Comparative FISH and molecular identification of new stripe rust resistant wheat-Thinopyrum intermedium ssp. trichophorum introgression lines
Jinbo Li, Qiheng Chen,Peng Zhng, To Lng, Smi Hoxh, Gungrong Li,b,*,Zujun Yng,b,*
aSchool of Life Science and Technology, University of Electronic Science and Technology of China,Chengdu 610054,Sichuan,China
bCenter for Informational Biology, University of Electronic Science and Technology of China,Chengdu 611731,Sichuan,China
cPlant Breeding Institute,The University of Sydney,Cobbitty, New South Wales 2570,Australia
Keywords:Disease resistance FISH GISH Stripe rust Triticum aestivum
ABSTRACT Thinopyrum intermedium (2n = 6x = 42, JJJsJsStSt) has been hybridized extensively with common wheat and has proven to be a valuable germplasm source for improving disease resistance, quality attributes, and yield potential in wheat. We characterized new disease resistant wheat-Th. intermedium derivatives A1082 and A5-5 using sequential multi-color fluorescence in situ hybridization (mc-FISH), genomic in situ hybridization (GISH), PCR-based landmark unique gene(PLUG)and intron targeting(IT)markers.A1082 was identified as a wheat-Th. intermedium 3J disomic addition line, and A5-5 was a T4BS·5JsL homozygous Robertsonian translocation line. Seventy-one and 106 pairs of primers amplified Th.intermedium-specific bands allowing chromosomes 3J and 5Js to be tracked, respectively. A new oligonucleotide probe, Oligo-6H-2-100, was developed for FISH labeling of the subterminal region of the long arm of chromosome 5Js. Both lines were highly resistant to stripe rust pathogen races prevalent in Chinese field screening nurseries. A5-5 also displayed a significant increase in tiller number compared to its wheat parent. The new lines can be exploited as useful germplasms for wheat improvement.
1. Introduction
Common wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD)is the staple carbohydrate source for 30% of the world population and is also the primary source of protein. The diseases leaf rust, stem rust and stripe rust are major constraints to increase wheat production in many countries.Among them, stripe rust, caused by Puccinia striiformis Westend. f. sp. tritici (Pst), may cause losses up to 70% [1,2]and even higher.Resistant cultivars are the most economical and environmentally friendly means to control the diseases.However, due to limited numbers of effective resistance genes in wheat cultivars and constantly evolving new pathogen races capable of overcoming deployed resistance genes, there is an urgent need to discover and exploit new sources of resistance.
Many wild relatives have been crossed with wheat, and a number of important resistance genes have been incorporated into widely cultivated wheat cultivars [3,4]. Thinopyrum intermedium (Host) Barkworth & D.R. Dewey (2n = 6x = 42,JJJsJsStSt) is a perennial wild species consisting of two subspecies, namely intermediate wheatgrass, ssp.intermedium, and a close pubescent relative, ssp. trichophorum[5]. Th. intermedium has superior resistance to many wheat diseases and can be easily crossed with wheat, making it a potential source of germplasm for wheat improvement[6,7].A considerable number of disease resistance genes have been transferred from Th.intermedium to wheat,including Pm40 and Pm43 for powdery mildew (Blumeria graminis f. sp. tritici)resistance [8,9], Yr50 for stripe rust (Pst) resistance [10], Sr44 for stem rust(Puccinia graminis f.sp.tritici)resistance[11],Lr38 for leaf rust (P. recondita Roberge ex Desmaz. f. sp. tritic)resistance [12], Bdv2 and Bdv3 for Barley yellow dwarf virus(BYDV) resistance [13,14], and Wsm1 for Wheat streak mosaic virus(WSMV)resistance[15].
The production of wheat-alien partial amphiploids is a key step for transferring potentially useful traits from wild relatives to wheat [16]. Several wheat-Th. intermedium partial amphiploids with useful agronomic traits have been developed and characterized by molecular cytogenetic methods[17-19]. For example, the wheat-Th. intermedium partial amphiploids TAF46 and Zhong 5 and their respective derivative lines(L series and Z series)have been studied extensively[6,20-22]. It was reported that the long arm of chromosome 7Ai-1 in TAF46-derived disomic addition line L1 carried resistance to WSMV [23]and BYDV [13,23,24]. A pair of alien chromosomes in the Zhong 5-derived disomic addition lines(Z1, Z2, or Z6) carried a BYDV resistance gene that was different from the one in TAF46; disomic addition line Z4 carried resistance to leaf rust,stem rust and stripe rust[6,21].Lang et al.[25]reported that line Z4 probably carried a gene(s)for stripe rust resistance effective at the adult stage in the centromeric region of chromosome 7Js. Hence, it should be worthwhile to explore wheat-Th. intermedium partial amphiploids with different origins for wheat improvement. Selection in progenies of crosses with polyploid Thinopyrum species, including Th. intermedium, often stabilize as 2n = 56 partial amphiploids with the full wheat chromosome content and a mixed genome from the related species. Thus partial amphiploids derived from the same cross can be genetically variable because of different chromosome combinations. A further source of genetic variation will come from different accessions of those species.
Conner et al. [26]reported that Th. intermedium ssp.trichophorum displayed high levels of resistance to wheat diseases,including rusts, root rot and WSMV. Yang and colleagues [27]reported wheat-Th. intermedium ssp. trichophorum partial amphiploid TE-3 with resistance to several foliar diseases and unique seed storage proteins. Later, members of the same laboratory characterized a series of rust-resistant lines derived from TE-3[28-30], and found a novel Th. intermedium St-chromosomespecific HMW-GS gene[31].
In the present study, we used mc-FISH, GISH, molecular markers and field evaluations to: (1) develop new wheat-Th.intermedium introgression lines; (2) identify the alien chromosomes by mc-FISH and GISH analyses; (3) develop specific markers for tracking the alien chromosomes or chromosome segments; and (4) evaluate the stripe rust responses, agronomic traits, and potential of the lines for use in wheat improvement.
2. Materials and methods
2.1. Plant materials
Common wheat cultivar Chinese Spring(CS)and Mianyang11(MY11), wheat-Th. intermedium ssp. trichophorum partial amphiploid(2n = 50 to 56)TE-3[27],and derived lines A1082 and A5-5 were used in the study. TE-3 was developed from BC2F4progenies of the cross of T. durum/Th. intermedium ssp.trichophorum//CS [27]. A1082 and A5-5 were selected from BC1F6progenies of the cross of MY11/TE-3//MY11. MY11 and TE-3 were used as the controls for assessing stripe rust response in the field, morphology, and molecular marker analysis, and CS was used as the susceptible control for assessing stripe rust resistance at the adult stage.These plant materials are held by the Laboratory of Molecular and Cell Biology, Center for Informational Biology, School of Life Science and Technology, University of Electronic Science and Technology of China in Chengdu. Wheat-Th. intermedium partial amphiploid TAF46 and derived disomic addition line L2 were kindly provided by Dr. Bernd Friebe, Wheat Genetic Resource Center,Kansas State University, USA.
2.2. FISH and GISH analyses
Preparation of mitotic metaphase squashes of seedling root tips was described by Lang et al.[25].The protocol of nondenaturing FISH (ND-FISH) using synthesized probes was described by Fu et al. [32]. Oligo probes Oligo-pSc119.2, OligopTa535, Oligo-(GAA)7, and Oligo-3A1 were used to detect the wheat chromosomes[25,33].Probe Oligo-pSt122 is specific for terminal regions of Th. intermedium chromosomes [34]. In addition,a new oligo probe,Oligo-6H-2-100(5′CGAGG TCCCC GTAAC CGGAC CCCGA AGTTC CCCGA ACGTT 3′), was designed using the Tandem Repeats Finder (TRF) algorithm[35]following Lang et al. [36]. Oligo probes were synthesized by Shanghai Invitrogen Biotechnology Co. Ltd. (Shanghai,China). The synthetic oligo probe sequences were either 5′end-labeled with 6-carboxyfluorescein(6-FAM)for green or 6-carboxytetramethylrhodamine (Tamra) for red signals (Table S1). FISH images were taken with a Zeiss Axio Imager epifluorescence microscope. Images were captured with a Retiga EXi CCD (charge-coupled device) camera (QImaging,Surrey, BC, Canada) operated with Image-Pro Plus version 7.0 software(Media Cybernetics Inc.,Bethesda,MD,USA).
After stripping off the oligo probes, the same slides were analyzed by GISH as described in Zhang et al. [37]. Total genomic DNA from Th. intermedium (Cytogenetic stock accession C05.05,University of Sydney)and Pseudoroegneria stipifolia(PI 314058, The National Small Grains Collection (NSGC),USDA-ARS, ID) were labeled with biotin-16-dUTP (Roche Diagnostics Australia,Castle Hill,New South Wales,Australia)using nick translation.Unlabeled total genomic DNA of wheat was used as blocker. The probe to blocker ratio was ~1:80.Signals were detected with Fluorescein Avidin DN (Vector Laboratories, Burlingame, CA, USA). Chromosomes were counterstained with DAPI and pseudo-colored red.
2.3. Agronomic trait evaluation
During the 2016-2017 and 2017-2018 growing seasons, all materials were planted with two replicates at the Xindu Experimental Station in Chengdu.Ten plants were grown in 1.5 m rows,spaced 30 cm apart.Ten plants of each line were sampled to evaluate agronomic traits, including plant height, spike length, number of spikelets per spike, number of spikes per plant,1000-kernel weight,10-kernel length and 10-kernel width. One-way ANOVA (post-hoc Tukey's test)was used to compare differences among means. Statistical analyses were performed with SPSS 20.0 software (IBM Corporation, New York, USA) and the significance level was set at P <0.05.
We inoculated all materials for evaluation of stripe rust response at the heading stage with a mixture of Pst races CYR32, CYR33, and CYR34 provided by the Institute of Plant Pathology,Gansu Academy of Agricultural Sciences,Lanzhou,Gansu. When rust was fully developed on the susceptible parent MY11 and the susceptible control CS, infection types were recorded based on a 0-4 scale, where 0, 0;, 1, 2, 3, and 4 were considered as immune,very resistant,resistant,moderately resistant, moderately susceptible, and susceptible,respectively[38].
2.4. Molecular marker analysis
DNA was extracted from young leaves of CS, MY11, TE-3,A1082, A5-5, and L2. A total of 500 PCR-based landmark unique gene (PLUG)and 212 intron targeting(IT) primer pairs were selected and synthesized [39,40]. PCR was performed according to Li et al. [30]. PCR products were separated in 8%non-denaturing PAGE gels and visualized by silver staining.To improve levels of polymorphism, the PLUG products were digested with restriction enzymes as described by Ishikawa et al. [39]. The physical chromosomal locations of IT markers and PLUG markers with unknown locations were searched using the wheat genome database IWGSC RefSeq v1.0(https://urgi.versailles.inra.fr/blast/).
3. Results
3.1. Chromosome identification in partial amphiploid TE-3 using combined FISH and GISH analyses
Examination of somatic mitotic cells of 54 wheat-Th.intermedium ssp. trichophorum partial amphiploid TE-3 seedlings showed that chromosome numbers varied between 50 and 56, suggesting cytological instability (Figs. 1, S1). The chromosome number in different TE-3 lines was 2n = 50 (Fig.S1-A), 2n = 53 (Fig. 1-A), 2n = 55 (Fig. S1-E), or 2n = 56 (Fig. S1-G). Occasionally, one or two telocentric chromosomes were observed (Fig. S1-G, H). In previous studies, we identified chromosomes 1St,6St, 7St,2Js,4Js,or 4J in TE-3 [28-30,41].
FISH with multiple probes and GISH were used to further characterize the alien chromosomes in partial amphiploid TE-3 (Figs. 1, S1). Mc-FISH analysis using Oligo-pSc119.2, OligopTa535, Oligo-(GAA)7, Oligo-pSt122, Oligo-3A1, and Oligo-6H-2-100 easily distinguished all chromosomes of wheat and Th.intermedium in TE-3 (Figs. 1, S1S1). GISH analysis using Th.intermedium or Ps. stipifolia total genomic DNA as a probe indicated that TE-3 contained three pairs of St chromosomes,two pairs of J chromosomes, and three pairs of Jschromosomes (Figs. 1-D, E, S1-D). The karyotype of the alien chromosomes in TE-3 is shown in Fig. 1-E. Those alien chromosomes were confirmed, and the previously unknown chromosomes J-1 and Js-3 were identified as 3J and 5Js,respectively. Wheat chromosome 4B was missing, presumably compensated by two group 4 alien chromosomes.
3.2. Identification of wheat-Th. intermedium alien chromosome lines by sequential mc-FISH
During the 2015-2016 growing season, wheat-Th. intermedium derivative lines A1082 (2n = 44) and A5-5 (2n = 42) were selected from the BC1F6progenies that were highly resistant to stripe rust. During the 2016-2017 and 2017-2018 growing seasons, they were bagged to ensure self-fertilization and progenies were subjected to chromosome counting, FISH analysis and stripe rust testing. In October 2016, 20 A1082 seeds and 30 A5-5 seeds were germinated for collection of roots for FISH analysis, and were then transplanted at Xindu Experimental Station for evaluation of stripe rust responses at the adult stage.
Sequential mc-FISH with probes Oligo-pSc119.2, OligopTa535, Oligo-(GAA)7, and Oligo-pSt122 was conducted to detect the chromosome constitutions of the two lines(Figs.2,3).The FISH karyotype of wheat parent cv.MY11(Fig.S2)was constructed using probes Oligo-pSc119.2, Oligo-pTa535 and Oligo-(GAA)7.The chromosome number of all A1082 progenies was 2n = 44, while that of A5-5 was 2n = 42 confirming the cytogenetic stability of both lines.As shown in Fig.2-A,A1082 contained all the wheat chromosomes and a pair of J-1 chromosomes. The J-1 chromosome displayed clear OligopSc119.2 and faint Oligo-pTa535 signals at the telomeric region of short arm, as well as three distinct Oligo-pTa535 signals along the long arm in A1082(Fig.2-A,G).Furthermore,the telomeric region of the short arm showed very strong Oligo-pSt122 signals(Fig.2-B, G).
Similarly, mc-FISH with probes Oligo-pSc119.2 and OligopTa535 showed that A5-5 contained a pair of translocated chromosomes (Fig. 3). Sequential mc-FISH with Oligo-(GAA)7,Oligo-pSt122, Oligo-3A1 and Oligo-6H-2-100 showed that the translocated chromosome pair was a centric fusion involving wheat chromosome arm 4BS and the long arm of chromosome Js-3 (Fig. 3-B, C). The translocated chromosome had strong Oligo-pSc119.2 signals in the short arm telomeric region, clear Oligo-pSc119.2 signals and faint Oligo-pTa535 signals along the long arm, intensive Oligo-(GAA)7signals in the proximal region of 4BS, and faint Oligo-3A1 signals in the middle region of the Js-3L chromosome,as well as the distinct Oligo-pSt122 and Oligo-6H-2-100 signals in the distal region of the Js-3L chromosome.

Fig.1-Sequential FISH and GISH patterns of wheat-Th.intermedium partial amphiploid TE-3.The probes for FISH were OligopSc119.2(green) + Oligo-pTa535(red)(A),Oligo-pSt122(green) + Oligo-(GAA)7(red)(B),Oligo-3A1(green) + Oligo-6H-2-100(red)(C).The probe(green)for GISH analysis was Ps.stipifolia(D)total genomic DNA.The karyotype of alien chromosomes(E) was shown by the probes with Oligo-pSc119.2(green) + Oligo-pTa535(red),Oligo-pSt122(green) + Oligo-(GAA)7 (red),Oligo-3A1(green) + Oligo-6H-2-100(red),and Ps.stipifolia total genomic DNA(green),respectively.Bars(A-D),10 μm.Chromosome J-1 and Js-3 are designated as in Li et al.[30].
3.3. GISH analysis
GISH analysis was conducted on A1082 and A5-5. GISH using Th.intermedium total genomic DNA as probe labeled the entire chromosome J-1, and two stronger signals were observed in both telomere regions (Figs. S1S1-D, 2-C). GISH using genomic Ps.stipifolia DNA as a probe showed hybridization to the entire length of the same J-1 chromosome (Fig. 1-D), indicating that the alien chromosome in A1082 is a J-genome chromosome. Similarly, GISH using total genomic DNA of Th.intermedium as a probe labeled the entire length of the Js-3 chromosome (Fig. S1S1-D), whereas the probe using total genomic DNA of Ps. stipifolia hybridized to the entire chromosome that previously had clear hybridization signals in both telomeric regions (Fig. 1-D), indicating that the alien chromosome in A5-5 is a Js-genome chromosome. The FISH and GISH results (Fig. 3) thus demonstrate that the chromosome pair in A5-5 involves a wheat-Js-genome translocation.
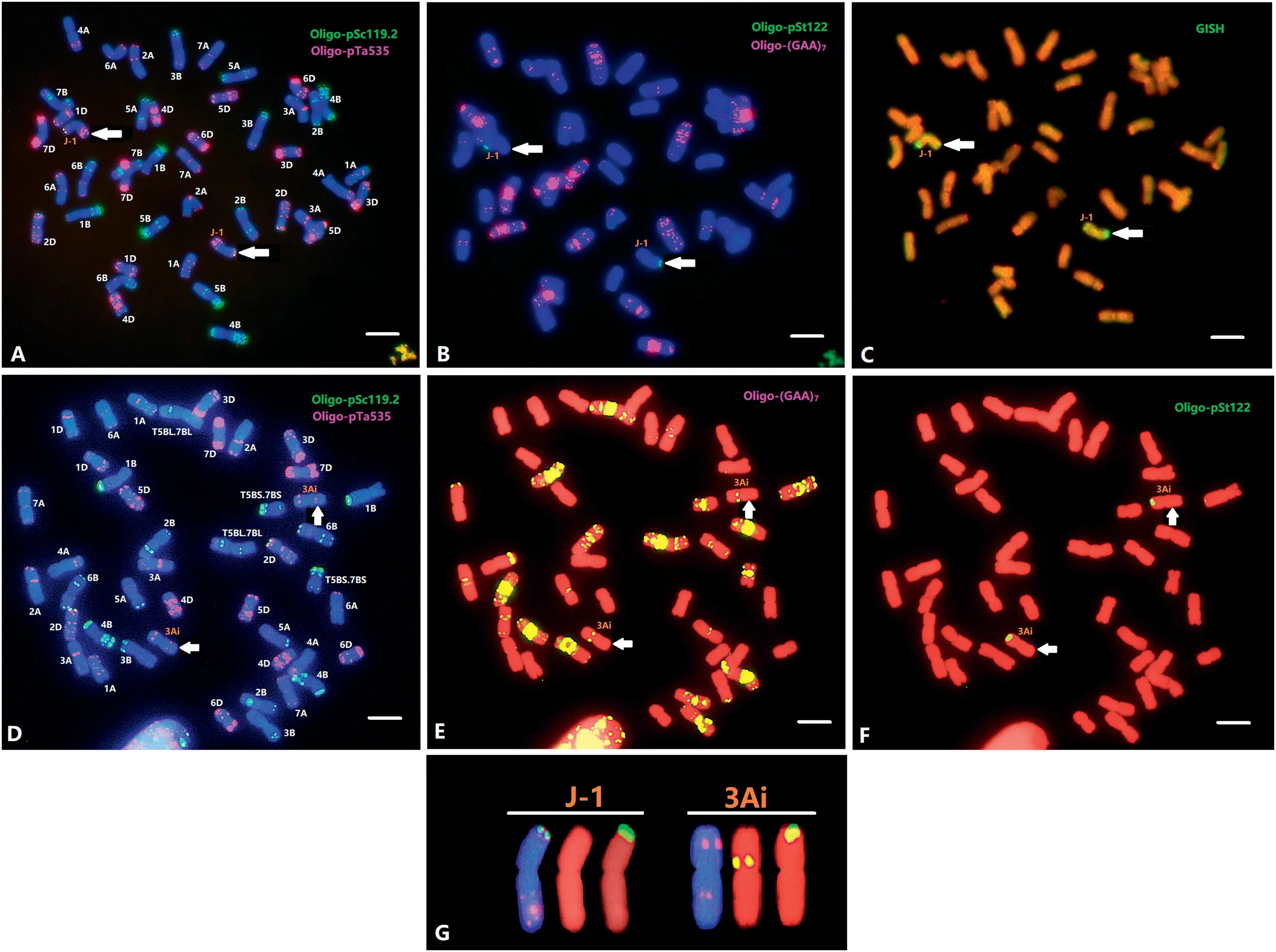
Fig.2- Sequential FISH and GISH analyses of metaphase chromosomes of A1082(A,B, C),and L2(D, E,F). The probes OligopSc119.2(green) + Oligo-pTa535(red)were used in FISH(A,D),and the probes Oligo-pSt122(green) + Oligo-(GAA)7(red)(B,E,F)were used in FISH.The probe(green)for GISH analysis(C)was Th. intermedium total genomic DNA.FISH karyotypes(G)of chromosomes J-1 and 3Ai are shown with the probes Oligo-pSc119.2(green) + Oligo-pTa535(red),Oligo-(GAA)7(red),and OligopSt122(green),respectively.Arrows point to the J-1 (A-C)and 3Ai-1 chromosomes(D-F),respectively.Bars (A-F),10 μm.
3.4. Molecular marker analyses
Six hundred and twenty primer pairs, including 500 PLUG and 120 IT primers [39,40], were used to establish the homoeologous relationships between the added Th.intermedium J-1 chromosomes and wheat chromosomes.Seventy-one pairs of primers (29 PLUG and 42 IT) from homoeologous group 3 amplified identical bands in partial amphipolid TE-3 and derived disomic addition line A1082(Table S2; Fig. 4) suggesting that the J-1 chromosome in A1082 belonged to the homoeologous group 3. The combined FISH,GISH and marker results (Figs. 1, 2, 4) confirmed that A1082 is a 3J disomic addition line.
We similarly screened 592 pairs of primers (500 PLUG and 92 IT) for all the seven wheat homoeologous groups; 106 primer pairs for the long arm of the homoeologous group 5,including 32 PLUG and 74 IT primers, generated identical banding patterns in partial amphiploid TE-3 and translocation line A5-5(Fig.S3,Table S3).Combining these results,we infer that A5-5 is a homozygous T4BS·5JsL Robertsonian translocation line.
3.5. Stripe rust and morphological evaluations
Both MY11 and CS were highly susceptible to stripe rust(IT 4),whereas TE-3 showed immunity (IT 0) (Fig. 5; Table 1). Line A1082 exhibited resistance (IT 0;) and translocation line A5-5 was moderately resistant (IT 2). These results suggest that chromosomes 3J and 5JsL might carry genes for resistance to stripe rust under field conditions.
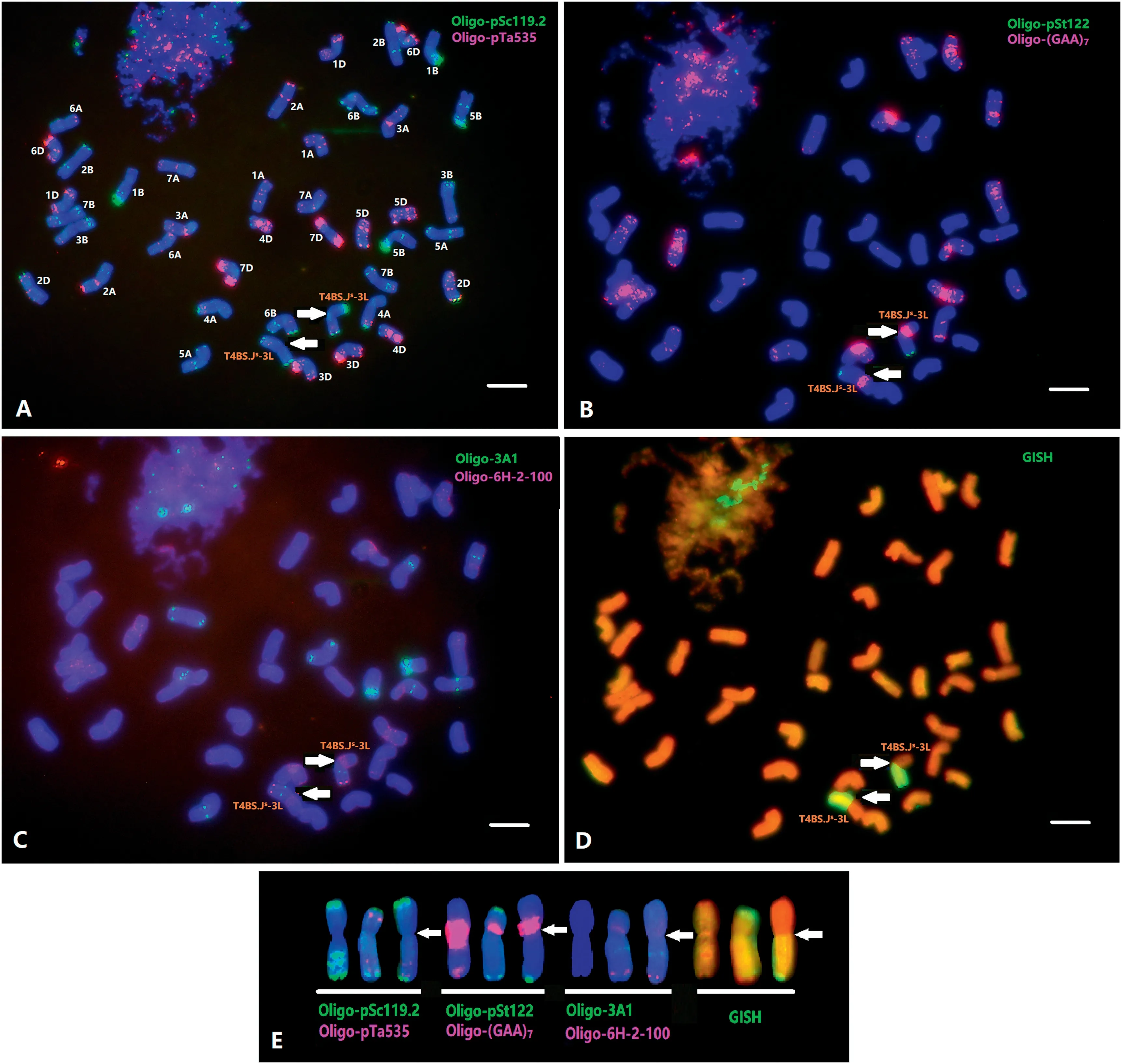
Fig.3- Sequential FISH and GISH analyses of metaphase chromosomes of A5-5.The probes were Oligo-pSc119.2(green) + Oligo-pTa535(red)(A),Oligo-pSt122(green) + Oligo-(GAA)7(red)(B), Oligo-3A1(green) + Oligo-6H-2-100(red)(C),and total genomic DNA of Th.intermedium (D).The chromosomes in the karyotypes(E)were 4B,Js-3 and 4BS.Js-3L, respectively.Arrows point to the translocation breakpoint.Bars (A-D),10 μm.
In order to confirm that the stripe rust resistance in A5-5 came from Th. intermedium chromosome 5JsL, the resistant A5-5 as male parent was crossed to the susceptible cv.Chuanmai 28 (CM28) as female parent, and F1was then selfed to obtain the F2population. All of F1plants were resistant to stripe rust. In March 2019, the stripe rust responses of F2population (51 plants) were evaluated at the heading stage at the Xindu Experimental Station. We randomly selected four pairs of 5JsL-specific primers (CINAU1359,CINAU1363,CINAU1364,and CINAU1435)to amplify the genomic DNA of 36 resistant and 15 susceptible plants. The 5JsL-specific bands were amplified in all the resistant plants,but not in all the susceptible plants(Fig.S4).In addition,we also observed that 11 plants that were highly resistant to stripe rust carried one or two chromosomes 3J, while 25 sib-plants without chromosome 3J were highly susceptible (Fig. 5). Therefore, the Th. intermedium chromosomes 5JsL and 3J were responsible for the stripe rust resistance.
As shown in Table 1, numbers of spikelets per spike and 10-kernel width of line A5-5 were similar to those of wheat parent MY11 and CS.Spike length and tiller number per plant in A5-5 were greater than for MY11 and CS. Kernel weight(37.6 g) of A5-5 was significantly heavier than that of MY11(34.4 g) and CS (26.5 g). Ten-kernel length of A5-5 (74.3 mm)was significantly longer than that of MY11(63.1 mm)and CS(59.2 mm). Post-hoc Tukey's test showed that spike length,tiller number per plant, 1000-kernel weight, as well as 10-kernel length of A5-5 differed significantly from those of wheat parent MY11 and CS(P <0.05,Table 1),indicating that chromosome 5JsL might carry a favorable gene(s) for grain yield.

Fig.4- PCR amplifications of IT markers in wheat-Th. intermedium lines.(A)CINAU1159;(B)CINAU1175;(C)CINAU1206;(D)CINAU1233;(E)CINAU1262;(F)CINAU1176.M,marker;1,CS;2,MY11;3,TE-3;4,A1082;5,L2.Arrows point to the specific bands for A1082 or the identical bands for A1082 and L2,and stars indicate specific bands for L2.
4. Discussion
Th. intermedium ssp. trichophorum was initially used in wide hybridization for crop improvement by Gupta and Fedak [42].

Fig.5- Stripe rust responses on flag leaves of (L to R)Chinese Spring,TE-3,MY11,A5-5,A1082 and the sib-line without chromosome 3J after inoculation with a urediniospore mixture of Pst races CYR32,CYR33,and CYR34.
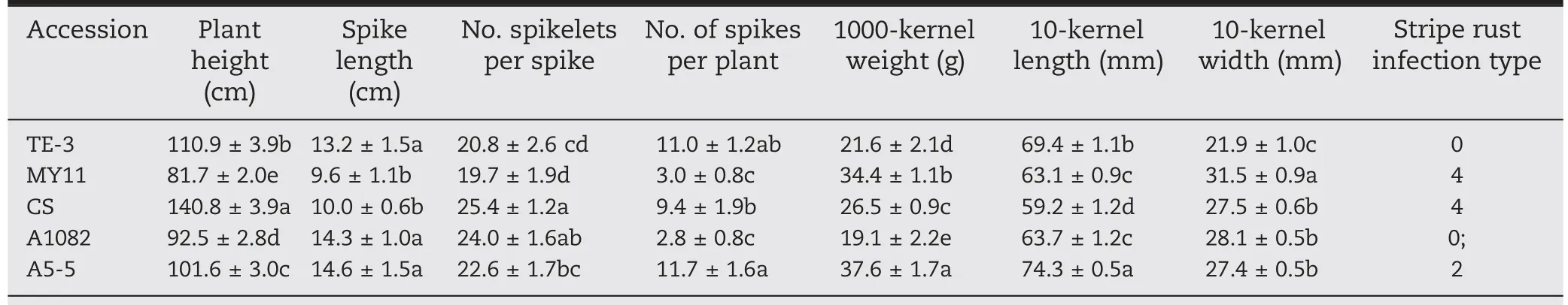
Table 1-Agronomic traits of wheat-Th. intermedium derivative lines and their parents.
Conner et al. [26]reported that wheat-Th. intermedium ssp.trichophorum partial amphiploid ‘Agrotana' (2n = 56) was resistant to common root rot (caused by Cochliobolus sativus(Ito and Kurib) Drechs. ex Dastur), wheat curl mite (Aceria tosichella Keifer),and the vector of Wheat streak mosaic virus.A wheat-Th. intermedium ssp. trichophorum partial amphiploid TE-3 produced by Yang et al.[43]showed resistance to several wheat diseases, including all prevalent races of Pst in China.Yang et al.[27]further characterized TE-3 by mitotic chromosome counts,meiotic pairing behavior,C-banding,GISH,seed storage proteins, and disease response. The current work completes the identification of all eight alien chromosomes in TE-3 and deletion of chromosome 4B from the wheat genome(Fig.1).
After introgressing alien chromosomes or chromosomal segments into wheat, it is important to efficiently characterize the alien contribution. GISH is a powerful and reliable technique for determining genomic origin, size of introgressed fragments and breakpoint positions of the introgressions [44]. FISH is also an efficient tool for differentiating wheat and alien chromosomes [30]. Recently developed FISH analysis using synthetic oligos as probes provides an even more precise method for studying plant chromosomes[30,45,46].Previously,mc-FISH analysis mainly utilized plasmid probes pSc119.2 and pTa535 (or pAs1) to determine the genomic constitution of wheat wide-cross derivatives[19,47]. The development of numerous new oligo probes enables precisely detecting structural variations in Triticeae species [25,36,48]. In comparison with FISH based on just Oligo-pSc119.2 and Oligo-pTa535 (or Oligo-pAs1) as oligo probes, hybridization signals generated by the currently available multiple oligos have greatly increased the resolving power of chromosome identification [25]. In this study, we developed a new oligo probe, Oligo-6H-2-100, to identify chromosome 5Js(Figs. 1, 3). Using multiple oligo probes,namely Oligo-pSc119.2, Oligo-pTa535, Oligo-pSt122 and Oligo-(GAA)7, in comparative oligo-FISH analysis we demonstrated that A1082 was a new wheat-Th. intermedium disomic addition line, in which chromosome 3J was distinctive from the 3Ai (3J) chromosome identified earlier in the partial amphiploid TAF46.
Molecular markers based on comparative genomic analysis have become a very attractive tool for identifying alien chromosomes in wheat backgrounds [39,40,49]. For example,PLUG and IT markers are practical tools for studying homoeologous relationships between alien and wheat chromosomes, because they were designed based on sequence conservation of orthologous genes and on intron polymorphisms among Triticeae species [39,40]. Several reports have verified the practicality of PLUG and IT markers in chromosome identification, genome research, and comparative analysis between wheat and its wild relatives[30,50,51]. The IT markers used in this study were based on polymorphisms in conserved orthologous genes between Dasypyrum villosum and wheat chromosomes [40]. In the current study, 42 of 120 (35.0%) D. villosum 3V-specific IT markers generated 3J-specific bands, and 80.4% (74 of 92) of 5VL-specific markers produced 5JsL-specific bands. The results also verify that the V and Jsgenomes have a close phylogenetic relationship[52]and confirmed our GISH results.
Disomic addition line L2 (2n = 44) was derived from the progenies of crosses between the partial amphiploid line TAF46 and wheat cv. ‘Vilmorin 27' [53]. The added pair of Th.intermedium chromosomes in L2 was designated 3Ai by Friebe et al. [17]. Based on the GISH patterns of Th. intermedium chromosomes using Ps.strigosa(St genome)genomic DNA as a probe,Chen et al.[22]showed that the 3Ai chromosome in L2 belonged to the J genome of Th. intermedium. In order to compare the 3J chromosomes in A1082 and L2,sequential mc-FISH and molecular marker analyses were performed. First,sequential mc-FISH with Oligo-pSc119.2, Oligo-pTa535, Oligo-(GAA)7and Oligo-pSt122 was performed on metaphase cells of L2 (Fig. 2-D-F). The chromosome number of L2 was 2n = 44,including all 42 wheat chromosomes and a pair of 3Ai (3J)chromosomes. Probes Oligo-pSc119.2 and Oligo-pTa535 showed a pair of chromosomes with distinct Oligo-pTa535 signals in the interstitial region of the short arm and faint Oligo-pTa535 signals in the middle of long arm (Fig. 2-D, G)without green Oligo-pSc119.2 signals. In addition, the chromosome 3Ai (3J) displayed clear Oligo-(GAA)7signals at the proximal region of the short arm and strong Oligo-pSt122 signals at the telomeric region of the short arm (Fig. 2-E, F).Thus,the hybridization patterns produced by multiple probes revealed that chromosome 3J in A1082 was different from the 3Ai(3J)chromosome in L2(Fig.2-G).One hundred and twenty nine primer pairs (120 IT and 9 PLUG) from homoeologous group 3 were chosen to compare the chromosome 3Ai (3J) in L2 and the chromosome 3J in A1082. Seventy-six pairs of primers (58.9%) failed to produce Th. intermedium-specific polymorphic bands between A1082 and L2; 34 pairs (26.4%)generated identical bands indicating some commonalities between 3J and 3Ai(3J);and 19 pairs(14.7%)were polymorphic between the two lines, demonstrating that the two chromosomes 3J were genetically different (Fig. 4). In combination with the mc-FISH results, this indicated that A1082 was a distinctive, new 3J disomic addition line.
Stripe rust is widely regarded as one of the most destructive wheat diseases worldwide. Of the >80 designated wheat stripe rust resistance genes, only Yr50 was transferred from Th. intermedium [10]. We confirmed a new wheat-Th.intermedium 3J disomic addition line (A1082) that is highly resistant to stripe rust,and a T4BS·5JsL translocation line(A5-5) that is moderately resistant. In addition, chromosome 5JsL in A5-5 might carry a favorable gene(s)for grain yield.
It is well known that the disomic addition and substitution lines,as well as whole-arm translocation lines,can seldom be used as cultivars due to incomplete compensation for the missing wheat chromosomes or chromosome arms and deleterious effects often referred to as linkage drag [3]. The next step in progress with the materials developed here is production of lines containing smaller alien segments containing the genes of economic interest [54]. The lines have been subjected to60Coγ irradiation or crossed with the high pairing CS ph1b mutant as means of developing such lines.
5. Conclusions
A wheat-Thinopyrum intermedium ssp. trichophorum chromosome 3J disomic addition line and a homozygous T4BS·5JsL Robertsonian translocation line were developed and characterized by molecular cytogenetics and markers. Both lines carry gene(s) for resistance to stripe rust. A new oligonucleotide probe, Oligo-6H-2-100, was developed for specific identification of chromosome 5Js.
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.06.001.
Declaration of Competing Interest
Authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to Prof. Robert A.McIntosh, The University of Sydney, for reviewing the manuscript. This work was funded by the National Key Research and Development Program of China (2016YFD0102000), Applied and Basic Project(2016JY0075) from Science and Technology Department of Sichuan Province, China, and National Natural Science Foundation of China(No.31171542).
- The Crop Journal的其它文章
- Positional cloning of PmCH1357 reveals the origin and allelic variation of the Pm2 gene for powdery mildew resistance in wheat
- The Crop Journal 作物学报(英文版) (Started in 2013, Bimonthly)
- Wheat powdery mildew resistance gene Pm64 derived from wild emmer(Triticum turgidum var.dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5
- Wheat breeding in northern China: Achievements and technical advances
- Breed ing w heat for resistance to Fusarium head blight in the Global North: China,USA,and Canad a
- Pyramiding disease resistance genes in elite winter wheat germplasm for Western Canada

