were observed for the eight agronomic traits investigated. These results suggest that it is feasible to improve FHB resistance of winter wheat without reducing yield potential by introgressing Fhb1 resistance allele into FHB-susceptible cultivars in the YHWZ.
1. Introduction
Fusarium head blight(FHB),which occurs mainly in warm and humid regions during wheat flowering,is a devastating fungal disease of wheat (Triticum aestivum L.) and other small-grain cereal crops worldwide [1-3]. It is caused primarily by the fungus Fusarium graminearum Schw.Epidemics of FHB not only result in reduction of grain yield and end-use quality,but also endanger food and feed safety owing to contamination with mycotoxins, especially deoxynivalenol [3-5]. In China, past FHB epidemics occurred mainly in the Middle and Low Yangtze Winter Wheat Zone (MLWZ). However, the disease has expanded into the northern wheat production regions,including the Yellow-Huai River Valleys Winter Wheat Zone(YHWZ) and North Winter Wheat Zone (NWWZ), a development that may be associated with changes in cropping systems and climate warming in those areas [6]. FHB epidemics occurred in nine years between 2001 and 2012 on>3.3 Mha of wheat in China, and in seven years in the same period on >0.67 Mha solely in Henan province, the largest wheat-producing province in the southern YHWZ [6,7].Currently, all of the cultivars grown in the YHWZ are highly susceptible to FHB except for a few moderately susceptible ones[8].Given that it is difficult to control FHB epidemics with fungicides and agronomic practices alone, improvement of wheat resistance to FHB is becoming an urgent breeding objective in the YHWZ.
Conventional wheat breeding for FHB resistance is limited by time-consuming,labor-intensive FHB screening in multiple environments. Molecular marker-assisted selection (MAS)provides an efficient way to accelerate FHB-resistant breeding. Resistance to FHB is a complex trait with a quantitative mode of inheritance [9]. It is controlled by a few major QTL/genes and many modifying genes with minor effects influenced by environmental factors [10,11]. In the last two decades, >250 QTL for FHB resistance have been identified on all wheat chromosomes[10,12].Most of them confer type II resistance (resistance to fungal spread). Of the large number of FHB-resistant QTL reported,only three have been validated as major QTL,including Fhb1 on chromosome arm 3BS[13-16],Fhb2 on 6BS [15,17], and Fhb5 on 5AS [18-21]. Fhb1, a wellrecognized major QTL for FHB resistance, was identified originally in the spring wheat cultivar ‘Sumai 3' and its derivative lines [13-15,18]and explained 20%-60% of phenotypic variance in mapping populations[14,15,18,22].Recently,a functional molecular marker for Fhb1 has been developed[16], providing a reliable method to track the target gene during MAS.
Fhb1 has been widely used in breeding programs to improve local FHB resistance in the USA, Canada, Australia,and Japan [23-26]. Based on MAS using the SSR markers Xgwm389 and Xbarc147,Zhou et al.[27]reported a reduction in the percentage of scabbed spikelets from 70%-80%to 30%-40%for improved lines carrying Fhb1 compared to plants without Fhb1 after introducing Fhb1 from Ning 7840, a wheat line derived from Sumai 3, into the susceptible soft red winter wheat line IL89-7978 from the USA.Near-isogenic lines(NILs)carrying the Fhb1 resistance allele reduced disease severity by 23% and infected kernels by 27% in harvested kernels following MAS using the SSR markers Xgwm493, Xbarc133,and Xgwm533 [28]. In China, several spring cultivars carrying Fhb1 have been released, but no winter wheat cultivar carrying the gene is available for wheat breeders and growers[6]. Wheat breeders in the YHWZ have attempted to increase FHB resistance by MAS using the diagnostic marker of Fhb1.Zhang et al. [8]reported that the mean number of diseased spikelets and disease index in backcross progenies carrying Fhb1 resistance allele were respectively 8.1 and 28.4% lower than those values in the recurrent parent Zhoumai 16,demonstrating the potential of Fhb1 for improving FHB resistance in wheat cultivars adapted to the YHWZ.
Despite the great efforts made to introgress Fhb1 from Sumai 3 and other resistant sources into wheat cultivars, a potential risk is the introgression of not only the desirable Fhb1 resistance allele but also undesirable loci [29,30]. The Fhb1 donors are mainly from the spring wheat cultivars adapted to the MLWZ of China and have different plant types and agronomic characters from those adapted to the YHWZ. Numerous studies have been performed to test the activity of Fhb1 in spring wheat, but its effects on FHB resistance in different genetic backgrounds and agronomic traits in winter wheat,particularly in cultivars adapted to the YHWZ,are still unknown.Such information will be useful for deployment of this gene in the YHWZ.The aims of the present study were to:1)compare the effects of Fhb1 from three donor cultivars in different genetic backgrounds on FHB resistance and 2) evaluate the effects of Fhb1 on agronomic traits in different winter wheat backgrounds.
2. Materials and methods
2.1. Plant materials
Dwarf-male-sterile (DMS) wheat was developed by crossing a Taigu male sterile line with dominant Ms2 [31]to Aibian 1,carrying the reduced-height gene Rht-D1c [32]. Both genes arose from natural mutations in common wheat,and they are closely linked, with only 0.18% recombination, on the short arm of chromosome 4D in DMS wheat.When the DMS wheat is crossed with any normal male-fertile parent, there is always a clear segregation of 1:1 for dwarf male-sterile plants and tall male-fertile plants in the F1generation. The dwarfmale-sterile plants are selected as female parents for further hybridization,and the tall male-fertile plants are advanced to pure lines to develop new cultivars.DMS wheat has been used in breeding programs to increase the efficiency of crossing without the need for manual emasculation [33]. Wheat cultivar Zhoumai 16 has been one of the dominant parents in the YHWZ in recent years.DMS-Zhoumai 16 was developed by backcrossing the DMS wheat to the recurrent parent Zhoumai 16. DMS-Zhoumai 16, carrying the dominant male sterile gene Ms2 and the dwarfing gene Rht-D1c,is an isogenic line of Zhoumai 16. The dwarf-male-sterile plants of DMSZhoumai 16 were crossed separately to the Fhb1 donor parents Ningmai 9, Ningmai 13, and Jianyang 84, and their F1dwarfmale-sterile plants were backcrossed to Zhoumai 16 to develop three BC1F1populations[8].Dwarf-male-sterile plants heterozygous at Fhb1 were selected from the BC1F1populations using the Fhb1 diagnostic marker [16], and further backcrossed in 2016 to the Zhoumai 16-derived cultivars Lunxuan 136 (pedigree: Zhoumai 16/Zhengmai 9023//Zhoumai 16) and Lunxuan 13 (pedigree: Zhoumai 16/Shimai 12//Zhoumai 16) resulting in six BC2F1populations: DMSZhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 136 (population 1),DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 136 (population 2), DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 136 (population 3), DMS-Zhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 13 (population 4), DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 13(population 5),and DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 13(population 6). The backcross populations were evaluated for FHB resistance in the BC2F2generation and for the effects of Fhb1 on agronomic traits in the BC2F1and BC2F2generations in the field, with Zhoumai 16 (highly susceptible), Huaimai 20(moderately susceptible),Yangmai 158(moderately resistant),and Sumai 3 (highly resistant)as controls.
2.2. Analysis of Fhb1 alleles
The Fhb1-specific marker primers TaHRC-GSM-F (5′-ATTCCTACTAGCCGCCTGGT-3′) and TaHRC-GSM-R (5′-ACTGGGGCAAGCAAACATTG-3′) were used to identify Fhb1 resistance and susceptibility alleles[16].A 20 μL PCR reaction mixture consisted of 1 μL 50-100 ng μL-1of DNA, 1 μL 10 μmol-1each of the forward and reverse primers, 10 μL 2 × Taq PCR Master Mix (Sangon Biotech Col. Ltd., Shanghai,China) and 5 μL sterile ddH2O using a Biometra T3000 Thermocycler (ABI, New York, NY, USA). PCR was performed with an initial denaturation at 94 °C for 5 min, 35 cycles of 94 °C for 30 s, annealing at 64 °C for 30 s, and 72 °C for 2 min with a final extension at 72 °C for 10 min.The PCR fragments were separated by electrophoresis in 2%agarose gels at 150 V for 30 min and visualized by staining with GeneFinder(Bio-V,Xiamen,Fujian,China).
2.3. Field assessment of FHB resistance
Field assessment of FHB resistance was performed only in the BC2F2generation. BC2F2seeds derived from 60 BC2F1plants heterozygous at Fhb1 (10 random plants per population) in the six populations, recurrent parents, and the controls were sown in 2017 on the Experimental Farm of Nanping Academy of Agricultural Science, Jianyang, Fujian province (27°33′N, 118°12′E), where warm and prolonged wet weather during anthesis favors FHB development. The experiment used a randomized complete block design. In the BC2F2generation the seeds from each of the 60 BC2F1plants were sown in six rows with two replications, and those of the recurrent parents Huaimai 20,Yangmai 158,and Sumai 3 were sown at 60-row intervals as the controls.Each row was 1 m long with 10 seedlings per row and the distance between rows was 30 cm. In 2018, all BC2F2plants were genotyped using the Fhb1 diagnostic marker, and plants homozygous for Fhb1 alleles were assessed for number of infected spikelets on 3-4 spikes from each plant.Owing to later occurrence of FHB in 2018, FHB scoring was delayed to day 30 after anthesis based on the appearance of the controls in the field[34].Disease severity(%)was calculated as the percentage of infected spikelets per spike[13].
2.4. Field trials and agronomic trait measurement
Field experiments were conducted under high-yield conditions on the Experimental Farm of the Chinese Academy of Agricultural Sciences in Xinxiang, Henan province (35°31′N,113°85′E) in the 2016/2017 and 2017/2018 wheat-growing seasons. In this area, the environmental conditions during anthesis are usually not suitable for FHB infection. When it has occurred, FHB has never caused visible damage, so that the area provides a suitable environment for evaluating the effect of Fhb1 on agronomic traits. The soil in this farm is a clay loam.The BC2F1seeds per population were sown in a 6.0-m row plot at 25 cm between rows. The BC2F2seeds from 10 BC2F1heterozygous Fhb1 plants per population, which were also used to evaluate FHB resistance in Nanping,were sown in a 6-row plot in 2-m rows with 20 seedlings per row and rows 25 cm apart using a randomized complete block design with two replications. All management practice followed the recommended practices for high yield production in the area but without application of any fungicide[35].
At physiological maturity, all selected plants according to breeding aims were uprooted from the plots, dried naturally,and used for trait measurement. Plant height (cm) was measured from the base of the stem to the tip of the spike,excluding awns. Spike number per plant, spike length (cm),spikelet number per spike, and sterile spikelet number per spike were measured as described previously [36]. Kernel weight per spike (g) was calculated by dividing kernel weight per plant by spike number per plant.Kernel number per spike(KNPS) was calculated using as kernel weight per spike/thousand-kernel weight × 1000. Thousand-kernel weight (g)was determined by averaging values of three samples of 200 kernels and then multiplying by five. After phenotyping for agronomic traits, about 10 seeds of each plant harvested in each of the BC2F1and BC2F2generations were genotyped for the Fhb1 alleles with the diagnostic markers[16].
2.5. Data analysis
Analysis of variance (ANOVA) was used to evaluate differences in FHB resistance and agronomic traits in the BC2F2generation, and least significant difference (LSD) at P <0.05 using SAS 9.3 (SAS Institute, Cary, NC, USA) for multiple comparisons of FHB resistance among donors or populations.A t-test in Microsoft Excel 2016 was used to compare agronomic trait means in the BC2F1generation.
3. Results
3.1. Detection of Fhb1 alleles in the BC2F1 and BC2F2 generations
The gene-specific markers TaHRC-GSM-F and TaHRC-GSM-R amplified a 1.4 kb fragment from plants carrying the Fhb1 resistance allele and a 2.0 kb fragment from those carrying the Fhb1 susceptibility allele (Fig. 1). A total of 3200 BC2F2plants from the six populations were genotyped, and 1089 plants carried the homozygous Fhb1 genotypes, representing 132-212 plants per population among the six populations.These homozygous plants were evaluated for FHB resistance(Table 1).
A total of 634 plants in 2017 and 1313 plants in 2018 were selected from the six populations for assessing the effects of Fhb1 on agronomic traits (Table 1). Those plants were all genotyped using the Fhb1 diagnostic markers to identify their Fhb1 alleles. In 2017, 330 plants were Fhb1-heterozygous genotype (Fhb1-H) and 304 plants were Fhb1-susceptible genotype(Fhb1-S).In 2018,476 plants were Fhb1-H,323 plants were Fhb1-resistant genotype (Fhb1-R), and 514 plants were Fhb1-S.
3.2. Comparison of FHB resistance between Fhb1 alleles
The mean number of infected spikelets and disease severity were respectively 14.9% and 62.2% for the highly susceptible control Zhoumai 16, while the corresponding phenotypic values for the moderately susceptible control Huaimai 20,the moderately resistant control Yangmai 158 and the highly resistant control Sumai 3 were respectively 4.9, 1.7, and 1.3 and 22.7%, 9.3%, and 6.6% (Table 2). These sharp contrasts in FHB severity between resistant and susceptible controls showed that the disease pressure in the field disease nursery at the Jianyang site was appropriate for selecting FHB-resistant breeding materials.
Significant differences were found in mean number of infected spikelets and disease severity between Fhb1-R and Fhb1-S plants in both Lunxuan 136 and Lunxuan 13 backgrounds and in a joint analysis combining the two backgrounds(P <0.05)(Table 3).Compared to Fhb1-S plants,Fhb1-R plants showed numbers of infected spikelets reduced by respectively 0.6, 1.3, and 1.1 and disease severity reduced by 2.2%, 5.7%, and 4.3% in the Lunxuan 136 background (populations 1-3),Lunxuan 13 background(populations 4-6)and the joint analysis (populations 1-6) (P <0.05). A significant difference in FHB resistance was also observed between the two genetic backgrounds, and plants from the Lunxuan 136 background showed fewer infected spikelets and lower disease severity than those from the Lunxuan 13 background(Tables 2 and 3). The difference in the number of infected spikelets and disease severity between Fhb1-R and Fhb1-S plants was significant in only population 1 of the Lunxuan 136 background (Fig. 2-A-B), but in all three populations of the Lunxuan 13 background (Fig.2-G-L).

Fig.1-Detection of different alleles of Fhb1 gene using the Fhb1 diagnostic marker in the BC2F1(A)and BC2F2(B)generations.The 1.4 kb and 2.0 kb bands indicate the Fhb1 resistance and susceptibility alleles,respectively.
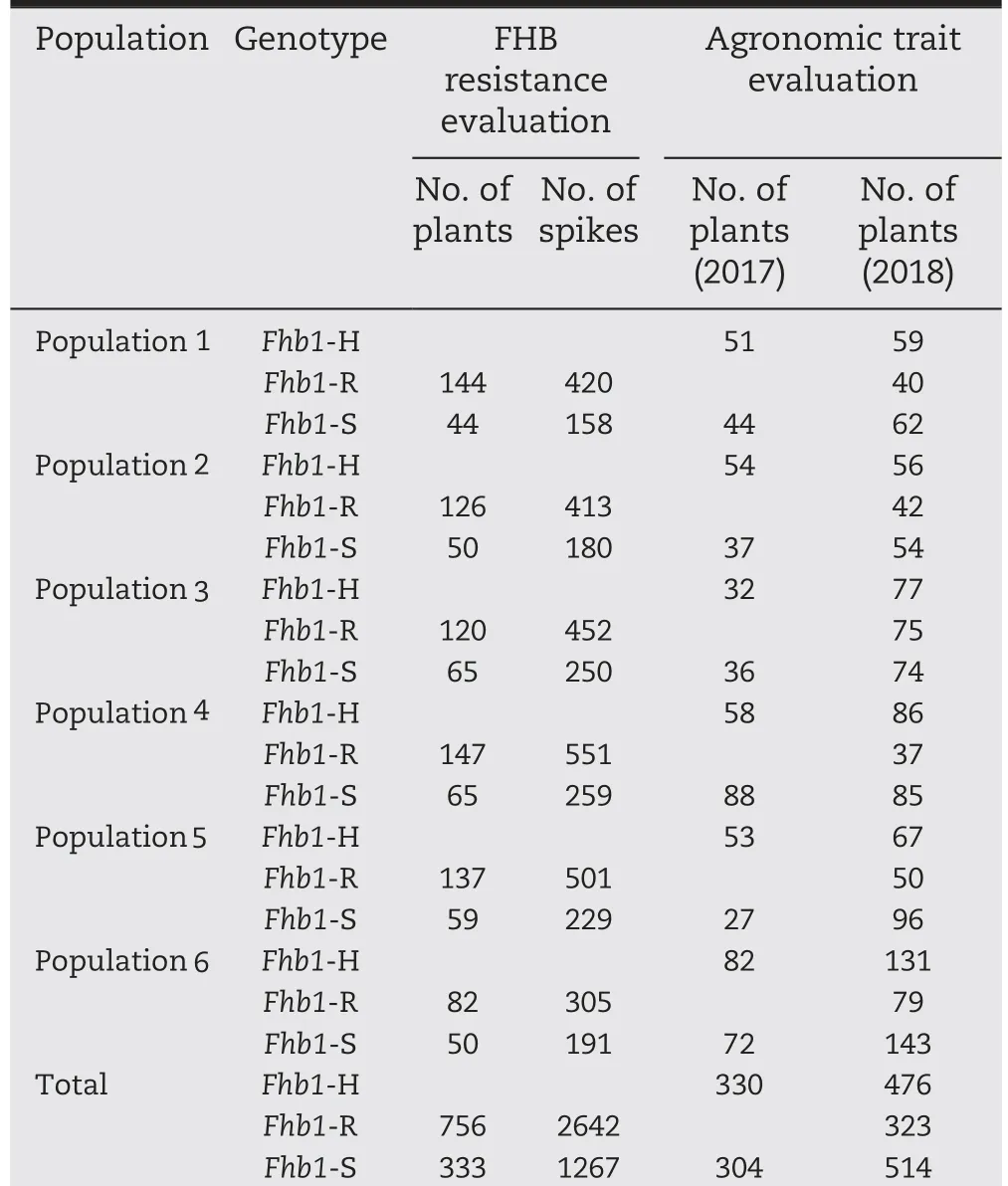
Table 1-Numbers of plants and spikes with different Fhb1 genotypes identified with a gene-functional marker and used for the evaluation of FHB resistance and agronomic traits.
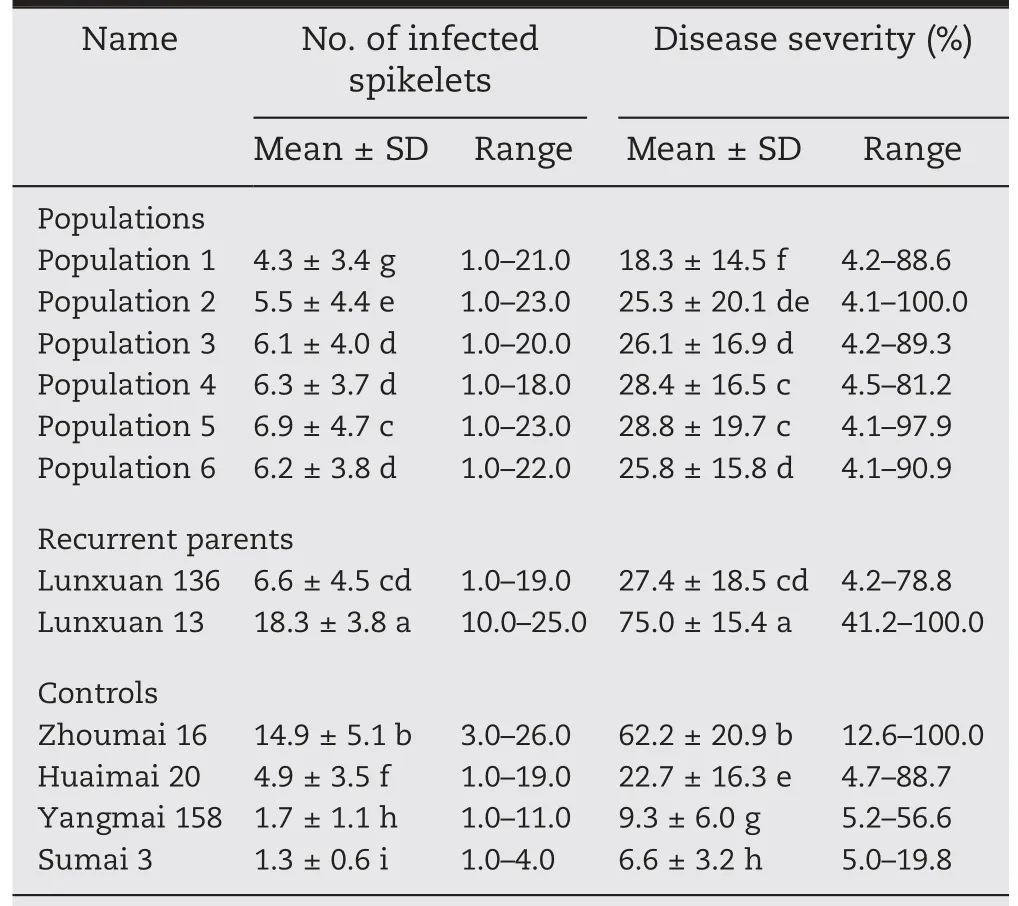
Table 2-Comparison of number of infected spikelets and disease severity among plants with Fhb1 resistance alleles from different donors of the Fhb1 gene.
Fhb1-R plants from the six populations showed significantly fewer infected spikelets and lower disease severity than their recurrent parents Lunxuan 136 and Lunxuan 13 and the highly susceptible control Zhoumai 16, but significantly more infected spikelets and higher disease severity than the moderately susceptible control Huaimai 20 in most cases(Table 2). The Fhb1-R plants from different donors differed in their FHB-resistant levels. The plants with the highest FHB resistance, which was significantly higher than that of Huaimai 20 but significantly lower than those of Yangmai 158 and Sumai 3, were observed in the BC2F2population produced using Ningmai 9 as the donor of the Fhb1 resistance allele and Lunxuan 136 as the recurrent parent. The frequencies of infected spikelet number and disease severity showed a skewed normal distribution in the six populations(Fig.3).In the populations with Lunxuan 136 as the recurrent parent,53%-73% and 69%-86% plants showed higher FHB resistance than Humai 20 and Lunxuan 136 respectively (Fig. 3-A-F),whereas in the populations with Lunxuan 13 as the recurrent parent,45%-52%and 97%-100%plants showed lower infected spikelet number and disease severity than Humai 20 and Lunxuan 13,respectively(Fig.3-G-L).
3.3. Effects of Fhb1 on agronomic traits
There was no significant difference in plant height,number of spikes per plant, spike length, number of spikelets per spike,number of sterile spikelets per spike,kernel weight per spike,number of kernels per spike, or thousand-kernel weight between Fhb1-H and Fhb1-S plants of populations 1 to 6 in 2017,nor in any of the agronomic traits except kernel number per spike among Fhb1-H, Fhb1-R, and Fhb1-S plants in 2018(Table 4). The difference in kernel number per spike in 2018 was found only between Fhb1-H and Fhb1-S plants and not between Fhb1-R and Fhb1-S plants in the Lunxuan 13 genetic background(Table 4).
In the Lunxuan 136 background, no significant difference between the plants with different Fhb1 genotypes was observed in any of the evaluated agronomic traits over the two years in the three populations,except for sterile spikelets per spike in 2017 and spike number per plant and kernel number per spike in 2018 (Table 5). Compared to Fhb1-S plants, Fhb1-H plants had fewer sterile spikelets per spike in population 1 (P <0.05) in 2017, but the difference was not significant between Fhb1-S and Fhb1-R plants in the same population.On average,Fhb1-S plants had 1.3 more spikes per plant than Fhb1-R plants in population 1 in 2018 (P <0.05). In population 2,the Fhb1-H plants had 4.5 more kernels per spike than Fhb1-S plants in 2018 (P <0.05); however, the difference between Fhb1-R and Fhb1-S plants was not significant.
In the Lunxuan 13 background, no significant difference was observed between plants with different Fhb1 genotypes for any agronomic trait except plant height in 2017 and spike length and spikelet number per spike in 2018(Table 6).Fhb1-S plants were 2.6 cm taller than Fhb1-H plants from population 4 in 2017 (P <0.05). Fhb1-S plants had 0.3 cm longer spikesthan the Fhb1-R plants and 0.4 more spikelets per spike than Fhb1-H plants (P <0.05) in population 6 in 2018. However, no significant difference in spikelet number per spike was observed between Fhb1-R and Fhb1-S plants in this population.
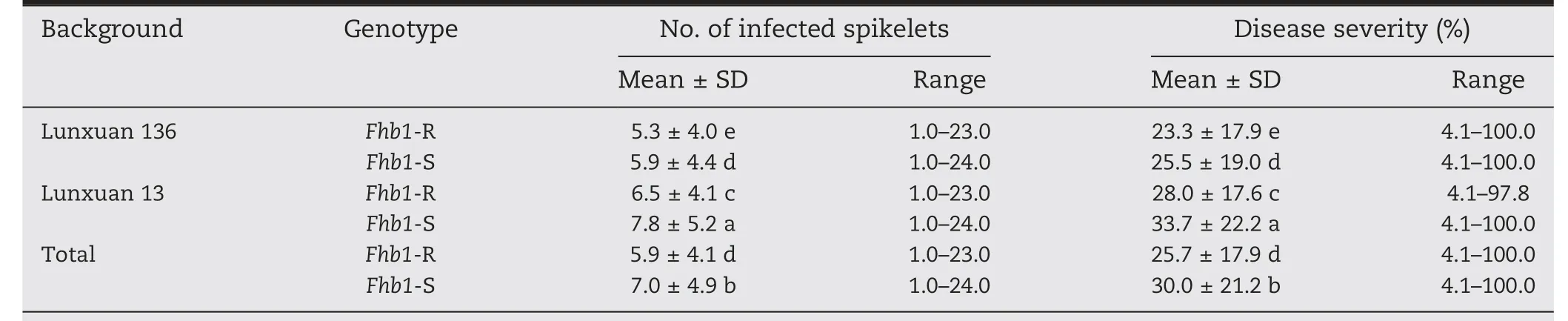
Table 3-Comparison of number of infected spikelets and disease severity between Fhb1-resistant and-susceptible plants.
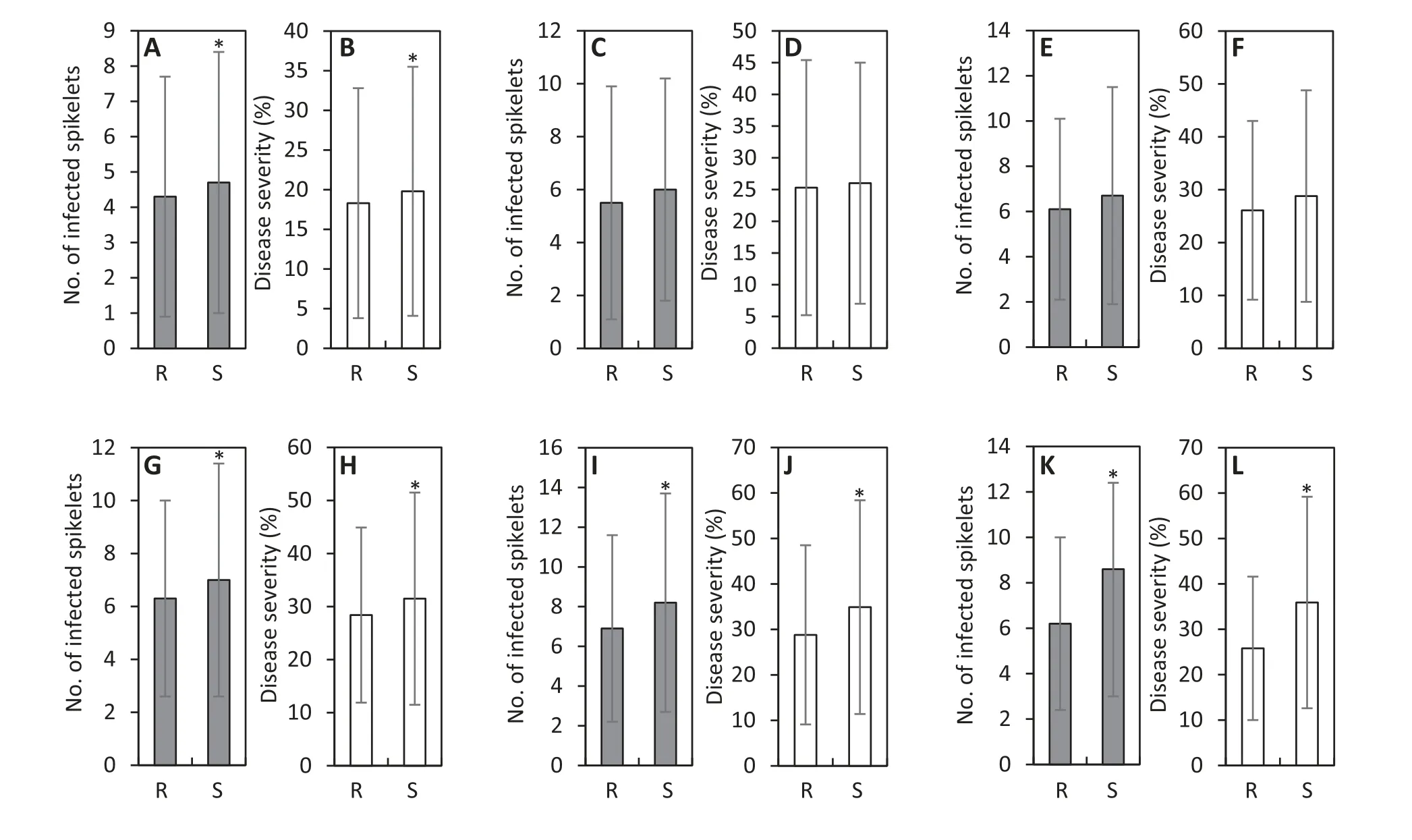
Fig.2- Comparison of number of infected spikelets and disease severity between Fhb1-resistant and-susceptible plants in population 1(A and B), population 2(C and D),population 3(E and F), population 4(G and H),population 5(I and J),and population 6(K and L).*Significant difference at P <0.05.R,Fhb1-resistant genotype;S,Fhb1-susceptible genotype;population 1,dwarf-male-sterile(DMS)-Zhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 136;population 2,DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 136;population 3, DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 136;population 4,DMSZhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 13;population 5, DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 13;population 6,DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 13.
4. Discussion
The genetic background of Fhb1 affected FHB resistance.Backcross progeny with the Lunxuan 136 background showed significantly fewer diseased spikelets and lower disease severity than those with the Lunxuan 13 background. The higher FHB resistance of Fhb1-S plants in the Lunxuan 136 background than that of Fhb1-R plants in the Lunxuan 13 background shows that the genetic background of the Fhb1 gene exerted a large effect on FHB resistance.This finding is in agreement with previous reports [28,37,38]. Compared to Lunxuan 13, Lunxuan 136 showed higher FHB resistance in this study. Lunxuan 136 and Lunxuan 13 share the common parent Zhoumai 16,but differ in one parent:Zhengmai 9023 in Lunxuan 136 and Shimai 12 in Lunxuan 13. Zhoumai 16 and Shimai 12 are highly susceptible to FHB,while Zhengmai 9023 is moderately susceptible or moderately resistant [39]. This finding suggests that the FHB resistance in Lunxuan 136 might be from Zhengmai 9023. Using the two Fhb1-flanking SSR markers, only marker Xgwm493 was detected in Zhengmai 9023, but the other marker Xgwm533 and the functional marker of Fhb1 did not amplify the target fragments (data not shown). A similar phenomenon was also found in the FHB-resistant wheat cultivar Lunxuan 22 (data not shown),which was developed by recurrent selection using DMS wheat as the maternal parent and Zhengmai 9023,Yangmai 158, and Yannong 144 as the paternal parents. It was speculated that the FHB resistance of Zhengmai 9023 may be associated with novel FHB-resistance gene(s). Thus, the higher FHB resistance of Fhb1-R plants in the Lunxuan 136 background may be due to additive interactions between Fhb1 and the unknown resistance gene(s)in Lunxuan 136,a notion consistent with the observation that Fhb1 in combination with other resistance genes together confers higher resistance than Fhb1 alone[37].
The effects of the Fhb1 from different donors on FHB resistance in the same recurrent parent differed.For example,the Fhb1-R plants from Ningmai 9 showed significantly higher FHB resistance than the Fhb1-R plants from other donors in the Lunxuan 136 background (Table 2). However, this difference did not appear in the Lunxuan 13 background, suggesting that the difference in the effects of Fhb1 on FHB resistance in different donors might be caused either by other undetected minor QTL in the donor parents or by an interaction between donor and recurrent parental backgrounds.
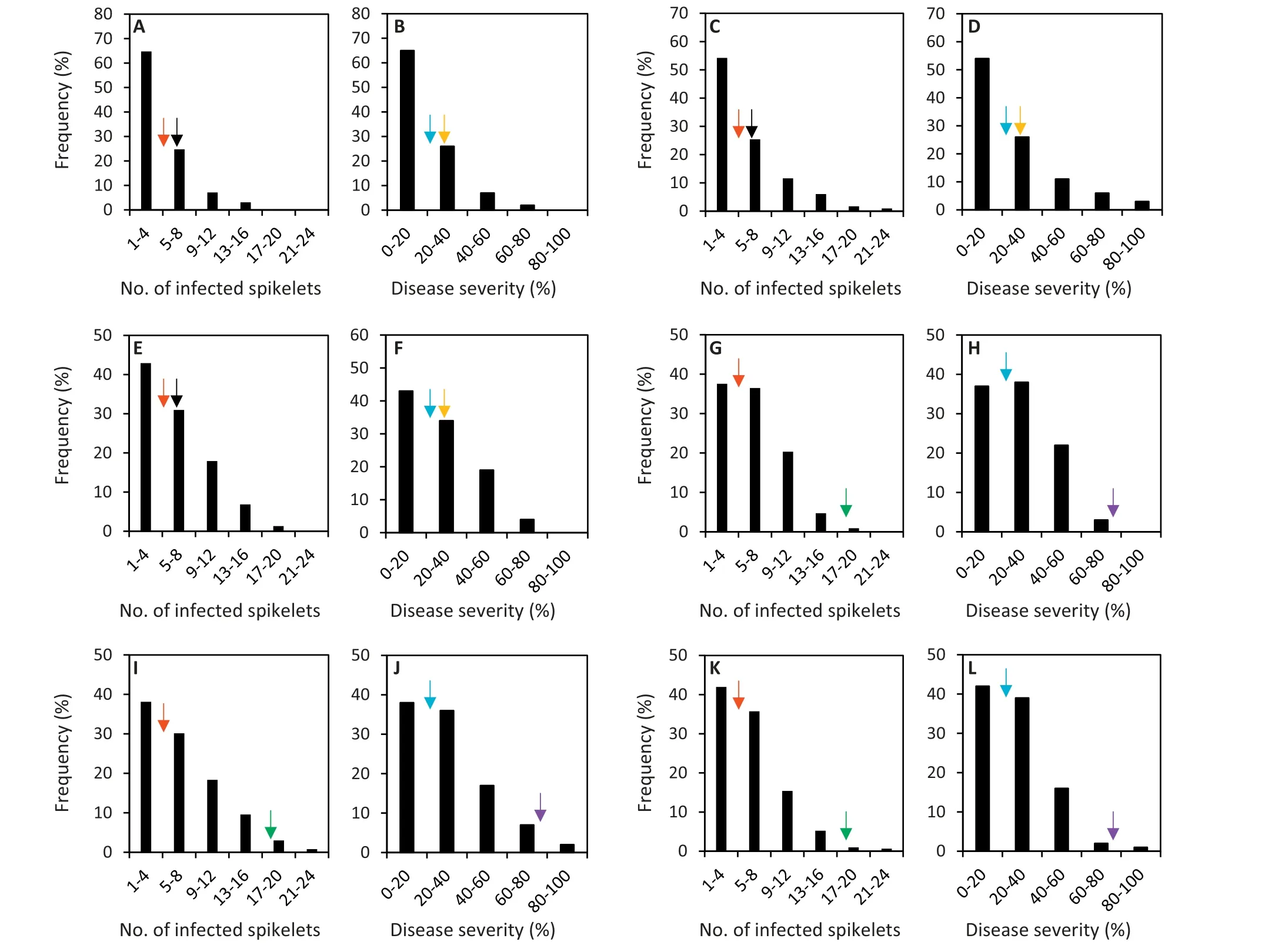
Fig.3- Frequency distribution of number of infected spikelets and disease severity in population 1(A and B),population 2(C and D),population 3(E and F),population 4(G and H),population 5(I and J),and population 6(K and L).The red,black,and green arrows represent the number of infected spikelets of Huaimai 20, Lunxuan 136,and Lunxuan 13, respectively.The blue,yellow,and purple arrows represent the disease severity of Huaimai 20,Lunxuan 136,and Lunxuan 13, respectively.Population 1,dwarf-male-sterile(DMS)-Zhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 136;population 2, DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 136;population 3,DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 136;population 4,DMS-Zhoumai 16/Ningmai 9//Zhoumai 16/3/Lunxuan 13; population 5,DMS-Zhoumai 16/Ningmai 13//Zhoumai 16/3/Lunxuan 13;population 6, DMS-Zhoumai 16/Jianyang 84//Zhoumai 16/3/Lunxuan 13.
The differences in FHB resistance between Fhb1-R and Fhb1-S plants were larger in the Lunxuan 13 than in the Lunxuan 136 background. This finding indicates that the effect of Fhb1 on disease resistance is greater in highly than in moderately susceptible cultivars. Compared to the moderately susceptible control Huaimai 20, only the Fhb1-R plants from population 1 in the Lunxuan 136 background showed significantly fewer infected spikelets and lower disease severity. However, the frequency distribution of infected spikelet number and disease severity showed marked skewing toward the FHB-resistant genotype in the six populations,and the majority of plants showed fewer infected spikelets and lower disease severity than Humai 20 in the six populations (Fig. 3). We have previously proposed that the optimal strategy of wheat FHB resistance breeding in the YHWZ is first to increase FHB resistance from highly to moderately susceptible (like Huaimai 20) and then further increase FHB resistance by pyramiding different resistance QTL to develop moderately to highly resistant cultivars.Based on the results of this study,transferring Fhb1 to a susceptible background could easily result in the selection of moderately susceptible or moderately resistant plants if other minor genes were present in the background.
Consistent and significant differences in the main agronomic traits investigated were not observed between plants with contrasting Fhb1 alleles in different genetic backgrounds.This finding indicates that Fhb1 does not exert negativeeffects on the expression of major agronomic traits, in agreement with previous studies in Germany [38]and the USA[40].Thus,selection of backcross progeny with increased FHB resistance and yields as high as those of recurrent parents should be practicable in the YHWZ.
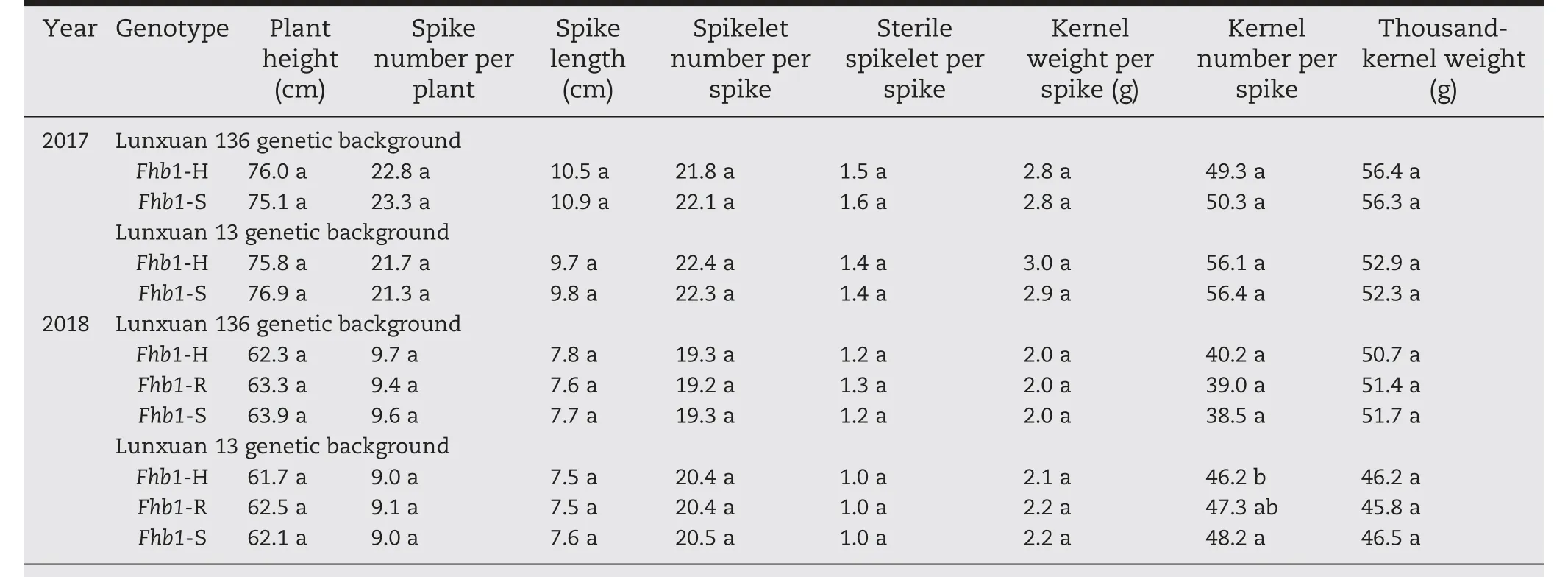
Table 4-Comparison of agronomic traits between two genotypes in 2017 and among three genotypes in 2018.
In conclusion, Fhb1 showed positive effects on FHB resistance in different genetic backgrounds of winter wheat adapted to the YHWZ.The effect of Fhb1 on disease resistance depended on the resistance levels of recurrent parents. The finding that Fhb1 showed negligible effect on agronomic traits suggests that it is feasible to improve FHB resistance of winter wheat without reducing yield potential by introgressing Fhb1 resistance allele into FHB-susceptible cultivars in the YHWZ.
Declaration of Competing Interest
Authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFD0101802,2017YFD010060), the National Natural Science Foundation of China (31771881, 31401468), and the Agricultural Science and Technology Innovation Program.
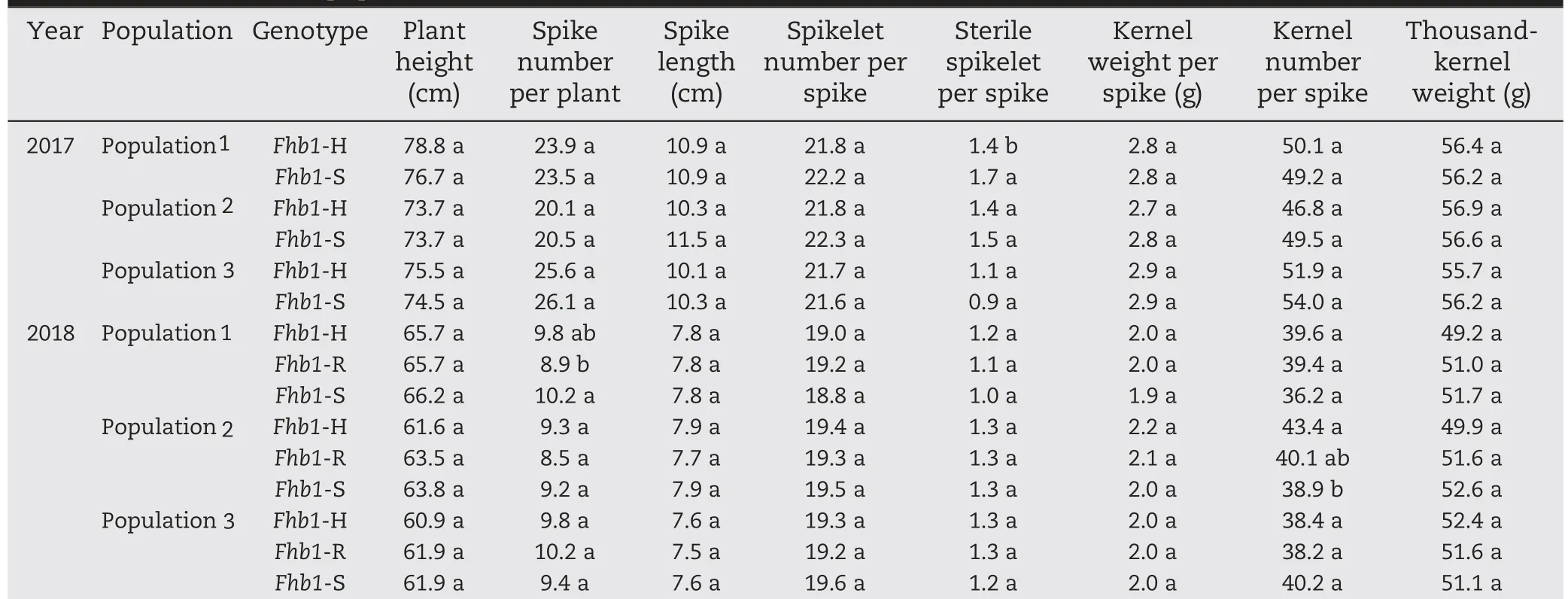
Table 5-Comparison of agronomic traits between two genotypes in 2017 and among three genotypes in 2018 in the three Lunxuan 136 backcross populations.
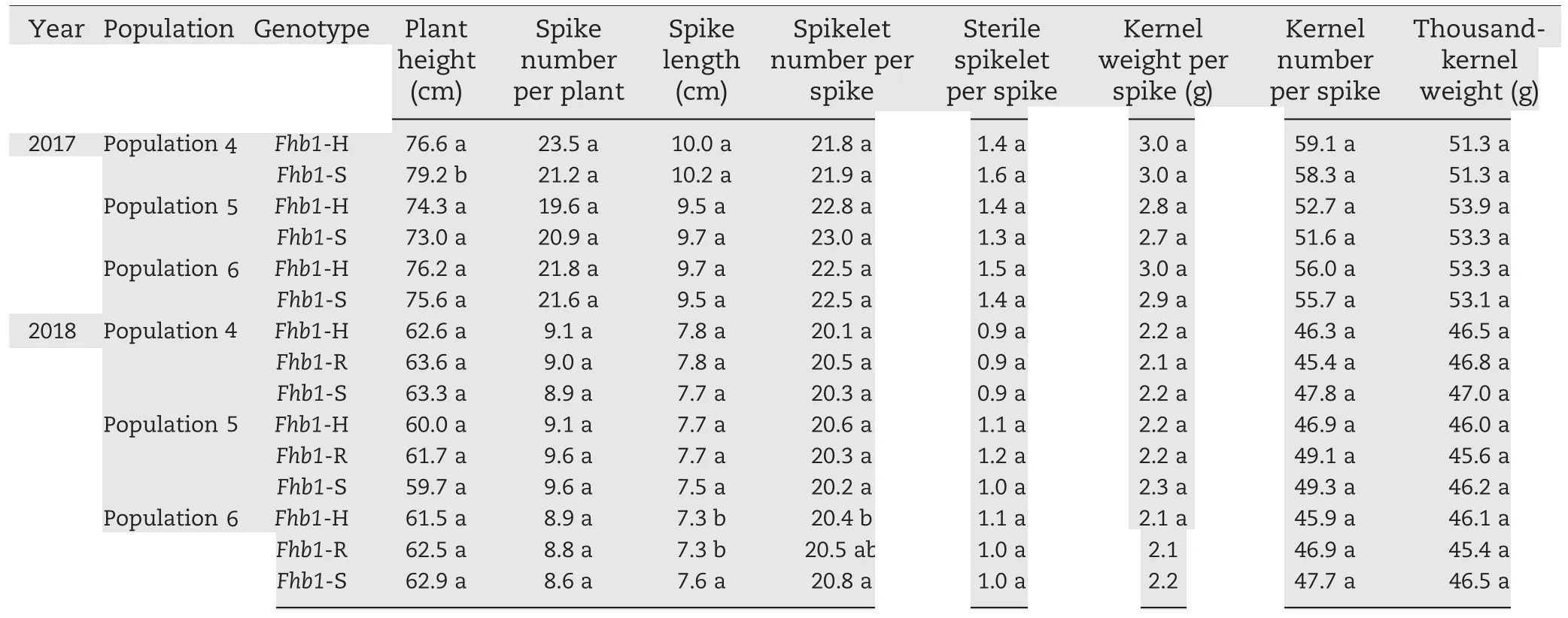
Table 6-Comparison of agronomic traits between two genotypes in 2017 and among three genotypes in 2018 in three Lunxuan 13 backcross populations.
- The Crop Journal的其它文章
- Positional cloning of PmCH1357 reveals the origin and allelic variation of the Pm2 gene for powdery mildew resistance in wheat
- The Crop Journal 作物学报(英文版) (Started in 2013, Bimonthly)
- Wheat powdery mildew resistance gene Pm64 derived from wild emmer(Triticum turgidum var.dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5
- Wheat breeding in northern China: Achievements and technical advances
- Breed ing w heat for resistance to Fusarium head blight in the Global North: China,USA,and Canad a
- Pyramiding disease resistance genes in elite winter wheat germplasm for Western Canada

